Study of Corrosion Resistance of Hybrid Structure of DP980 Two-Phase Steel and Laser-Welded 6013-T4 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methodology
2.2.1. X-Ray Fluorescence Spectroscopy—FRX
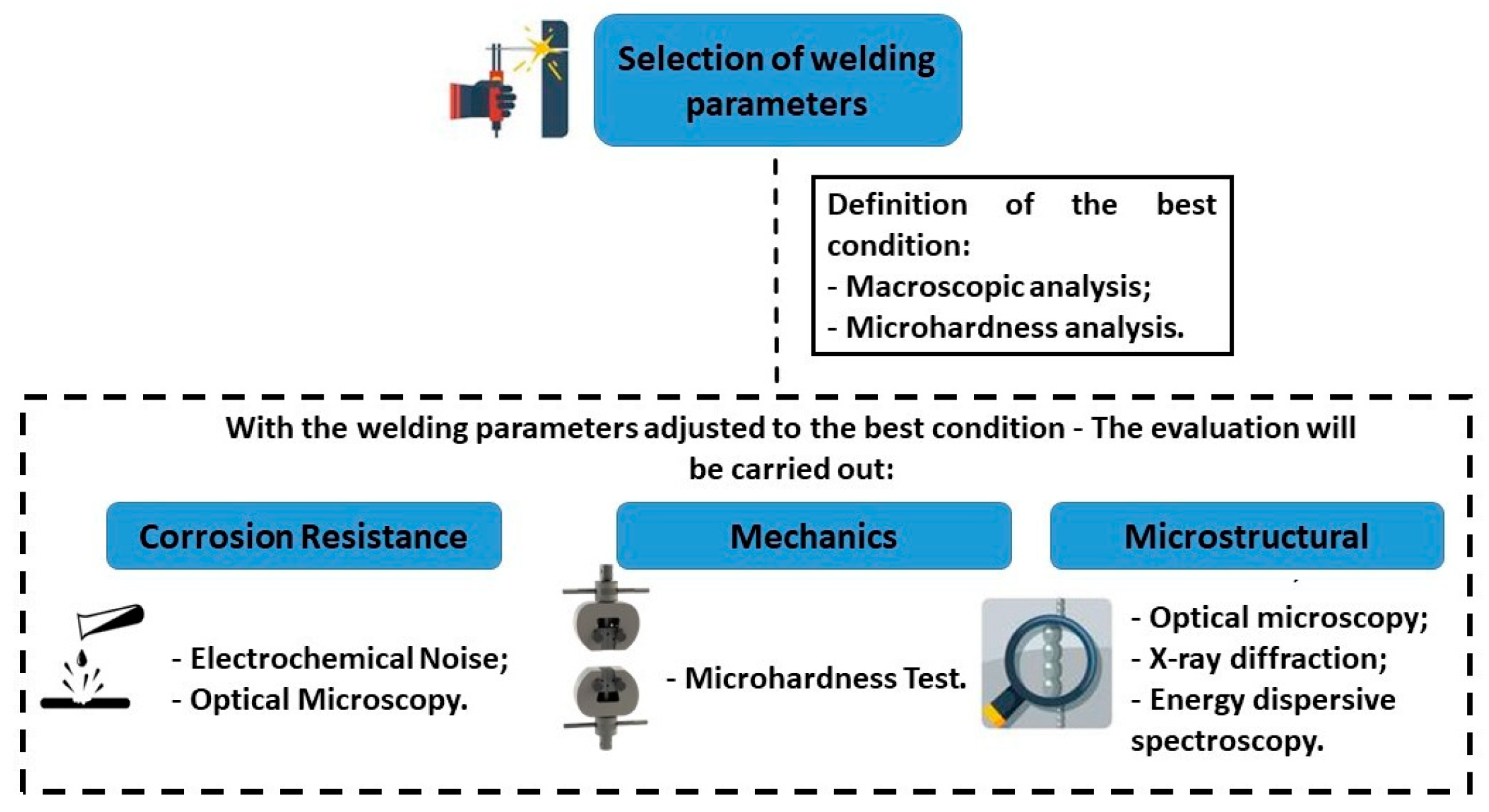
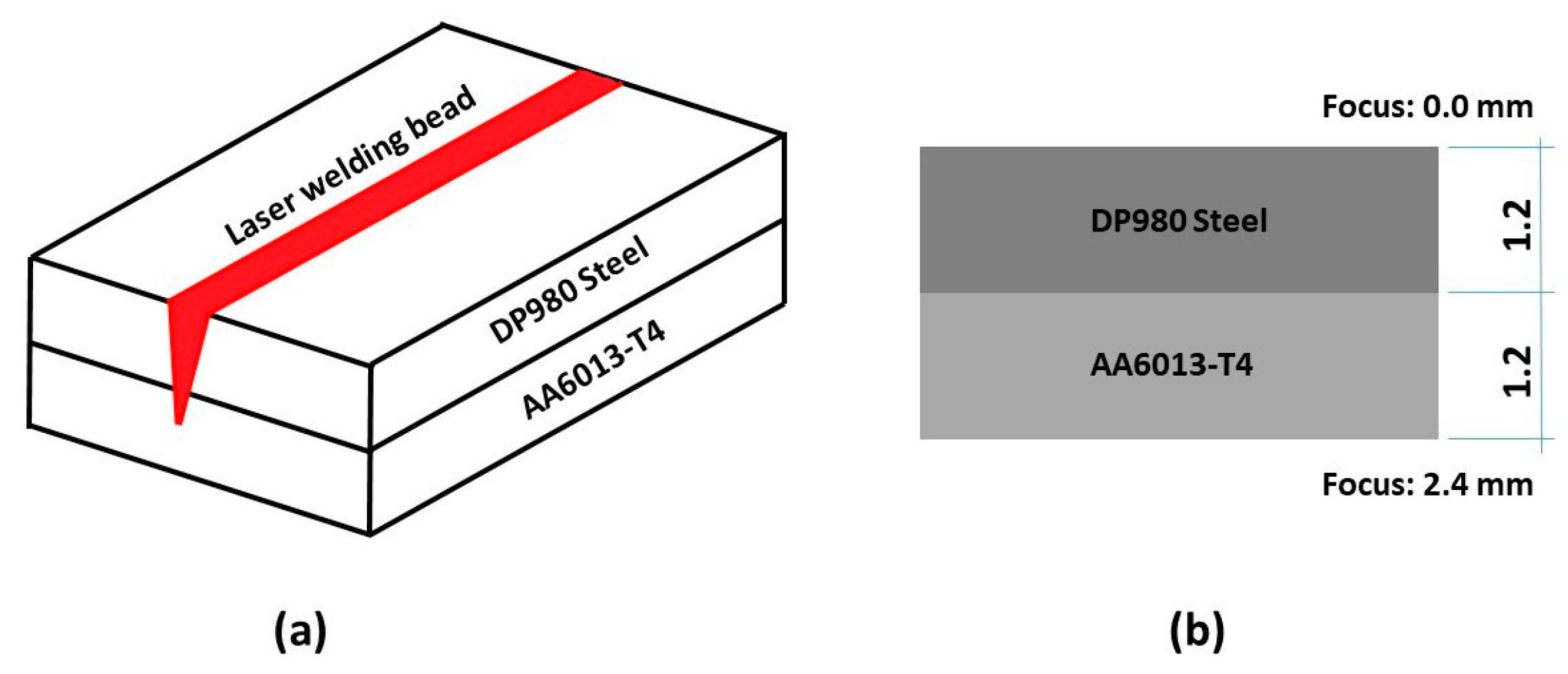
