Acute Bilateral Vestibular Neuropathy During Myocardial Infarction: A Case Report
Abstract
1. Introduction
1.1. Acute Vestibular Syndrome (AVS)
1.2. Vascular Supply of Labyrinth
1.3. Bilateral Vestibular Neuropathy and Similar Previous Reports
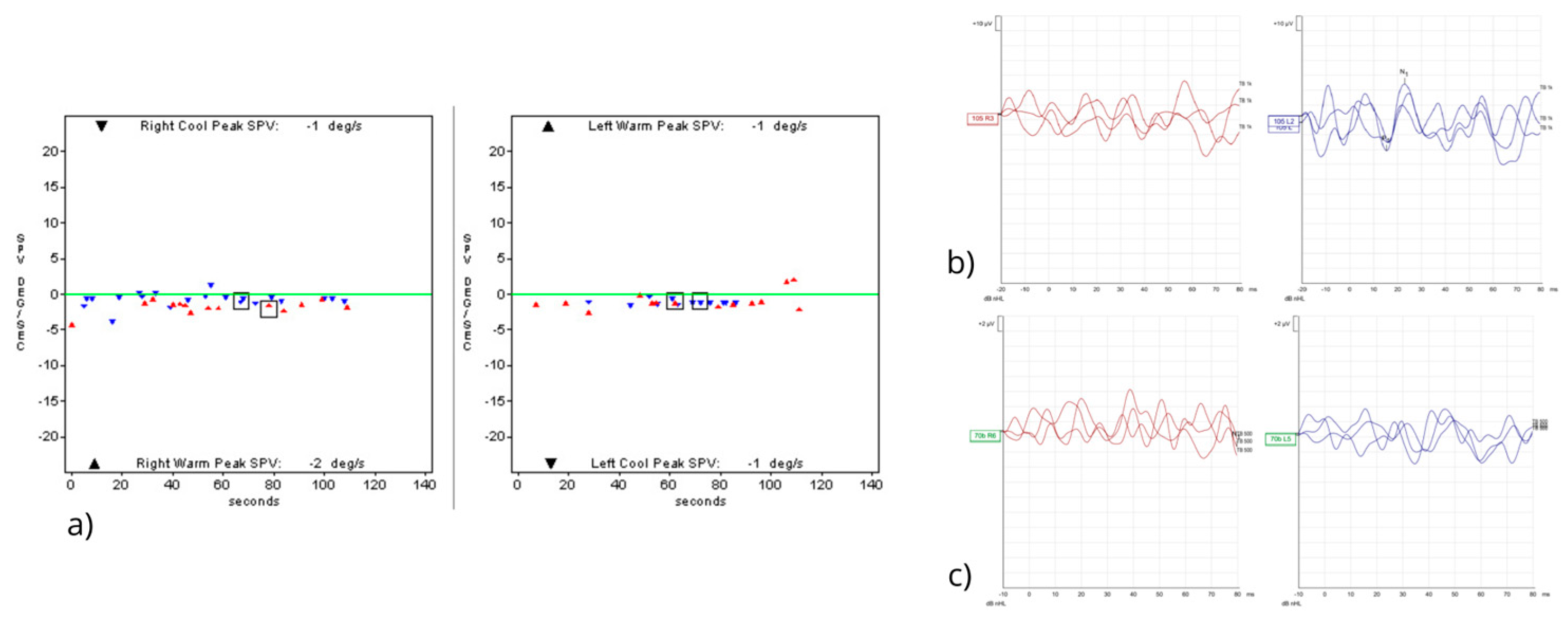
2. Case Report
3. Discussion
3.1. Rarity of the Condition and Pathophysiological Hypotheses
3.2. Clinical Features and Differential Diagnosis
3.3. Follow-Up, Outcome, and Comparison with the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABVN | Acute Bilateral Vestibular Neuropathy |
| AICA | Anterior Inferior Cerebellar Artery |
| AMI | Acute Myocardial Infarction |
| ASA | Acetylsalicylic Acid |
| ASC | Anterior Semicircular Canal |
| AVA | Anterior Vestibular Artery |
| AVS | Acute Vestibular Syndrome |
| BCG | Bacillus Calmette–Guerin |
| BSVN | Bilateral Sequential Vestibular Neuritis |
| BVH | Bilateral Vestibular Hypofunction |
| BVP | Bilateral Vestibulopathy |
| CCU | Cardiac Care Unit |
| CP | Computerized Posturography |
| CoG | Center of Gravity |
| DHI | Dizziness Handicap Inventory |
| ED | Emergency Department |
| HIMP | Head Impulse Paradigm |
| LSC | Lateral Semicircular Canal |
| MRI | Magnetic Resonance Imaging |
| NT-ProBNP | N-terminal prohormone of Brain Natriuretic Peptide |
| PSC | Posterior Semicircular Canal |
| PVA | Posterior Vestibular Artery |
| SHIMP | Suppression Head Impulse Paradigm |
| TIA | Transient Ischemic Attack |
| TURP | Transurethral Resection of the Prostate |
| VEMPs | Vestibular Evoked Myogenic Potentials |
| VN | Vestibular Neuritis |
| cVEMPs | cervical Vestibular Evoked Myogenic Potentials |
| oVEMPs | ocular Vestibular Evoked Myogenic Potentials |
| vHIT | video Head Impulse Test |
References
- Edlow, J.A.; Carpenter, C.; Akhter, M.; Khoujah, D.; Marcolini, E.; Meurer, W.J.; Morrill, D.; Naples, J.G.; Ohle, R.; Omron, R.; et al. Guidelines for Reasonable and Appropriate Care in the Emergency Department 3 (GRACE-3): Acute Dizziness and Vertigo in the Emergency Department. Acad. Emerg. Med. 2023, 30, 442–486. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Kim, J.-S. Approach to Dizziness in the Emergency Department. Clin. Exp. Emerg. Med. 2015, 2, 75–88. [Google Scholar] [CrossRef]
- Ängerud, K.H.; Ericsson, M.; Brännström, M.; Sederholm Lawesson, S.; Strömberg, A.; Thylén, I. Symptoms of Acute Myocardial Infarction as Described in Calls to Tele-Nurses and in Questionnaires. J. Cardiovasc. Nurs. 2023, 38, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Newman-Toker, D.E.; Camargo, C.A. ’Cardiogenic Vertigo’—True Vertigo as the Presenting Manifestation of Primary Cardiac Disease. Nat. Clin. Pract. Neurol. 2006, 2, 167–172, quiz 173. [Google Scholar] [CrossRef]
- Ortiz-Garcia, J.; Gomez, C.R.; Schneck, M.J.; Biller, J. Recent Advances in the Management of Transient Ischemic Attacks. Fac. Rev. 2022, 11, 19. [Google Scholar] [CrossRef]
- Picciotti, P.M.; Anzivino, R.; Galli, J.; Franceschi, F.; Conti, G.; Simeoni, B.; Covino, M. Clinical Evolution of Acute Vestibular Syndrome: Longitudinal Retrospective Analysis of Epidemiological Data and Prognostic Factors for Recovery. J. Pers. Med. 2023, 13, 407. [Google Scholar] [CrossRef]
