Odontogenic Maxillary Sinusitis: The Interface and Collaboration between Rhinologists and Dentists
Abstract
:1. Introduction
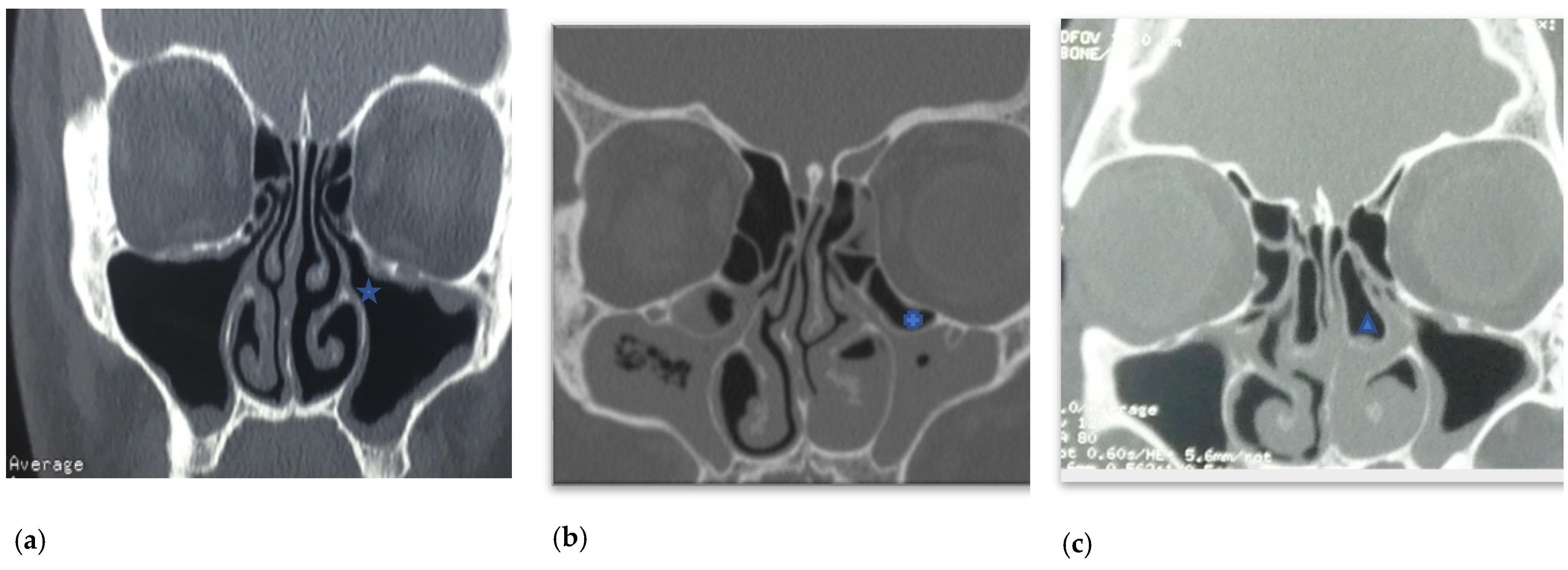
2. Aetiology
2.1. Iatrogenic
2.2. Endodontic and Periodontic Disease
2.3. Microbiology
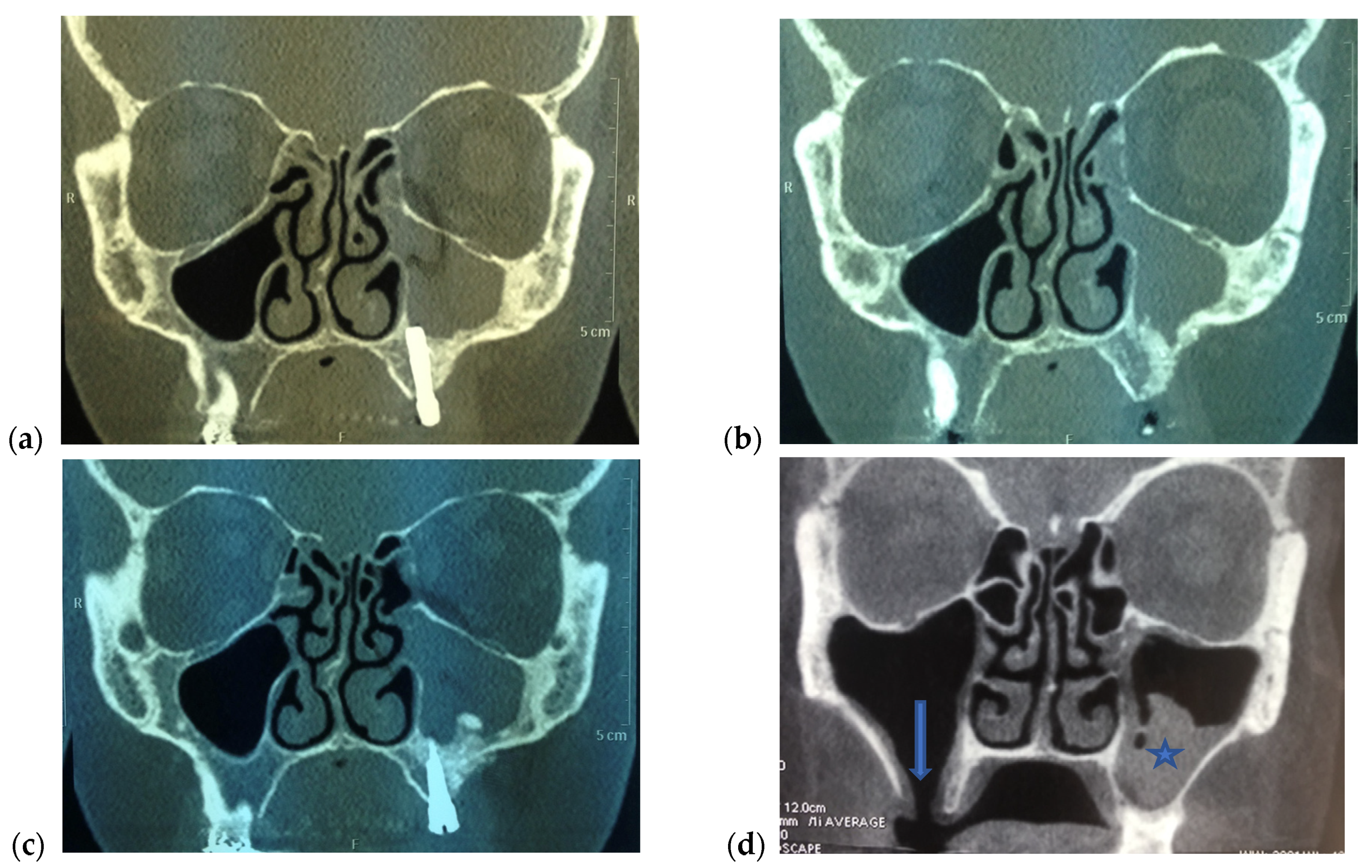
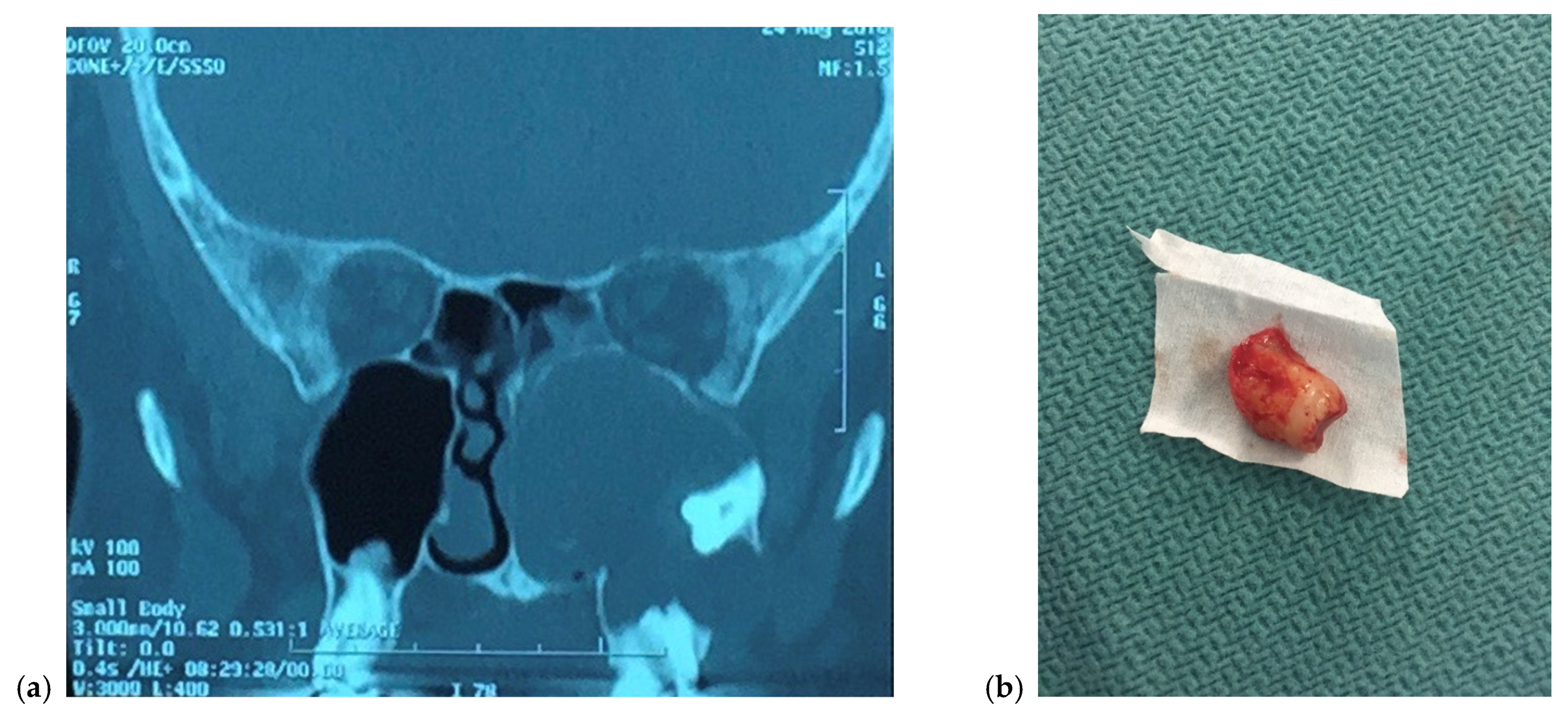
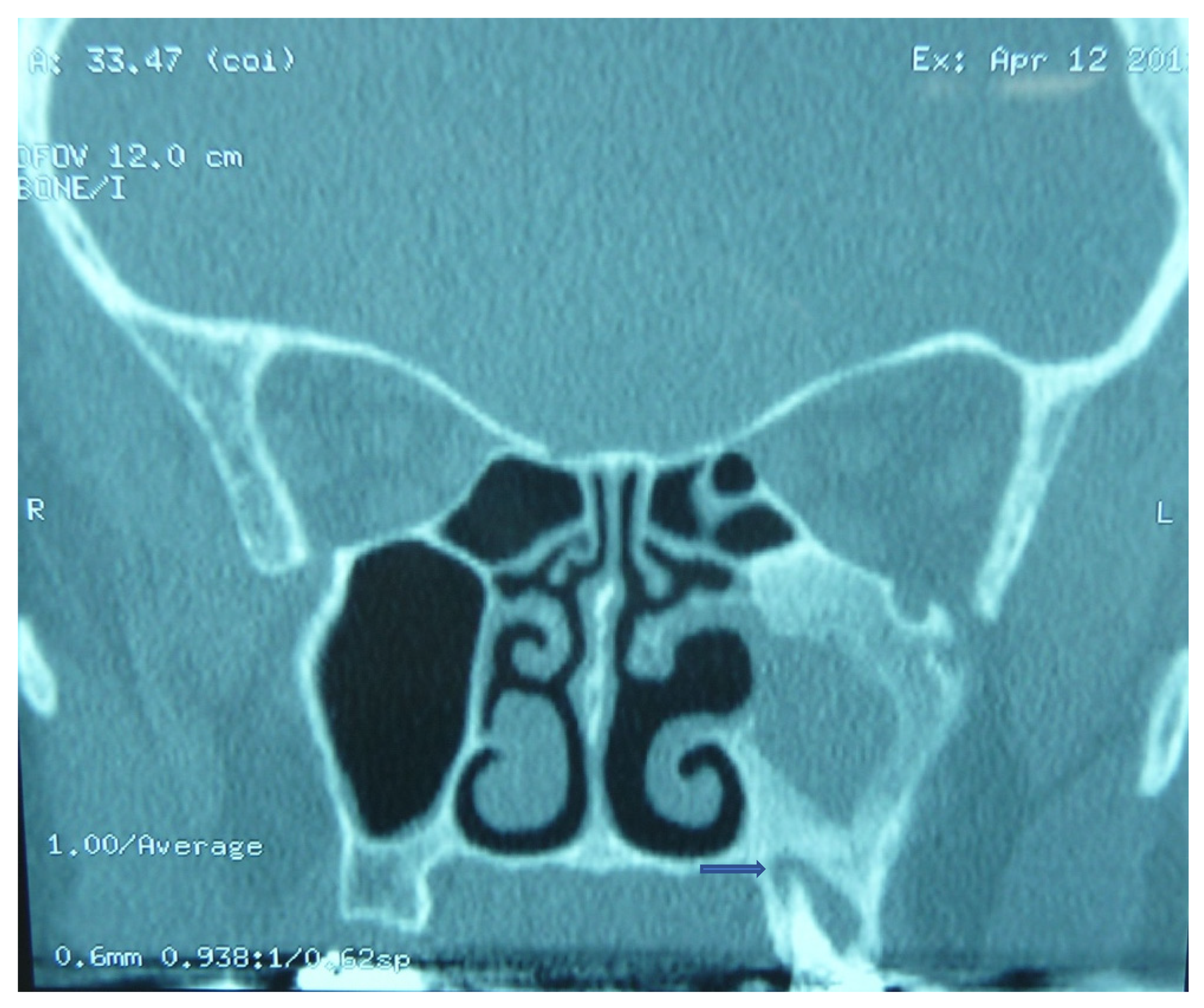
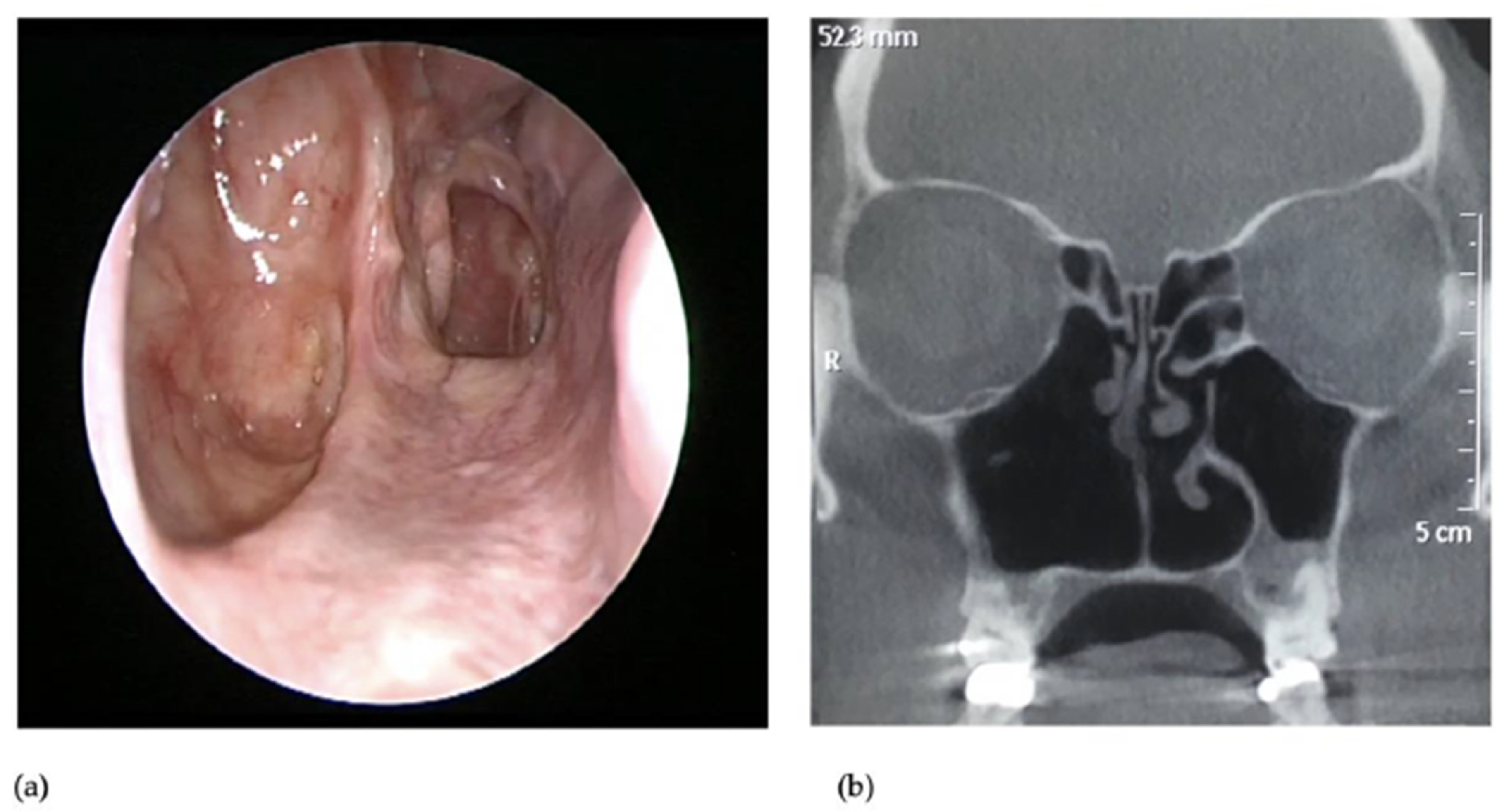
3. Presentation
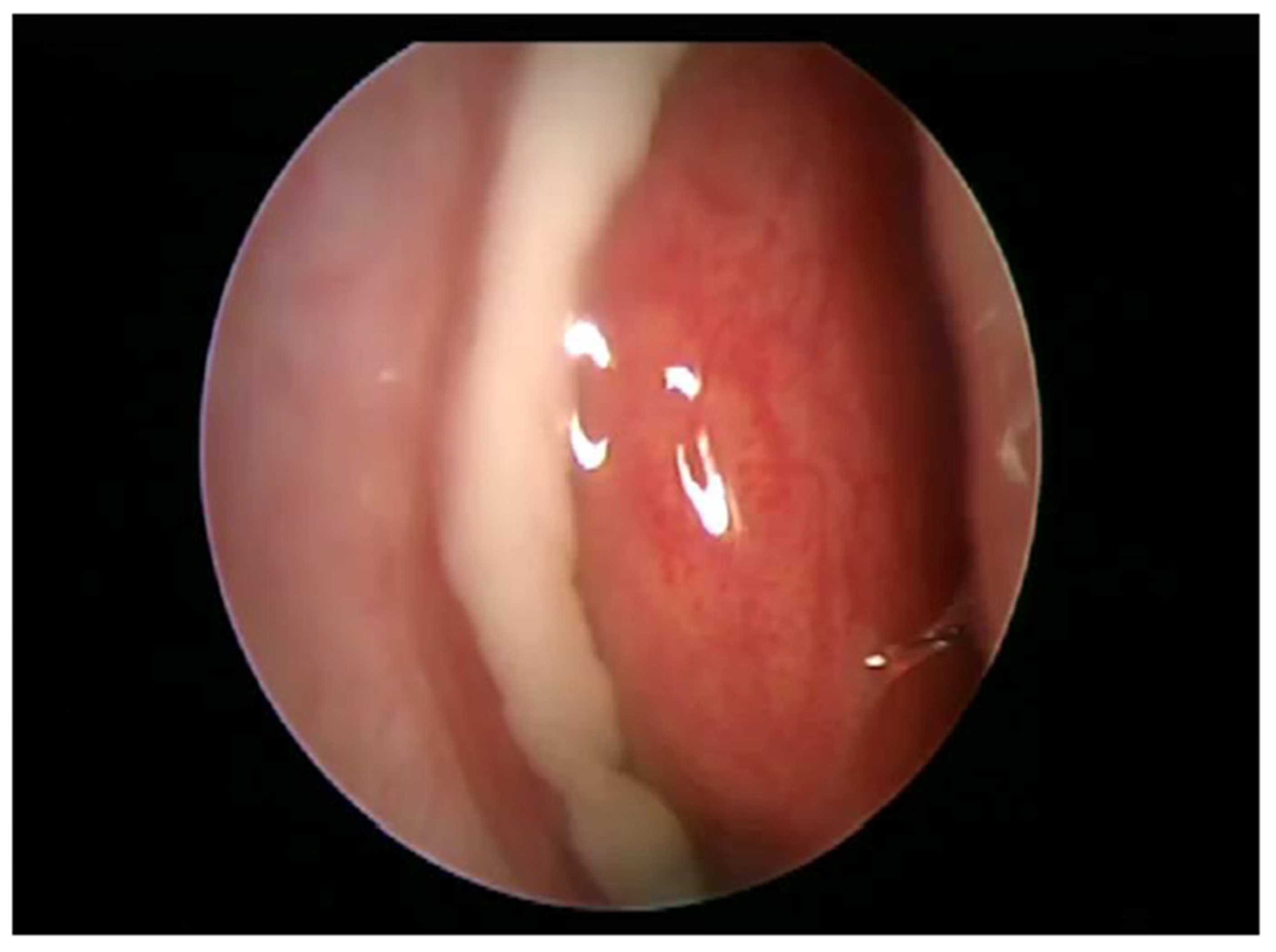
4. Management
5. Recommendations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puglisi, S.; Privitera, S.; Maiolino, L.; Serra, A.; Garotta, M.; Blandino, G.; Speciale, A. Bacteriological findings and antimicrobial resistance in odontogenic and non-odontogenic chronic maxillary sinusitis. J. Med. Microbiol. 2011, 60, 1353–1359. [Google Scholar] [CrossRef]
- Arias-Irimia, O.; Barona Dorado, C.; Santos Marino, J.; Martinez Rodriguez, N.; Martinez Gonzalez, J.M. Meta-analisis of the etiology of odontogenic maxillary sinusitis. Medicina Oral Patología Oral y Cirugía Buccal 2010, 15, e70–e73. [Google Scholar]
- Lechien, J.R.; Filleul, O.; De Araujo, P.C.; Hsieh, J.W.; Chantrain, G.; Saussez, S. Chronic Maxillary Rhinosinusitis of Dental Origin: A Systematic Review of 674 Patient Cases. Int. J. Otolaryngol. 2014, 2014, 465173. [Google Scholar] [CrossRef] [Green Version]
- Zirk, M.; Dreiseidler, T.; Pohl, M.; Rothamel, D.; Buller, J.; Peters, F.; Zöller, J.E.; Kreppel, M. Odontogenic sinusitis maxillaris: A retrospective study of 121 cases with surgical intervention. J. Cranio-Maxillofac. Surg. 2017, 45, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.S.; Sysolyatin, S.P.; Sysolyatin, P.G.; Melnikov, M.N. Chronic maxillary sinusitis of dental origin: Is external surgical approach mandatory? Laryngoscope 2002, 112, 1056–1059. [Google Scholar] [CrossRef]
