Extrusion 3D-Printed Kaolinite Ceramic Filters for Water Applications
Abstract
1. Introduction
2. Materials
3. Methodology
3.1. Printer Preparation
3.2. Printer Parameters
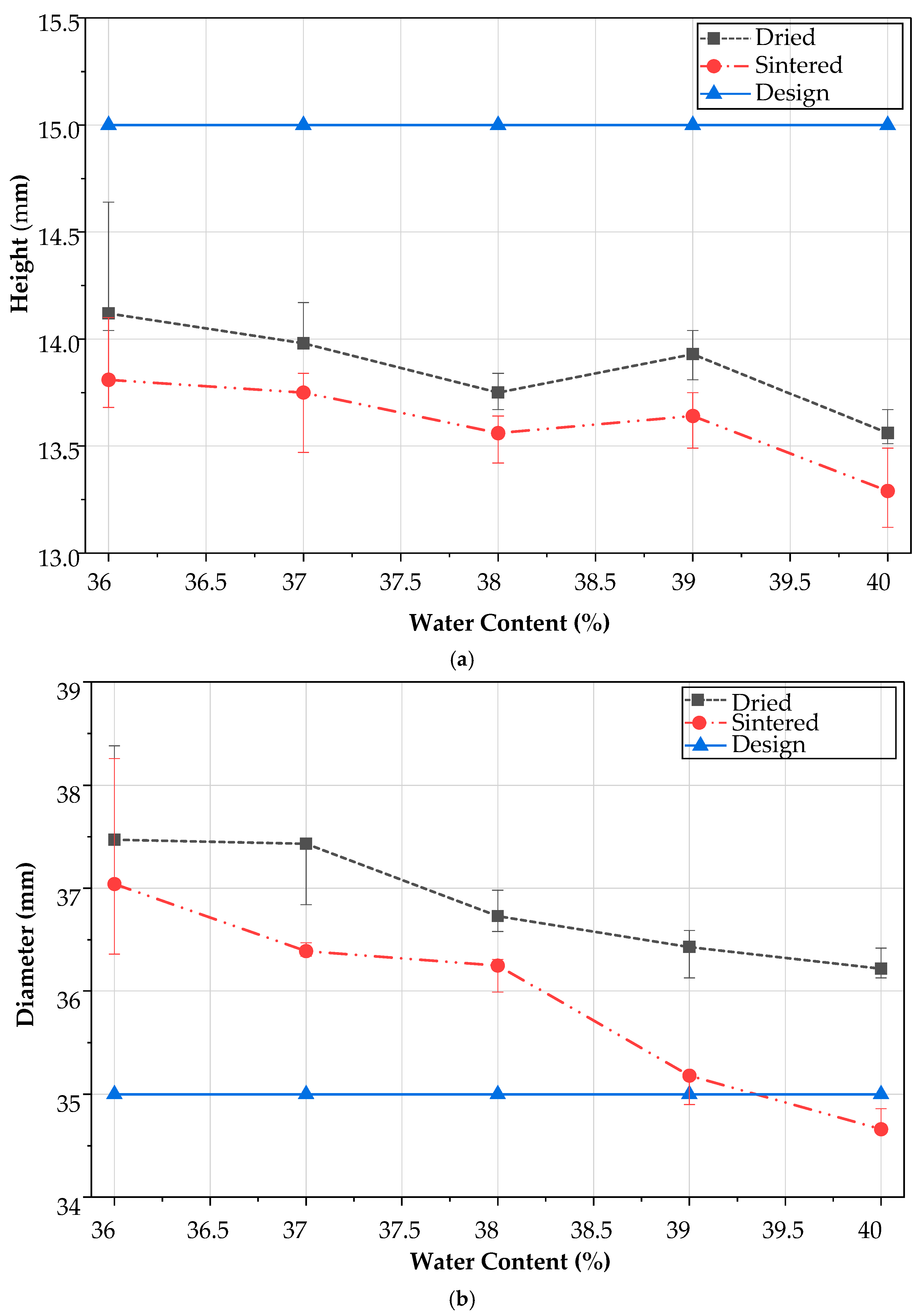
3.3. Effect of Water Content
3.4. Effect of Sintering on Dimension Stability
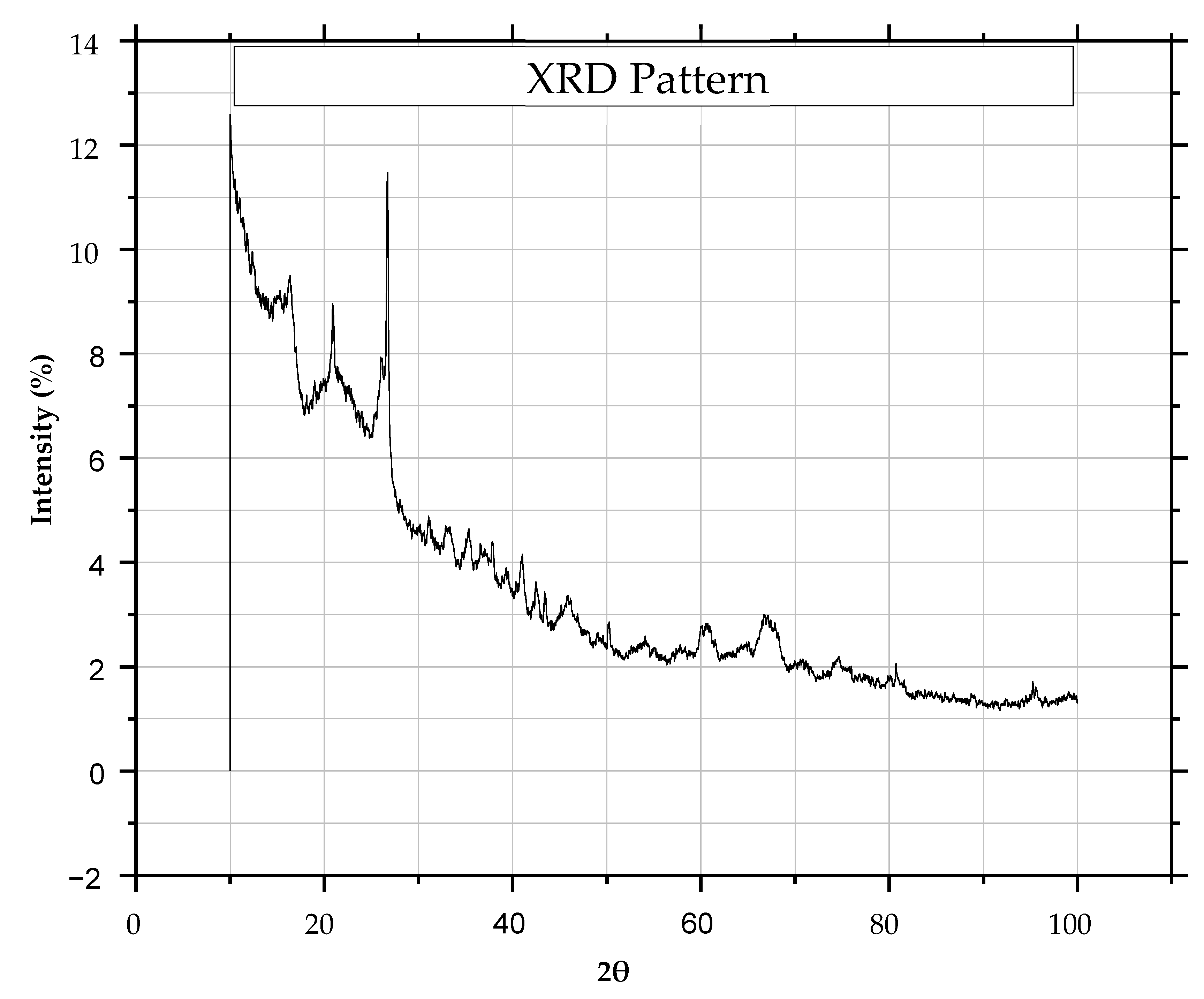
3.5. X-Ray Diffraction (XRD)
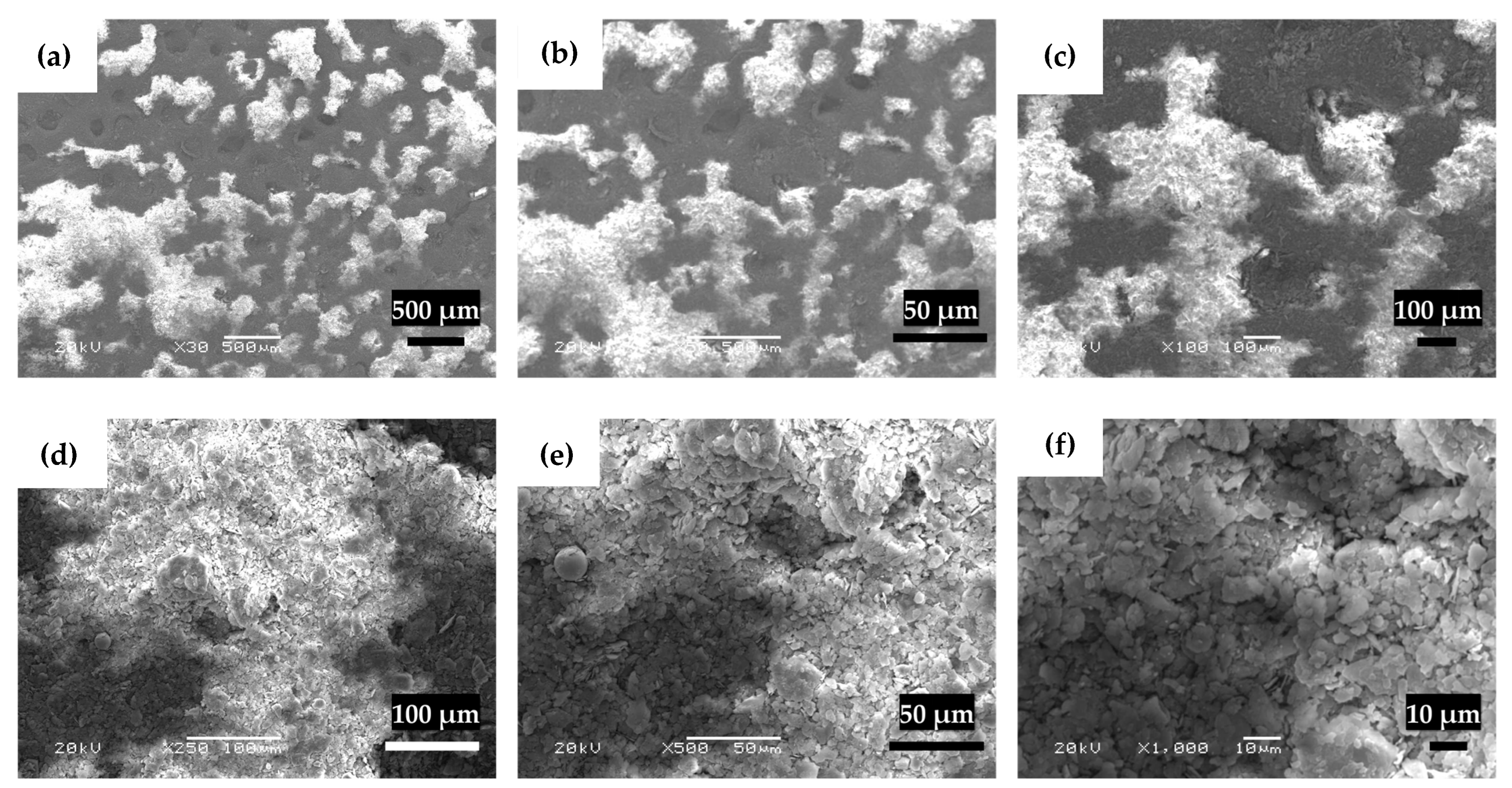
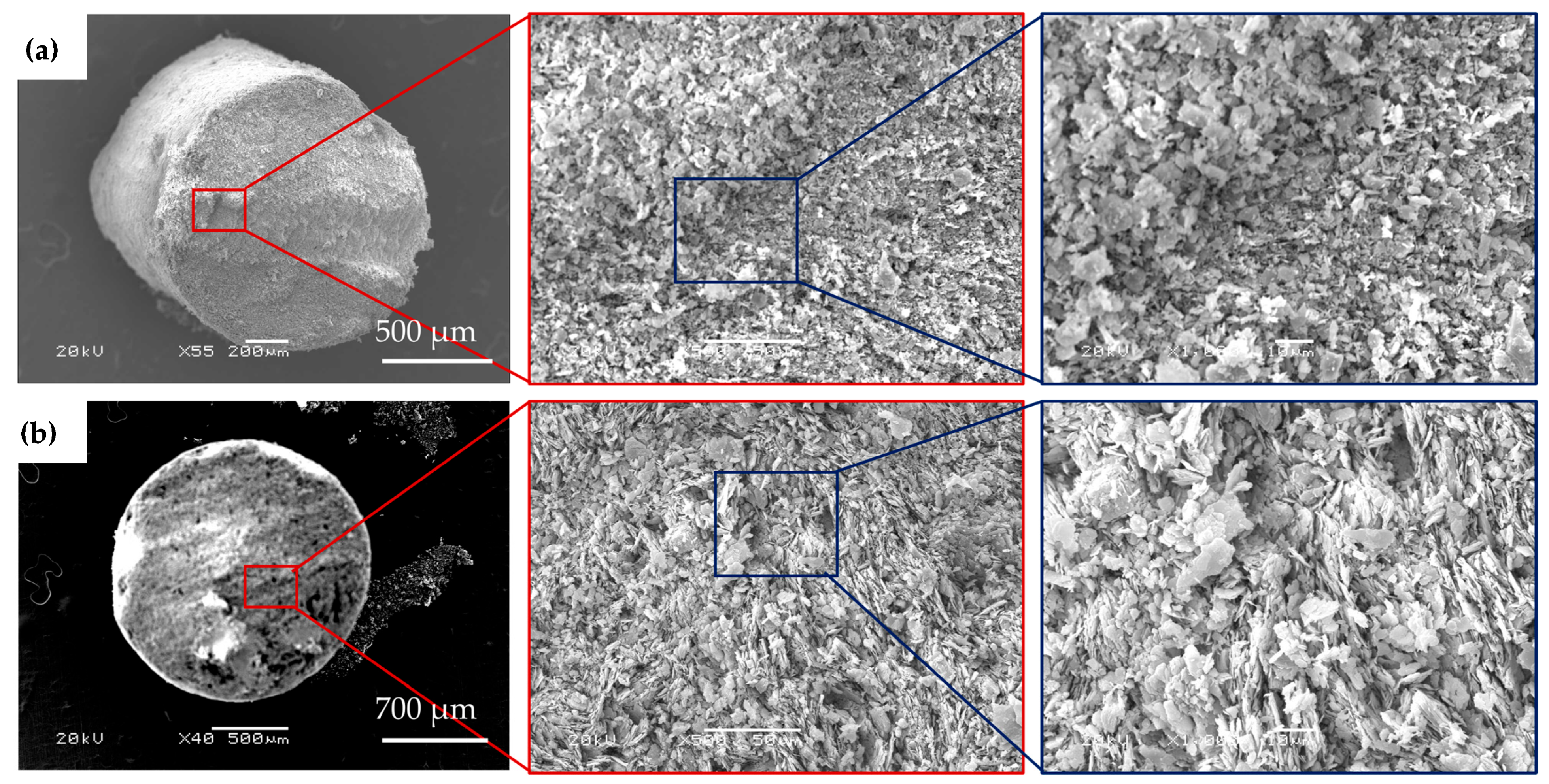
3.6. Scanning Electron Microscopy (SEM)
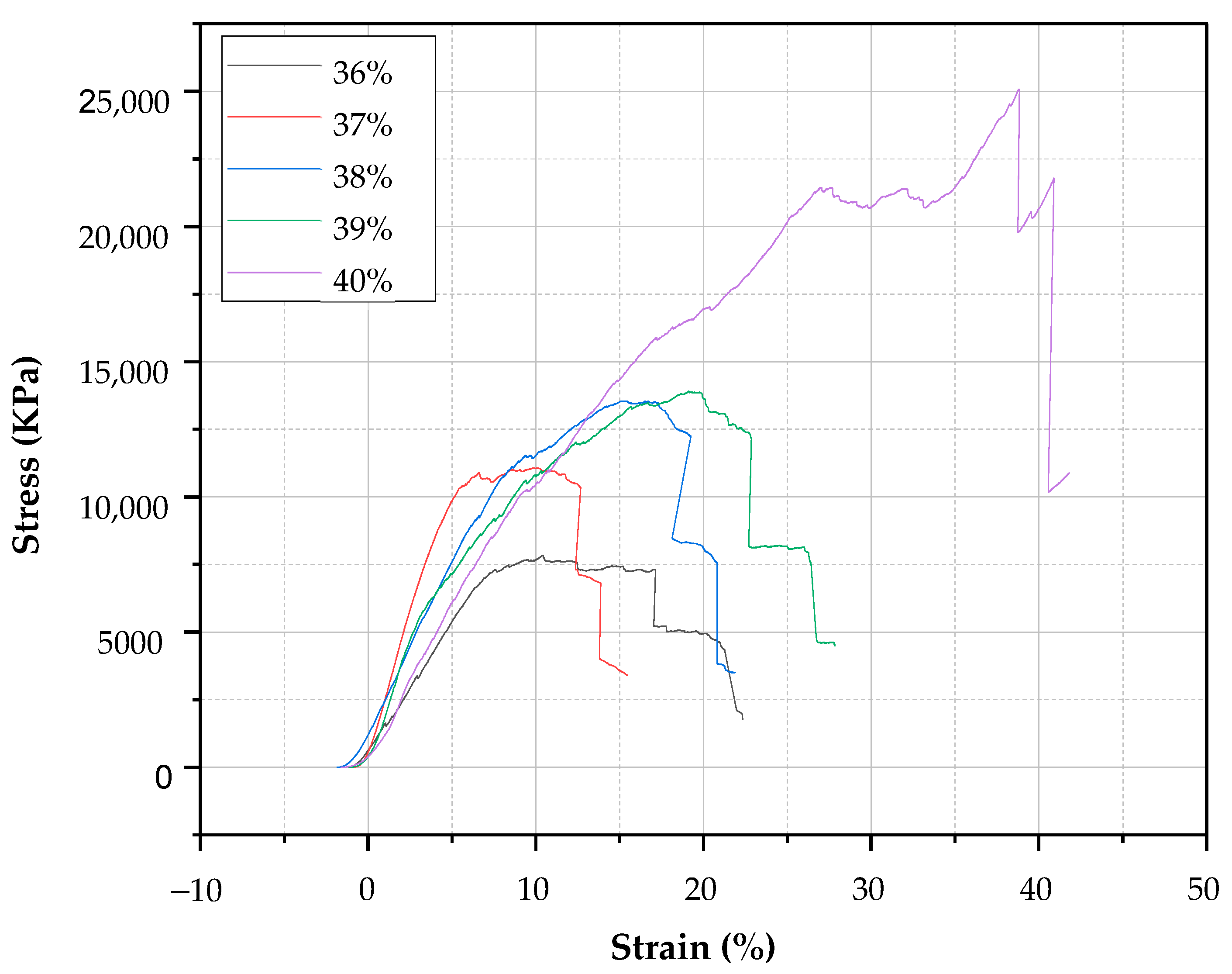
3.7. Compression Test
3.8. Water Filtration
3.8.1. Turbidity Test
3.8.2. Watercolor Test
3.8.3. pH Test
3.8.4. Conductivity Test
3.8.5. Mineral Concentration Test
3.8.6. Total Hardness and Calcium Hardness Test
3.8.7. M-Alkalinity Test
3.8.8. Microbiological Filtration
4. Results
4.1. Kaolinite Powder Size Distribution