- Edlow, J.A.; Gurley, K.L.; Newman-Toker, D.E. A New Diagnostic Approach to the Adult Patient with Acute Dizziness. J. Emerg. Med. 2018, 54, 469–483. [Google Scholar] [CrossRef]
- Strupp, M.; Bisdorff, A.; Furman, J.; Hornibrook, J.; Jahn, K.; Maire, R.; Newman-Toker, D.; Magnusson, M. Acute Unilateral Vestibulopathy/Vestibular Neuritis: Diagnostic Criteria. J. Vestib. Res. 2022, 32, 389–406. [Google Scholar] [CrossRef]
- Bae, C.H.; Na, H.G.; Choi, Y.S. Current Diagnosis and Treatment of Vestibular Neuritis: A Narrative Review. J. Yeungnam Med. Sci. 2022, 39, 81–88. [Google Scholar] [CrossRef]
- Simões, J.; Vlaminck, S.; Seiça, R.; Acke, F.; Miguéis, A. Vascular Mechanisms in Acute Unilateral Peripheral Vestibulopathy: A Systematic Review. ACTA Otorhinolaryngol. Ital. 2021, 41, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.L.; McGarvie, L.A.; Reid, N.; Young, A.S.; Halmagyi, G.M.; Welgampola, M.S. Vestibular Neuritis Affects Both Superior and Inferior Vestibular Nerves. Neurology 2016, 87, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-A.; Yi, H.-A.; Lee, H. Recent Advances in Cerebellar Ischemic Stroke Syndromes Causing Vertigo and Hearing Loss. Cerebellum 2016, 15, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Manzari, L.; Princi, A.A.; De Angelis, S.; Tramontano, M. Clinical Value of the Video Head Impulse Test in Patients with Vestibular Neuritis: A Systematic Review. Eur. Arch. Otorhinolaryngol. 2021, 278, 4155–4167. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Gioacchini, F.M.; Cassandro, E.; Tulli, M.; Ralli, M.; Re, M.; Cassandro, C. Clinical Application of cVEMPs and oVEMPs in Patients Affected by Ménière’s Disease, Vestibular Neuritis and Benign Paroxysmal Positional Vertigo: A Systematic Review. Acta Otorhinolaryngol. Ital. 2019, 39, 298–307. [Google Scholar] [CrossRef]
- Comacchio, F.; Mion, M.; Armato, E.; Castellucci, A. Sequential Vestibular Neuritis: Report of Four Cases and Literature Review. J. Audiol. Otol. 2021, 25, 89–97. [Google Scholar] [CrossRef]
- Lee, S.-U.; Kim, H.-J.; Kim, J.-S. Bilateral Vestibular Dysfunction. Semin. Neurol. 2020, 40, 40–48. [Google Scholar] [CrossRef]
- Yacovino, D.A.; Finlay, J.B.; Urbina Jaimes, V.N.; Verdecchia, D.H.; Schubert, M.C. Acute Bilateral Superior Branch Vestibular Neuropathy. Front. Neurol. 2018, 9, 353. [Google Scholar] [CrossRef]
- Ichijo, K.; Kinoshita, M.; Fujimoto, C.; Uranaka, T.; Kikkawa, Y.S.; Sugasawa, K.; Yamasoba, T.; Iwasaki, S. Acute Bilateral Vestibulopathy with Simultaneous Involvement of Both Superior and Inferior Vestibular Nerves. Auris Nasus Larynx 2020, 47, 905–908. [Google Scholar] [CrossRef]
- Lee, S.-U.; Kim, T.; Lee, E.-S. Acute Bilateral Vestibulopathy Associated With COVID-19. J. Clin. Neurol. 2022, 18, 247–249. [Google Scholar] [CrossRef]
- Martellucci, S.; Malara, P.; Pagliuca, G.; Castellucci, A. Lindsay-Hemenway Syndrome Involving the Horizontal Semicircular Canal: Some Considerations Upon Residual Canal Afferents in BPPV Secondary to an Ipsilateral Acute Unilateral Vestibulopathy. Otol. Neurotol. 2025, 46, 693–699. [Google Scholar] [CrossRef]