- Hoskison, E.; Daniel, M.; E Rowson, J.; Jones, N.S. Evidence of an increase in the incidence of odontogenic sinusitis over the last decade in the UK. J. Laryngol. Otol. 2011, 126, 43–46. [Google Scholar] [CrossRef]
- Little, R.E.; Long, C.M.; Loehrl, T.A.; Poetker, D.M. Odontogenic sinusitis: A review of the current literature. Laryngoscope 2018, 3, 110–114. [Google Scholar] [CrossRef]
- Saibene, A.M.; Pipolo, G.C.; Lozza, P.; Maccari, A.; Portaleone, S.M.; Scotti, A.; Borloni, R.; Messina, F.; Di Pasquale, D.; Felisati, G. Redefining boundaries in odontogenic sinusitis: A retrospective evaluation of extramaxillary involvement in 315 patients. Int. Forum Allergy Rhinol. 2014, 4, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Reddy, U.D.M.A.; Dev, B. Pictorial essay: Anatomical variations of paranasal sinuses on multidetector computed tomography-How does it help FESS surgeons? Indian J. Radiol. Imaging 2012, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.-M.; Chrysikos, D.; Samolis, A.; Tsakotos, G.; Troupis, T. Anatomical Variations of the Nasal Cavities and Paranasal Sinuses: A Systematic Review. Cureus 2021, 13, e12727. [Google Scholar] [CrossRef]
- Akay, G.; Yaman, D.; Karadag, O.; Güngör, K. Evaluation of the Relationship of Dimensions of Maxillary Sinus Drainage System with Anatomical Variations and Sinusopathy: Cone-Beam Computed Tomography Findings. Med. Princ. Pract. 2019, 29, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.H. Maxillary sinusitis of dental origin. Am. J. Orthod. Oral Surg. 1943, 29, B133–B151. [Google Scholar] [CrossRef]