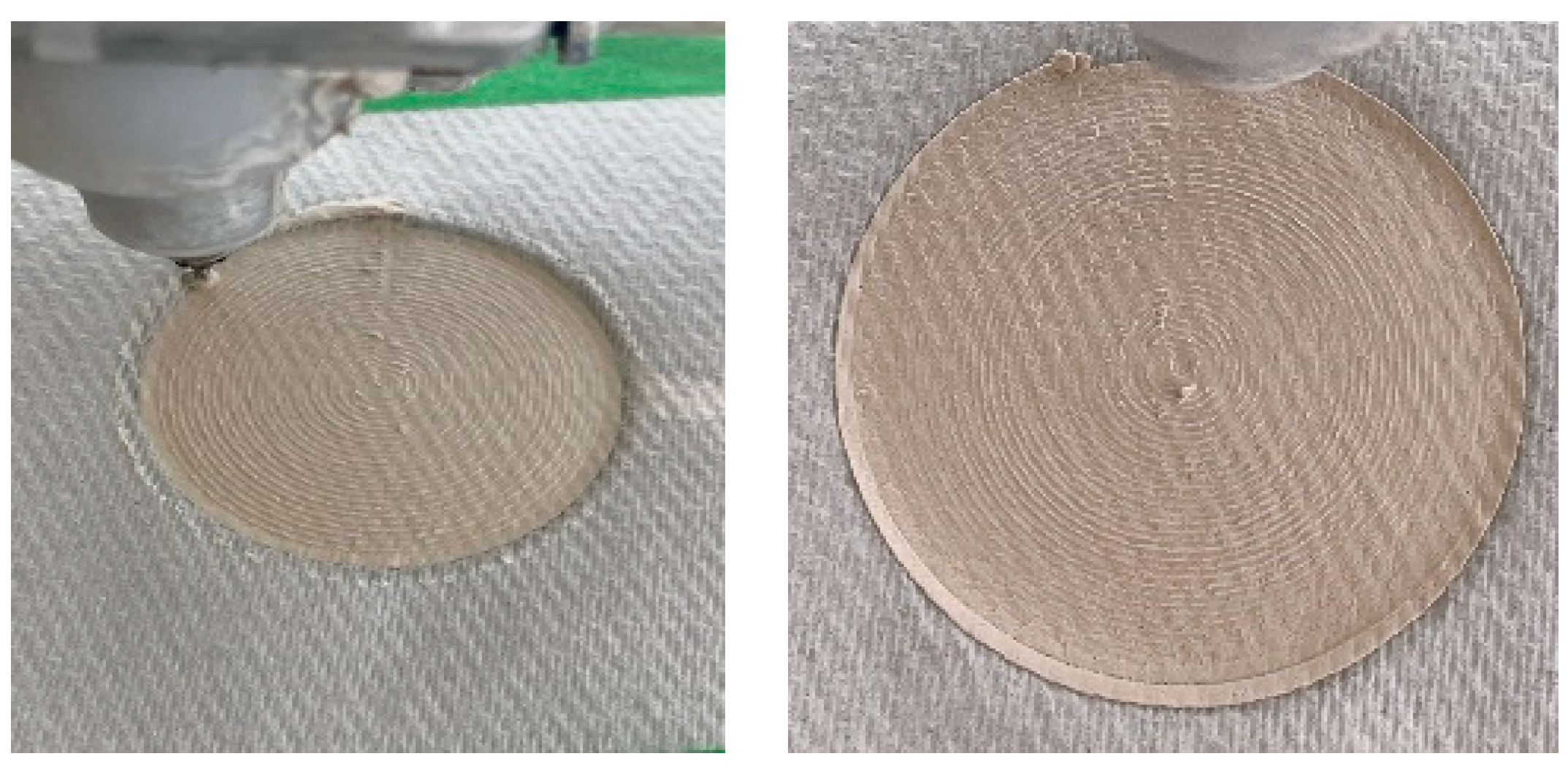
4.2. Printing Substrate
4.3. Dimension Stability After Sintering
4.4. X-Ray Diffraction (XRD)
4.5. Scanning Electron Microscopy (SEM)
4.6. Compression Test
4.7. Filtration Results
4.7.1. Turbidity Test
4.7.2. Watercolor Test
4.7.3. pH Test
4.7.4. Conductivity Test
4.7.5. Mineral Concentration Test
4.7.6. Total Hardness and Calcium Hardness Test
4.7.7. M-Alkalinity Test
4.7.8. Microbiological Filtration
4.7.9. Comparison with Traditional Filters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Motalebi, A.; Khondoker, M.A.H.; Kabir, G. A systematic review of life cycle assessments of 3D concrete printing. Sustain. Oper. Comput. 2024, 5, 41–50. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, X.; Shang, Y.; Xiong, K.; Xu, Z.; Guo, R.; Cai, S.; Zheng, C. Dense ceramics with complex shape fabricated by 3D printing: A review. J. Adv. Ceram. 