2.2.2. Choosing Welding Parameters
2.2.3. Vickers Hardness by Microindentation
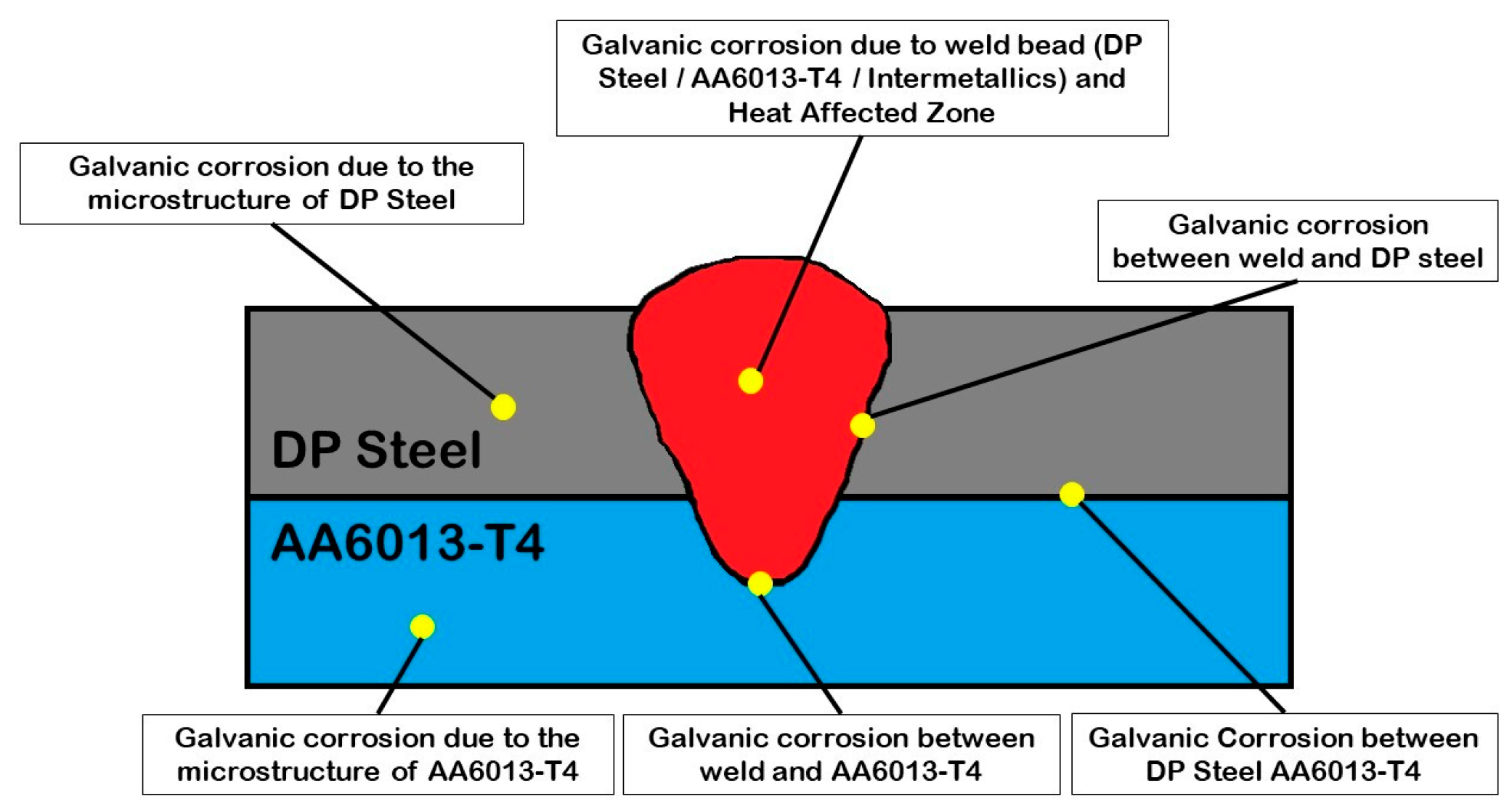
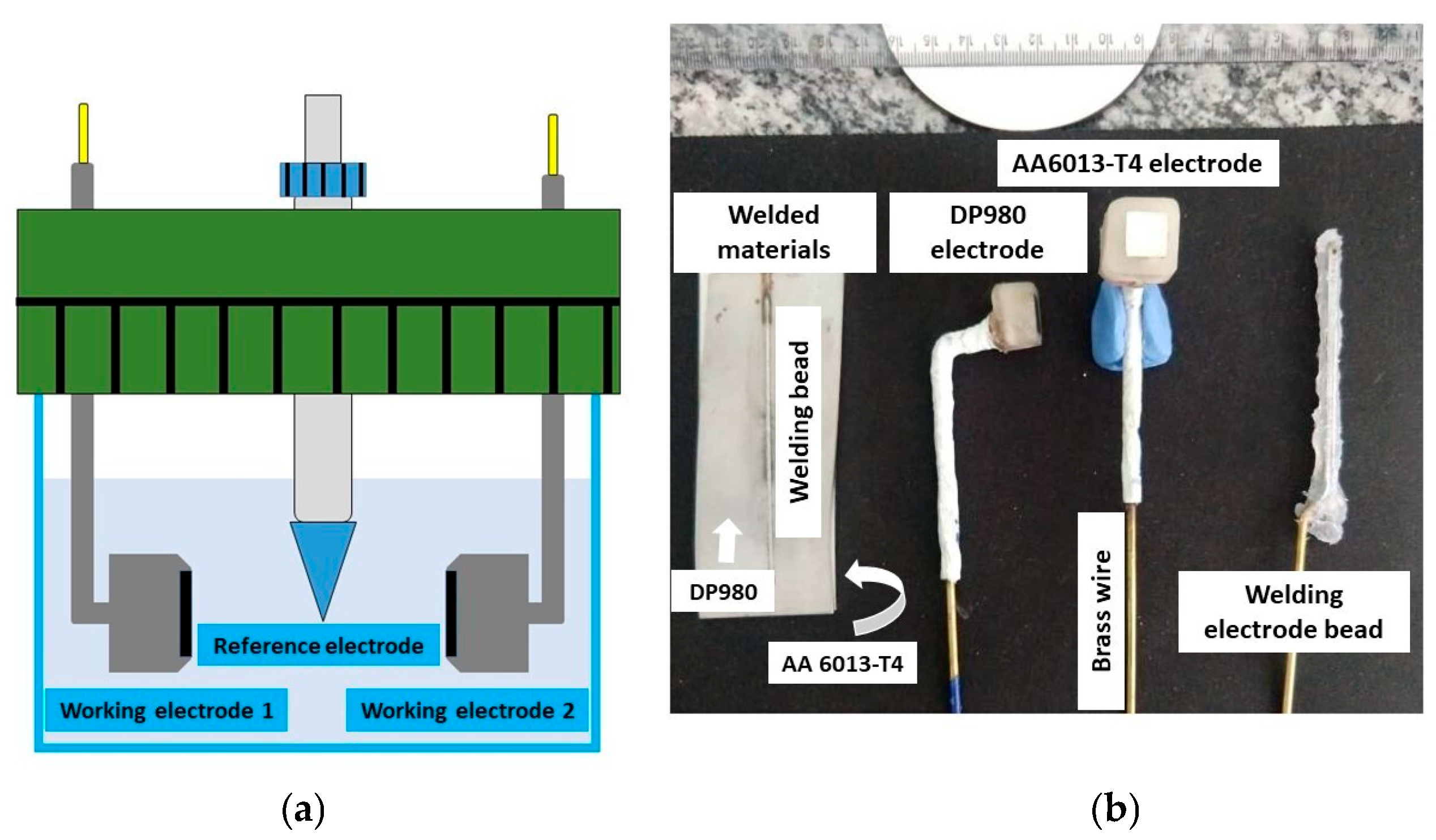