- Craig, J.R.; Dds, R.W.T.; Aghaloo, T.L.; Pokorny, A.T.; Gray, S.T.; Mattos, J.L.; Poetker, D.M. Management of odontogenic sinusitis: Multidisciplinary consensus statement. Int. Forum Allergy Rhinol. 2020, 10, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Psillas, G.; Papaioannou, D.; Petsali, S.; Dimas, G.G.; Constantinidis, J. Odontogenic maxillary sinusitis: A comprehensive review. J. Dent. Sci. 2020, 16, 474–481. [Google Scholar] [CrossRef]
- Yassin-Kassab, A.; Bhargava, P.; Tibbetts, R.J.; Griggs, Z.H.; Peterson, E.I.; Craig, J.R. Comparison of bacterial maxillary sinus cultures between odontogenic sinusitis and chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2021, 11, 40–47. [Google Scholar] [CrossRef]
- Galli, M.; DE Soccio, G.; Cialente, F.; Candelori, F.; Federici, F.R.; De Vincentiis, M.; Minni, A. Chronic maxillary sinusitis of dental origin and oroantral fistula: The results of combined surgical approach in an Italian university hospital. Bosn. J. Basic Med. Sci. 2020, 20, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Al-Dajani, M. Incidence, Risk Factors, and Complications of Schneiderian Membrane Perforation in Sinus Lift Surgery: A Meta-Analysis. Implant Dent. 2016, 25, 409–415. [Google Scholar] [CrossRef]
- Newsome, H.A.; Poetker, D.M. Odontogenic Sinusitis: Current Concepts in Diagnosis and Treatment. Immunol. Allergy Clin. N. Am. 2020, 40, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Akinyamoju, A.O.; Gbadebo, S.O.; Adeyemi, B.F. PERIAPICAL LESIONS OF THE JAWS: A REVIEW OF 104 CASES IN IBADAN. Ann. Ib. Postgrad. Med. 2014, 12, 115–119. [Google Scholar] [PubMed]