2021, 10, 195–218. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef] [PubMed]
- Rimašauskas, M.; Kuncius, T.; Rimašauskienė, R.; Simokaitis, T. Evaluation of Conditions for Self-Healing of Additively Manufactured Polymer Composites with Continuous Carbon Fiber Reinforcement. J. Manuf. Mater. Process. 2025, 9, 179. [Google Scholar] [CrossRef]
- Ihekweme, G.O.; Obianyo, I.I.; Orisekeh, K.I.; Kalu-Uka, G.M.; Nwuzor, I.C.; Onwualu, A.P. Plasticity characterization of certain Nigeria clay minerals for their application in ceramic water filters. Sci. Prog. 2021, 104, 00368504211012148. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chang, L.; Liu, W.; Xiong, Z.; Zhao, Y.; Zhang, J. Advances in mercury removal from coal-fired flue gas by mineral adsorbents. Chem. Eng. J. 2020, 379, 122263. [Google Scholar] [CrossRef]
- Sheikhi, M.; Arzani, M.; Mahdavi, H.R.; Mohammadi, T. Kaolinitic clay-based ceramic microfiltration membrane for oily wastewater treatment: Assessment of coagulant addition. Ceram. Int. 2019, 45, 17826–17836. [Google Scholar] [CrossRef]
- Pang, R.; Lai, M.K.; Teo, H.H.; Yap, T.C. Influence of Temperature on Interlayer Adhesion and Structural Integrity in Material Extrusion: A Comprehensive Review. J. Manuf. Mater. Process. 2025, 9, 196. [Google Scholar] [CrossRef]
- Revelo, C.F.; Colorado, H.A. 3D printing of kaolinite clay ceramics using the Direct Ink Writing (DIW) technique. Ceram. Int. 2018, 44, 5673–5682. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Przybyła, S.; Kwiatkowski, M.; Hebda, M. The Concept of Using 3D Printing Technology in Ceramic Foundry Filter Manufacturing. J. Mater. Eng. Perform. 