- Yacovino, D.A.; Zanotti, E.; Cherchi, M. The Spectrum of Acute Vestibular Neuropathy through Modern Vestibular Testing: A Descriptive Analysis. Clin. Neurophysiol. Pract. 2021, 6, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Kim, J.-S.; Murofushi, T.; Straumann, D.; Jen, J.C.; Rosengren, S.M.; Della Santina, C.C.; Kingma, H. Bilateral Vestibulopathy: Diagnostic Criteria Consensus Document of the Classification Committee of the Bárány Society. J. Vestib. Res. 2017, 27, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Bhat, J.S.; Sequeira, N.M.; Bhojwani, K.M. Ageing Effect on Air-Conducted Ocular Vestibular Evoked Myogenic Potential. Audiol. Res. 2015, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Comacchio, F.; Biancoli, E.; Poletto, E.; Bellemo, B.; Magnavita, P. Scientific Poster: “Neuropatia Vestibolare Bilaterale Acuta in Seguito ad Infarto del Miocardio: Un Case Report”. In Proceedings of the 111th National Congress of the Italian Society of Otorhinolaryngology (S.I.O.), Padova, Italy, 28–31 May 2025. [Google Scholar]

| Year, Author | Patient | Suspected Etiology | Outcomes | Key Findings |
|---|---|---|---|---|
| 2018, Yacovino et al. [17] | Male, 68 y | Viral (suspected, after upper respiratory tract infection) | Partial functional recovery documented at 1-year follow-up with updated instrumental assessment. | First documented case of ABVN of the superior branches of the vestibular nerves; supports bilateral VN as rare cause of BVH |
| 2019, Ichijo et al. [18] | Female, 36 y | Viral or autoimmune process (suspected, after fever; hypoparathyroidism) | Partial functional recovery documented at 1-year follow-up with updated instrumental assessment. | First reported case of ABVN involving both superior and inferior branches of the vestibular nerves |
| 2022, Lee et al. [19] | Male, 43 y | Viral (suspected, during SARS-CoV-2 infection) | Resolution of symptoms was confirmed through a telephone-based assessment at the 5-month follow-up. | First case linking COVID-19 to ABVN; incomplete vestibular instrumental assessment does not allow for topographic localization of the lesion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comacchio, F.; Biancoli, E.; Poletto, E.; Bellemo, B.; Magnavita, P. Acute Bilateral Vestibular Neuropathy During Myocardial Infarction: A Case Report. J. Otorhinolaryngol. Hear. Balance Med. 2025, 6, 15. https://doi.org/10.3390/ohbm6020015
Comacchio F, Biancoli E, Poletto E, Bellemo B, Magnavita P. Acute Bilateral Vestibular Neuropathy During Myocardial Infarction: A Case Report. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2025; 6(2):15. https://doi.org/10.3390/ohbm6020015
Chicago/Turabian StyleComacchio, Francesco, Elia Biancoli, Elisabetta Poletto, Barbara Bellemo, and Paola Magnavita. 2025. "Acute Bilateral Vestibular Neuropathy During Myocardial Infarction: A Case Report" Journal of Otorhinolaryngology, Hearing and Balance Medicine 6, no. 2: 15. https://doi.org/10.3390/ohbm6020015
APA StyleComacchio, F., Biancoli, E., Poletto, E., Bellemo, B., & Magnavita, P. (2025). Acute Bilateral Vestibular Neuropathy During Myocardial Infarction: A Case Report. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 6(2), 15. https://doi.org/10.3390/ohbm6020015