- Rajendra Santosh, A.B. Odontogenic Cysts. Dent. Clin. N. Am. 2020, 64, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Microbiology of Acute and Chronic Maxillary Sinusitis Associated with an Odontogenic Origin. Laryngoscope 2005, 115, 823–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saibene, A.M.; Vassena, C.; Pipolo, C.; Trimboli, M.; De Vecchi, E.; Felisati, G.; Drago, L. Odontogenic and rhinogenic chronic sinusitis: A modern microbiological comparison. Int. Forum Allergy Rhinol. 2015, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Emanuelli, E.; Robiony, M.; Zerman, N.; Polini, F.; Politi, M. Endoscopic Surgical Treatment of Chronic Maxillary Sinusitis of Dental Origin. J. Oral Maxillofac. Surg. 2007, 65, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Pinto, L.C.C.; Victor, F.L.; Da Silva, E.A.B.; Ribeiro, A.A.; Sarquis, M.I.D.M.; Camões, I.C.G. Aspergillus in endodontic infection near the maxillary sinus. Braz. J. Otorhinolaryngol. 2015, 81, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Elwany, S.; A Ibrahim, A.; A Hussein, W.K.; Medra, A.M.; Elwany, N. Ten-year experience with multidisciplinary diagnosis and treatment of odontogenic sinusitis. J. Laryngol. Otol. 2021, 135, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Longhini, A.B.; Ferguson, B.J. Clinical aspects of odontogenic maxillary sinusitis: A case series. Int. Forum Allergy Rhinol. 2011, 1, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Lee, S.J. Clinical Features and Treatments of Odontogenic Sinusitis. Yonsei Med. J. 2010, 51, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Workman, A.D.; Granquist, E.J.; Adappa, N.D. Odontogenic sinusitis: Developments in diagnosis, microbiology, and treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Nichols, B.G.; Poetker, D.M.; Loehrl, T.A. Odontogenic sinusitis: A case series studying diagnosis and management. Int. Forum Allergy Rhinol. 