2024, 33, 14426–14432. [Google Scholar] [CrossRef]
- del Bosque, A.; Fernández-Arias, P.; Vergara, D. Advances in the Additive Manufacturing of Superalloys. J. Manuf. Mater. Process. 2025, 9, 215. [Google Scholar] [CrossRef]
- Elsersawy, R.; Rahman, A.; Sakib-Uz-Zaman, C.; Khondoker, M.A.H. Multifunctional inks in aerosol jet printing: Performance, challenges, and applications. Front. Manuf. Technol. 2025, 5, 1558209. [Google Scholar] [CrossRef]
- Donyadari, R.; Ortiz, B.F.; Khondoker, M.A.H. Optimization of Printing Parameters for Extrusion 3D Printing of Ceramic Clay. In Proceedings of the 1st International Conference on Industrial, Manufacturing, and Process Engineering (ICIMP-2024), Regina, SK, Canada, 27–29 June 2024; p. 47. [Google Scholar] [CrossRef]
- Habib, M.A.; Elsersawy, R.; Khondoker, M.A.H. Pellet-Based Extrusion Additive Manufacturing of Lightweight Parts Using Inflatable Hollow Extrudates. J. Manuf. Mater. Process. 2025, 9, 37. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, Y.; Li, P.; Chen, J. Fine lattice structural titanium dioxide ceramic produced by DLP 3D printing. Ceram. Int. 2019, 45, 23007–23012. [Google Scholar] [CrossRef]
- Frontmatter. In Ceramic Materials; Wiley: Hoboken, NJ, USA, 2007. [CrossRef]
- ASTM C1424-15(2019); Test Method for Monotonic Compressive Strength of Advanced Ceramics at Ambient Temperature. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019. [CrossRef]
- 2024 Drinking Water Quality Report. Available online: https://www.regina.ca/home-property/water/water/quality-protection/ (accessed on 1 July 2025).