2.2.4. Corrosion Measurements
Electrochemical Noise Measurements and Analysis
Analysis of Electrochemical Noise Results
Characterization by Ray Diffraction X–DRX
Characterization by Energy Dispersive Spectroscopy
Conductivity and pH Analysis for Corrosion Measurements
3. Results and Discussion
3.1. Parameter Selection
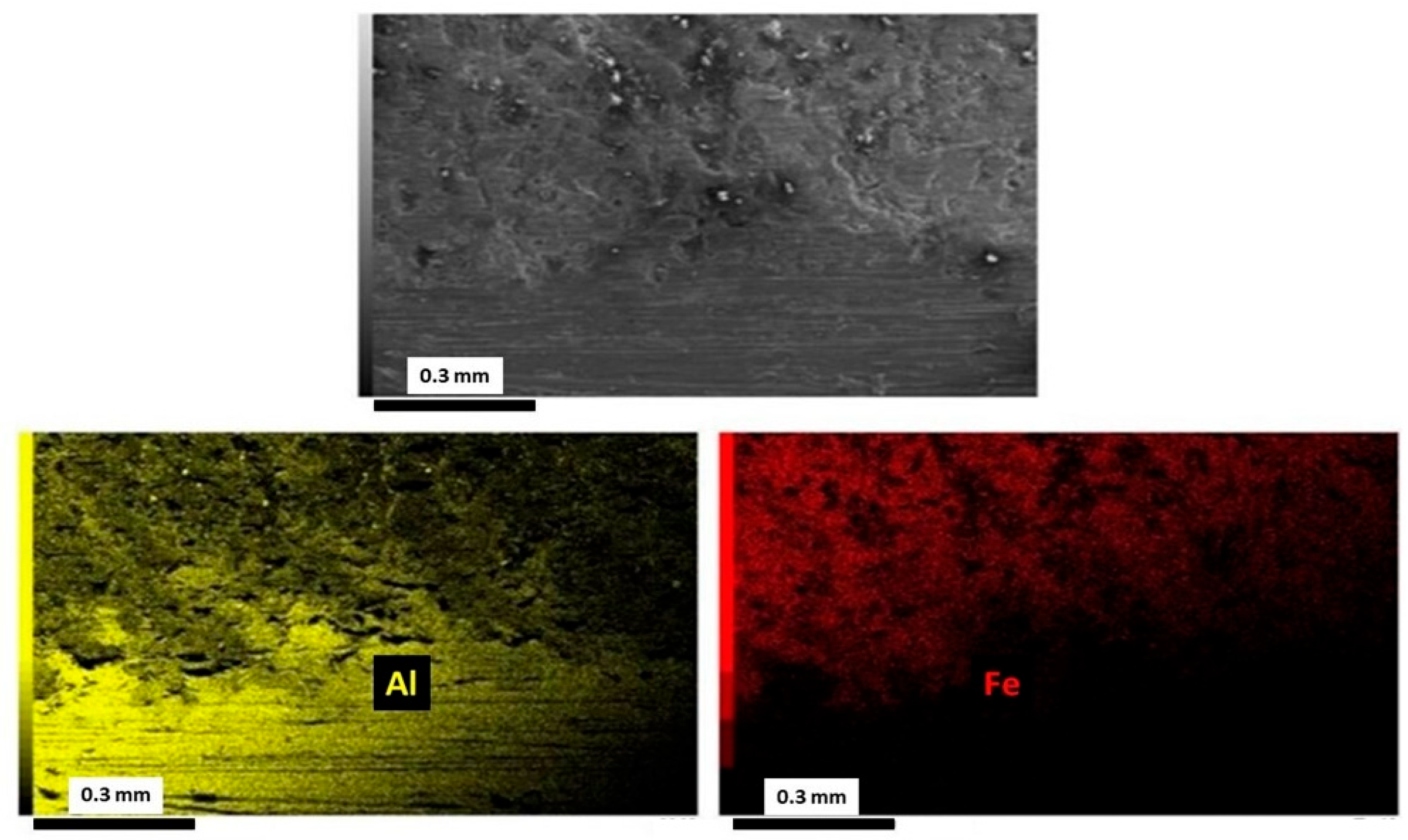
3.2. Microstructural Analysis of Welded Joints