2015, 5, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ikeda, T.; Yokoi, H.; Kohno, N. Association between odontogenic infections and unilateral sinus opacification. Auris Nasus Larynx 2015, 42, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Turfe, Z.; Ahmad, A.; Peterson, E.I.; Craig, J.R. Odontogenic sinusitis is a common cause of unilateral sinus disease with maxillary sinus opacification. Int. Forum Allergy Rhinol. 2019, 9, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, A.; Tataryn, R. Clinical and radiologic findings in a case series of maxillary sinusitis of dental origin. Int. Forum Allergy Rhinol. 2013, 3, 973–979. [Google Scholar] [CrossRef]
- Costa, F.; Emanuelli, E.; Franz, L.; Tel, A.; Robiony, M. Single-step surgical treatment of odontogenic maxillary sinusitis: A retrospective study of 98 cases. J. Cranio-Maxillofacial Surg. 2019, 47, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Nakai, Y.; Samukawa, Y.; Miyake, M.; Hoshikawa, H. Assessment of Simultaneous Surgery for Odontogenic Sinusitis: Endoscopic Sinus Surgery with Endoscopic Apicoectomy. J. Craniofacial Surg. 2019, 30, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.R.; McHugh, C.I.; Do, Z.H.G.; Peterson, E.I. Optimal timing of endoscopic sinus surgery for odontogenic sinusitis. Laryngoscope 2019, 129, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, R.A.; Hoehle, L.P.; Smeets, R.; Heiland, M.; Caradonna, D.S.; Gray, S.T.; Sedaghat, A.R. Impact of odontogenic chronic rhinosinusitis on general health-related quality of life. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Ungar, O.J.; Yafit, D.; Kleinman, S.; Raiser, V.; Safadi, A. Odontogenic sinusitis involving the frontal sinus: Is middle meatal antrostomy enough? Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Huang, C.-C.; Chang, P.-H.; Chen, C.-W.; Wu, C.-C.; Fu, C.-H.; Lee, T.-J. The Characteristics and New Treatment Paradigm of Dental Implant–related Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2013, 27, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Crovetto-Martinez, R.; Martin-Arregui, F.; Zabala-Lopez-De-Maturana, A.; Tudela-Cabello, K.; La Torre, M.C.-D. Frequency of the odontogenic maxillary sinusitis extended to the anterior ethmoid sinus and response to surgical treatment. Medicina Oral Patología Oral y Cirugía Buccal 2014, 19, e409–e413. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, J.L.; David, R.M.; Lensing, S.Y.; Samant, R.S.; Kumar, M.; Van Hemert, R.L.; Angtuaco, E.J.; Fitzgerald, R.T. Root Cause Analysis: An Examination of Odontogenic Origins of Acute Maxillary Sinusitis in Both Immunocompetent & Immunocompromised Patients. J. Comput. Assist. Tomogr. 2017, 41, 484–488. [Google Scholar] [CrossRef]
- Turfe, Z. Odontogenic sinusitis is a common cause of unilateral maxillary sinus and middle meatal polypoid disease. Int. Forum Allergy Rhinol. 2019, 9, S97. [Google Scholar] [CrossRef] [PubMed]
- Haiderali, Z. The role of CBCT in implant dentistry: Uses, benefits and limitations. Br. Dent. J. 2020, 228, 560–561. [Google Scholar]
- Meltzer, E.O.; Bachert, C.; Staudinger, H. Treating acute rhinosinusitis: Comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. J. Allergy Clin. Immunol. 2005, 116, 1289–1295. [Google Scholar] [CrossRef]