- Co-Op Refinery. Available online: https://www.fcl.crs/our-business/refinery (accessed on 1 July 2025).
- Sachan, A.; Penumadu, D. Identification of microfabric of kaolinite clay mineral using X-ray diffraction technique. Geotech. Geol. Eng. 2007, 25, 603–616. [Google Scholar] [CrossRef]
- Yanu, C.A.; Nguiamba, N.; Sieliechi, J.M.; Ngassoum, M.B. Elaboration of Ceramic Pot Filter from Kaolinite (Cameroon Clay) for the Elimination of Suspended Particles from Domestic Drinking Water. J. Mater. Sci. Chem. Eng. 2023, 11, 43–60. [Google Scholar] [CrossRef]
- Yusuf, K.O.; Abadunmi, T.; Adeola, A.; Giegbefumwen, S.O. A Simple Ceramic Water Filter for Water Purification in the Rural Area. Technium 2023, 12. [Google Scholar] [CrossRef]
- Heikal, G. Removal of heavy metals using kaolinite and Bentonite filter media. Int. J. Curr. Eng. Technol. 2018, 8, 1535–1541. [Google Scholar] [CrossRef]



| Mix (g) | Water (wt%) | Kaolinite (wt%) | Water/Kaolinite Ratio |
|---|---|---|---|
| 700 | 36 | 64 | 0.56 |
| 700 | 37 | 63 | 0.59 |
| 700 | 38 | 62 | 0.61 |
| 700 | 39 | 61 | 0.64 |
| 700 | 40 | 60 | 0.67 |
| 36% |  |  |  |
| 37% |  |  |  |
| 38% |  |  |  |
| 39% |  |  |  |
| 40% |  |  |  |
 |  |  |  |
| Fiber Glass | Aluminum Foil | Cardstock Paper | Tissue Paper |
| Water (wt%) | Designed Height | Height | H’ | Designed Diameter | Diameter | D’ |
|---|---|---|---|---|---|---|
| 36% | 15 | 14.12 | 13.81 | 35 | 37.43 | 36.36 |
| 14.04 | 13.68 | 38.38 | 37.04 | |||
| 14.64 | 14.1 | 37.47 | 38.26 | |||
| 37% | 13.98 | 13.84 | 37.43 | 36.39 | ||
| 13.98 | 13.75 | 36.84 | 36.34 | |||
| 14.17 | 13.47 | 37.47 | 36.47 | |||
| 38% | 13.84 | 13.42 | 36.98 | 35.99 | ||
| 13.75 | 13.64 | 36.73 | 36.31 | |||
| 13.67 | 13.56 | 36.58 | 36.25 | |||
| 39% | 13.81 | 13.49 | 36.43 | 35.18 | ||
| 13.93 | 13.64 | 36.59 | 34.9 | |||
| 14.04 | 13.75 | 36.13 | 35.18 | |||
| 40% | 13.56 | 13.49 | 36.22 | 34.64 | ||
| 13.67 | 13.29 | 36.42 | 34.86 | |||
| 13.51 | 13.12 | 36.13 | 34.66 |
| Water% | Maximum Force (KN) | Area (m2) | Max Stress (KN/m2) | Max Strain (%) | Force at Peak 1 | Force at Peak 2 | Force at Peak 3 |
|---|---|---|---|---|---|---|---|
| 36% | 9.71 | 0.001096235 | 8858 | 17.35 | 9.52 | 9.71 | |
| 10.1 | 0.001077536 | 9373 | 20.57 | 1.9 | 2.15 | 3.71 | |
| 8.09 | 0.001077536 | 7508 | 22.34 | 7.74 | 8.09 | ||
| 37% | 11.56 | 0.001100347 | 10,506 | 13.96 | 11.53 | 11.56 | 11.38 |
| 13.23 | 0.001037193 | 12,756 | 15.47 | 13.23 | ----- | ----- | |