3.3. Vickers Hardness Characterization by Microindentation of Welded Joint
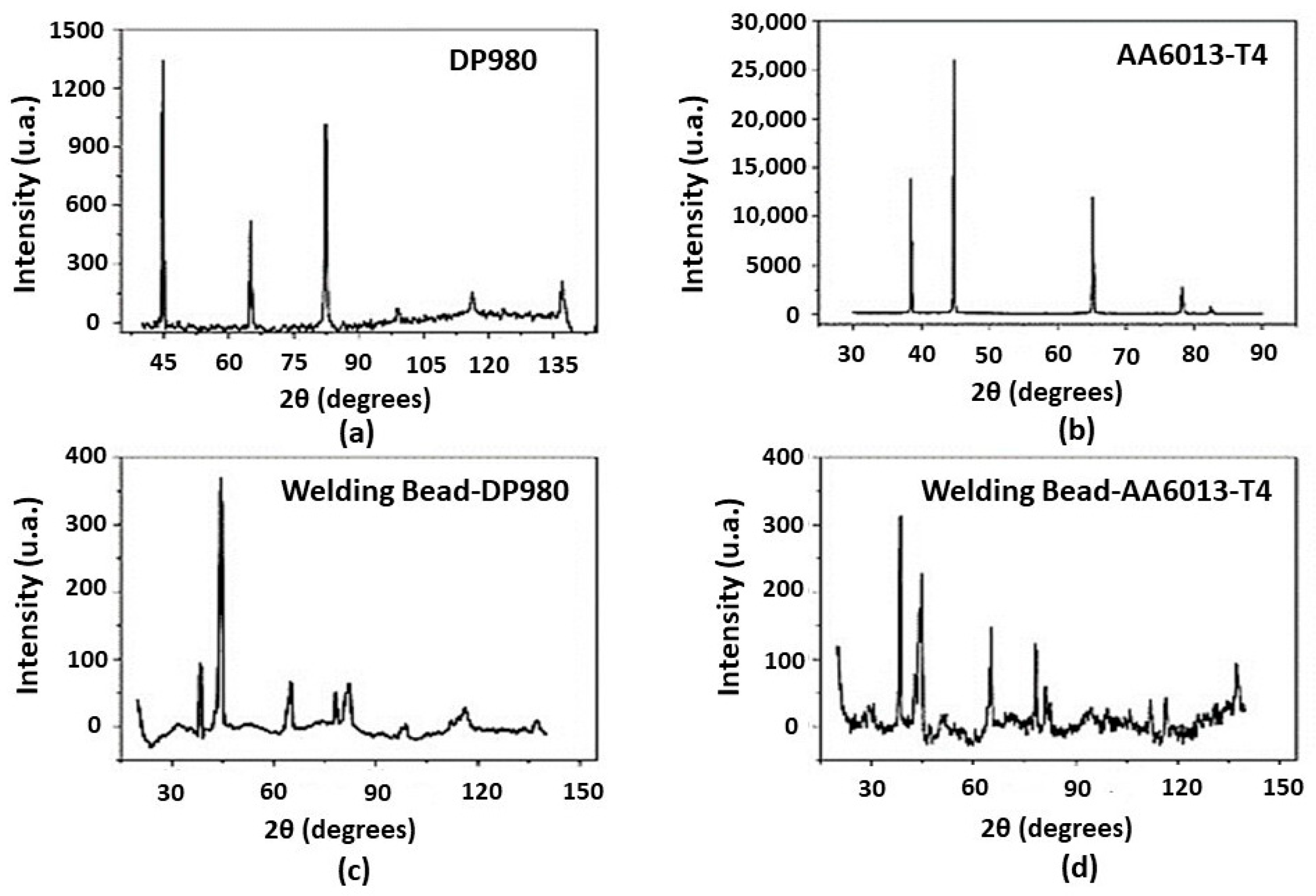
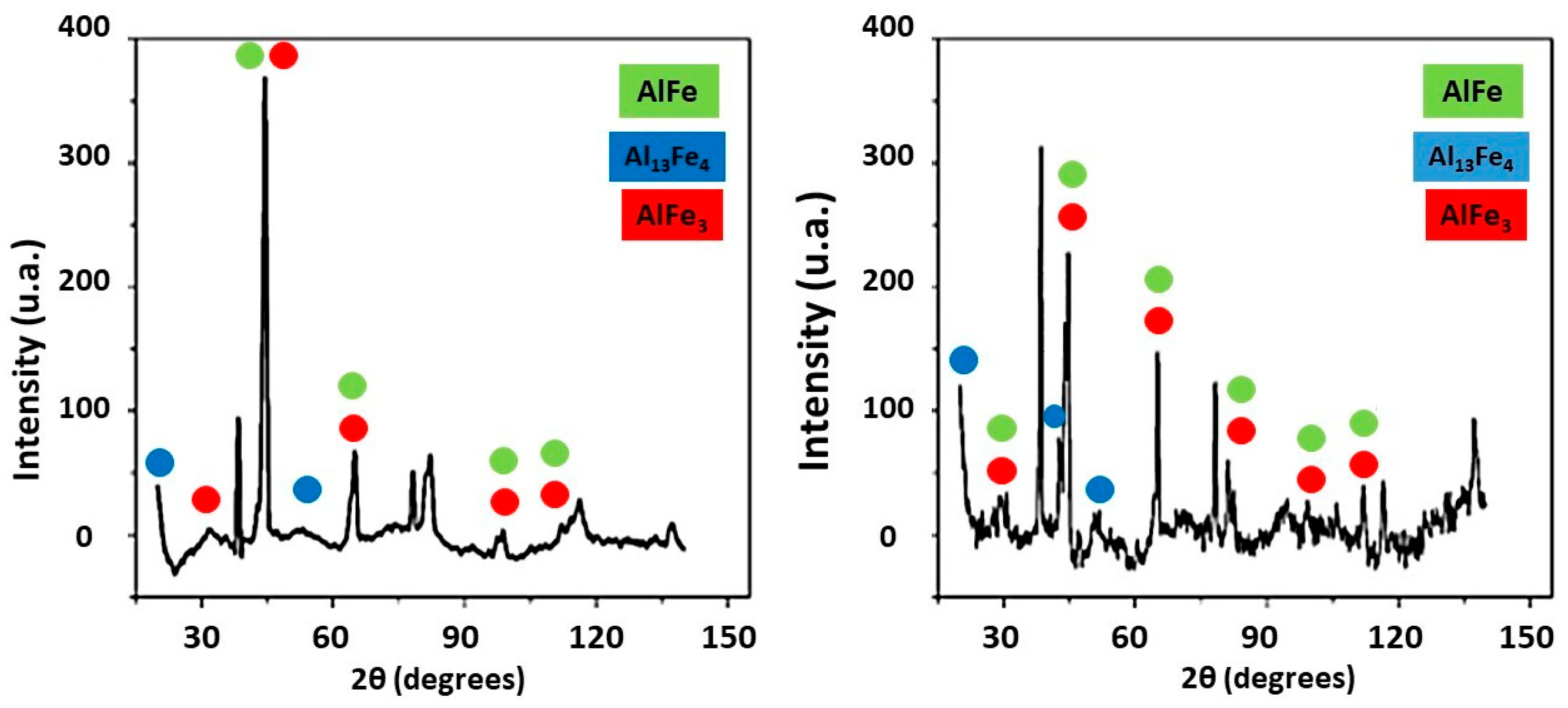