- Mattos, J.L.; Ferguson, B.J.; Lee, S. Predictive factors in patients undergoing endoscopic sinus surgery for odontogenic sinusitis. Int. Forum Allergy Rhinol. 2016, 6, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Tomomatsu, N.; Uzawa, N.; Aragaki, T.; Harada, K. Aperture width of the osteomeatal complex as a predictor of successful treatment of odontogenic maxillary sinusitis. Int. J. Oral Maxillofac. Surg. 2014, 43, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Felisati, G.; Chiapasco, M.; Lozza, P.; Saibene, A.M.; Pipolo, G.C.; Zaniboni, M.; Biglioli, F.; Borloni, R. Sinonasal Complications Resulting from Dental Treatment: Outcome-Oriented Proposal of Classification and Surgical Protocol. Am. J. Rhinol. Allergy 2013, 27, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.J.; George, S.; Preethi, S.; Arunraj, V.S. A Comparative Study Between Endoscopic Middle Meatal Antrostomy and Caldwell-Luc Surgery in the Treatment of Chronic Maxillary Sinusitis. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.J.; Luong, A.; Yao, W.C.; Citardi, M.J. Management of Odontogenic Cysts by Endonasal Endoscopic Techniques: A Systematic Review and Case Series. Am. J. Rhinol. Allergy 2018, 32, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, J.S.; Kim, H.T.; Lee, C.H.; Park, Y.H.; Bae, J.H. Clinical features and treatment outcomes of dental implant-related paranasal sinusitis: A 2-year prospective observational study. Clin. Oral Implant. Res. 2015, 27, e100–e104. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.K.D.; Lebowitz, R.A.; Giacchi, R.J.; Glickman, R.; Jacobs, J.B. Chronic Sinusitis Complicating Sinus Lift Surgery. Am. J. Rhinol. 2001, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Andric, M.; Saranovic, V.; Drazic, R.; Brkovic, B.; Todorovic, L. Functional endoscopic sinus surgery as an adjunctive treatment for closure of oroantral fistulae: A retrospective analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 510–516. [Google Scholar] [CrossRef]