| 11.79 | 0.001044627 | 11,286 | 13.3 | 11.79 | 11.42 | ----- | |
| 38% | 14.63 | 0.001034911 | 14,136 | 21.48 | 11.61 | 14.63 | ----- |
| 15.38 | 0.001105055 | 13,918 | 21.05 | 15.38 | ----- | ----- | |
| 14.45 | 0.001105055 | 13,076 | 21.9 | 11.89 | 14.45 | 14.14 | |
| 39% | 13.91 | 0.000935904 | 14,863 | 23.17 | 11.31 | 11.62 | 12.23 |
| 9.66 | 0.000928868 | 10,400 | 26.09 | 6.37 | 8.35 | 9.13 | |
| 16.04 | 0.000956623 | 16,767 | 27.83 | 15.91 | 16.04 | ----- | |
| 40% | 14.25 | 0.000897801 | 15,872 | 39.02 | 6.87 | 10.15 | 12.41 |
| 30.36 | 0.000928868 | 32,685 | 41.82 | 25.66 | 27.85 | ----- | |
| 33.9 | 0.000972034 | 34,875 | 41.24 | 25.49 | 23.96 | ----- |
| Water Sample | Refinery Water | City Water | 36% | 37% | 40% |
|---|---|---|---|---|---|
| Turbidity (NTU) | 29.3 ± 1.9 | 0.67 ± 0.11 | 4.1 ± 0.3 | 3.8 ± 1.4 | 2.9 ± 0.2 |
| Color (455 nm) | 470 ± 20 | 32 ± 4 | 153 ± 11 | 180 ± 19 | 201 ± 15 |
| pH | 6.38 ± 0.15 | 7.44 ± 0.03 | 7.26 ± 0.05 | 7.16 ± 0.05 | 7.18 ± 0.05 |
| Conductivity (mS) | 8.02 ± 0.59 | 0.915 ± 0.025 | 4.5 ± 0.35 | 6.8 ± 0.455 | 6.52 ± 0.655 |
| Iron (ppm) | 0.735 ± 0.045 | 0.205 ± 0.015 | 0.48 ± 0.075 | 0.24 ± 0.12 | 0.28 ± 0.03 |
| Silica (ppm) | 39.5 ± 3.5 | 9.1 ± 0.4 | 61 ± 10 | 74.8 ± 7.5 | 82.4 ± 5.05 |
| Manganese (ppm) | 1.15 ± 0.05 | 0.115 ± 0.015 | 0.6 ± 0.06 | 0.7 ± 0.125 | 0.4 ± 0.075 |
| TH | 292 ± 12 | 25 ± 3 | 116 ± 3 | 138 ± 3 | 144 ± 12 |
| CaH | 135 ± 5 | 8 ± 1 | 42 ± 3 | 38 ± 3 | 35 ± 2 |
| M-Alkalinity | 1.85 ± 0.15 | 24.5 ± 1.15 | 11 ± 2 | 10.5 ± 0.5 | 12 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsersawy, R.; Donyadari, R.; Khondoker, M.A.H. Extrusion 3D-Printed Kaolinite Ceramic Filters for Water Applications. J. Manuf. Mater. Process. 2025, 9, 278. https://doi.org/10.3390/jmmp9080278
Elsersawy R, Donyadari R, Khondoker MAH. Extrusion 3D-Printed Kaolinite Ceramic Filters for Water Applications. Journal of Manufacturing and Materials Processing. 2025; 9(8):278. https://doi.org/10.3390/jmmp9080278
Chicago/Turabian StyleElsersawy, Rawan, Romina Donyadari, and Mohammad Abu Hasan Khondoker. 2025. "Extrusion 3D-Printed Kaolinite Ceramic Filters for Water Applications" Journal of Manufacturing and Materials Processing 9, no. 8: 278. https://doi.org/10.3390/jmmp9080278
APA StyleElsersawy, R., Donyadari, R., & Khondoker, M. A. H. (2025). Extrusion 3D-Printed Kaolinite Ceramic Filters for Water Applications. Journal of Manufacturing and Materials Processing, 9(8), 278. https://doi.org/10.3390/jmmp9080278