- Saibene, A.M.; Collurà, F.; Pipolo, C.; Bulfamante, A.M.; Lozza, P.; Maccari, A.; Arnone, F.; Ghelma, F.; Allevi, F.; Biglioli, F.; et al. Odontogenic rhinosinusitis and sinonasal complications of dental disease or treatment: Prospective validation of a classification and treatment protocol. Eur. Arch. Oto-Rhino-Laryngol. 2018, 276, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Lin, W.; Yuan, W.; Chen, L. New Insight on Pathophysiology, Diagnosis, and Treatment of Odontogenic Maxillary Sinusitis. J. Nanomater. 2021, 2021, 9997180. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandakumar, B.S.; Niles, N.N.A.; Kalish, L.H. Odontogenic Maxillary Sinusitis: The Interface and Collaboration between Rhinologists and Dentists. J. Otorhinolaryngol. Hear. Balance Med. 2021, 2, 8. https://doi.org/10.3390/ohbm2040008
Nandakumar BS, Niles NNA, Kalish LH. Odontogenic Maxillary Sinusitis: The Interface and Collaboration between Rhinologists and Dentists. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2021; 2(4):8. https://doi.org/10.3390/ohbm2040008
Chicago/Turabian StyleNandakumar, Beeshman Saireuben, Naomi Natasha Amalee Niles, and Larry Hilton Kalish. 2021. "Odontogenic Maxillary Sinusitis: The Interface and Collaboration between Rhinologists and Dentists" Journal of Otorhinolaryngology, Hearing and Balance Medicine 2, no. 4: 8. https://doi.org/10.3390/ohbm2040008
APA StyleNandakumar, B. S., Niles, N. N. A., & Kalish, L. H. (2021). Odontogenic Maxillary Sinusitis: The Interface and Collaboration between Rhinologists and Dentists. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 2(4), 8. https://doi.org/10.3390/ohbm2040008