3.4. XRD and EDS Analysis
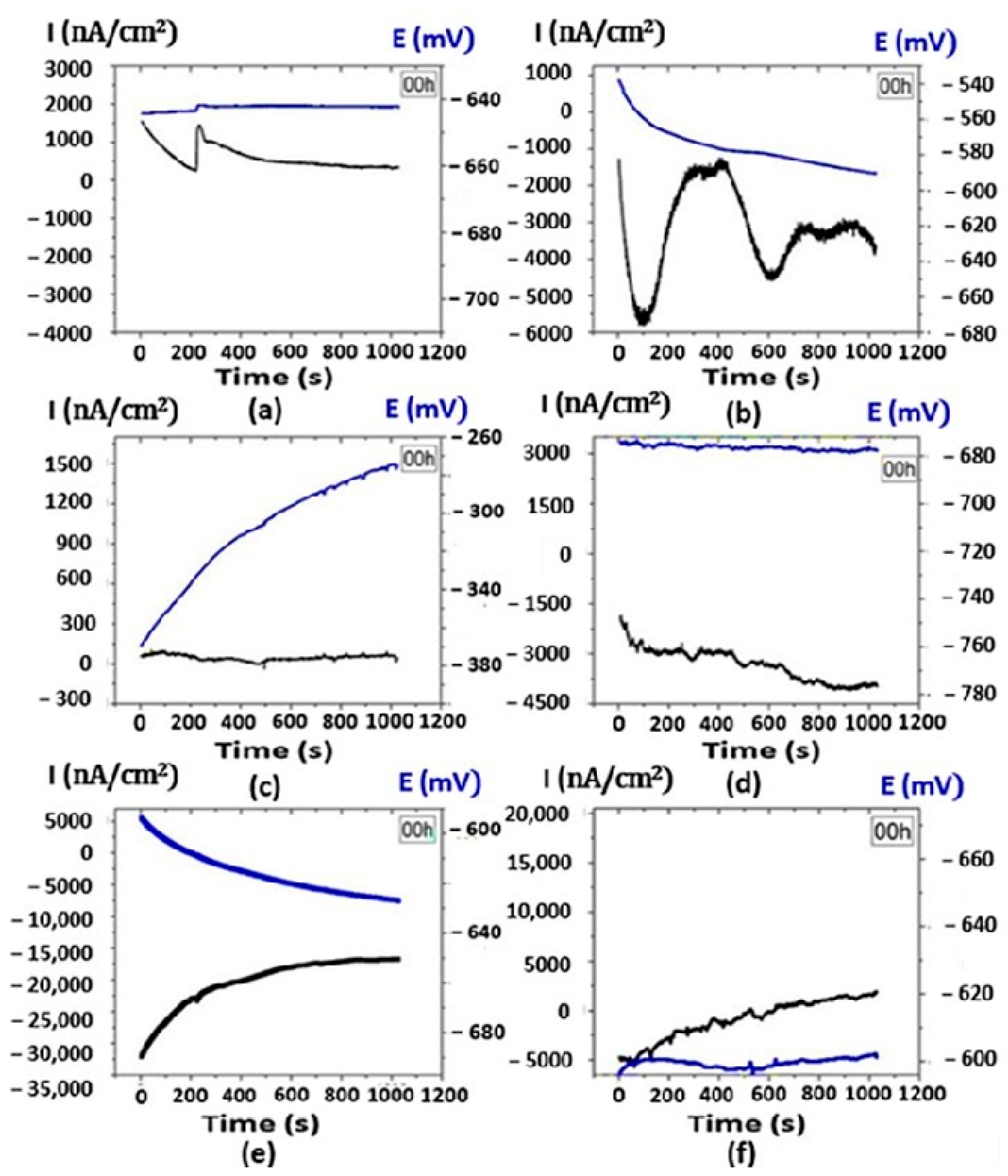
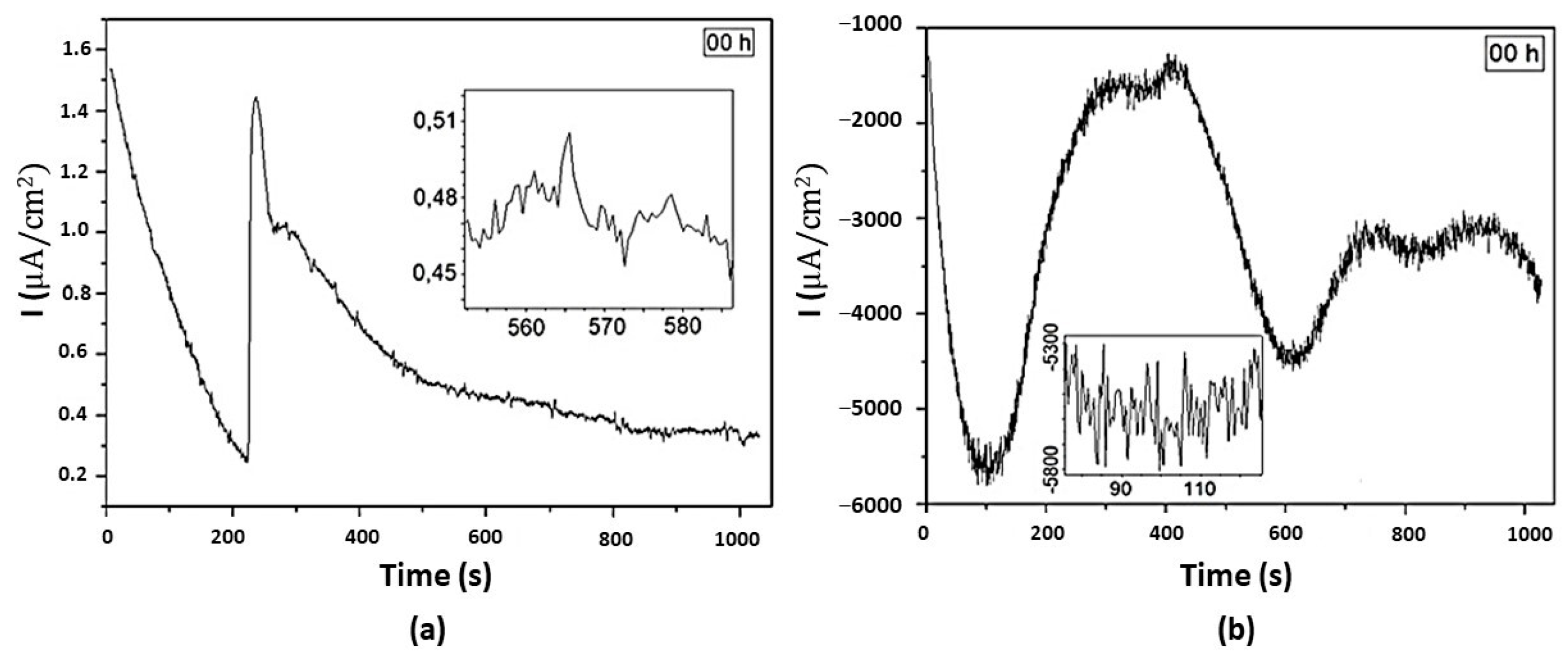
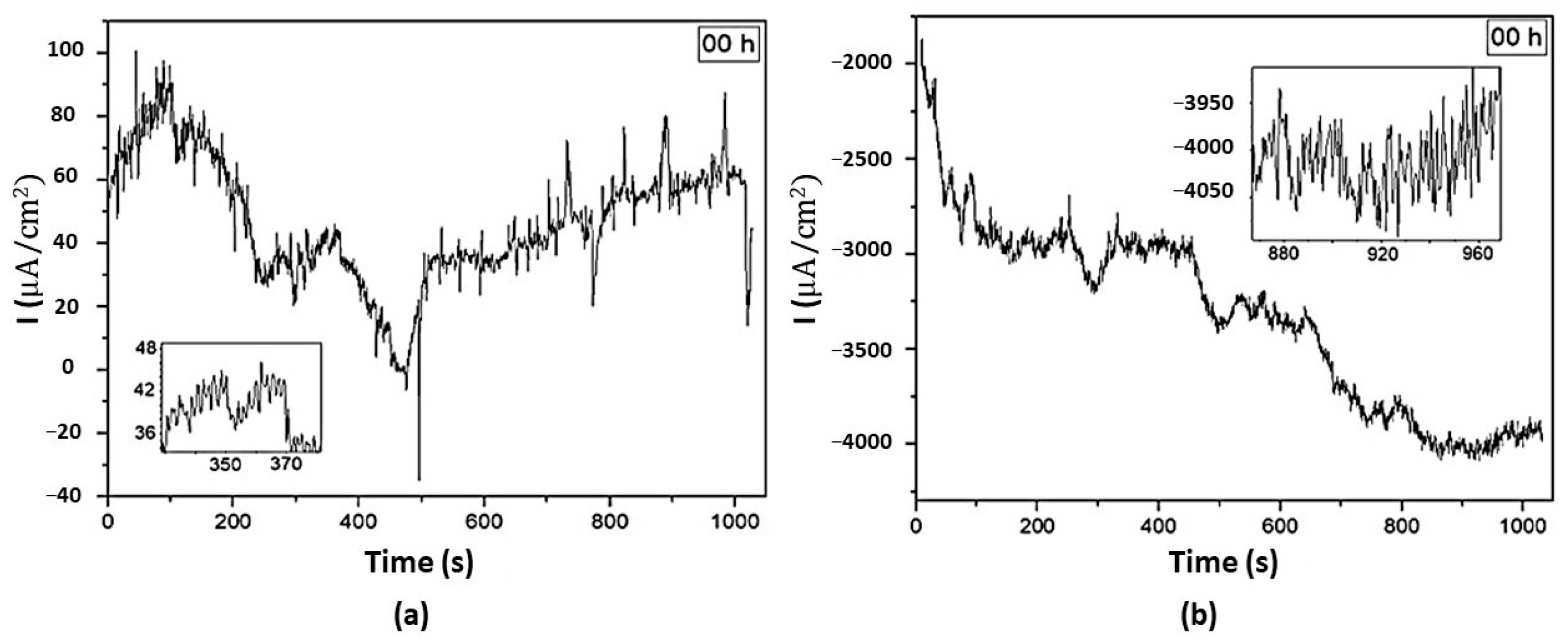
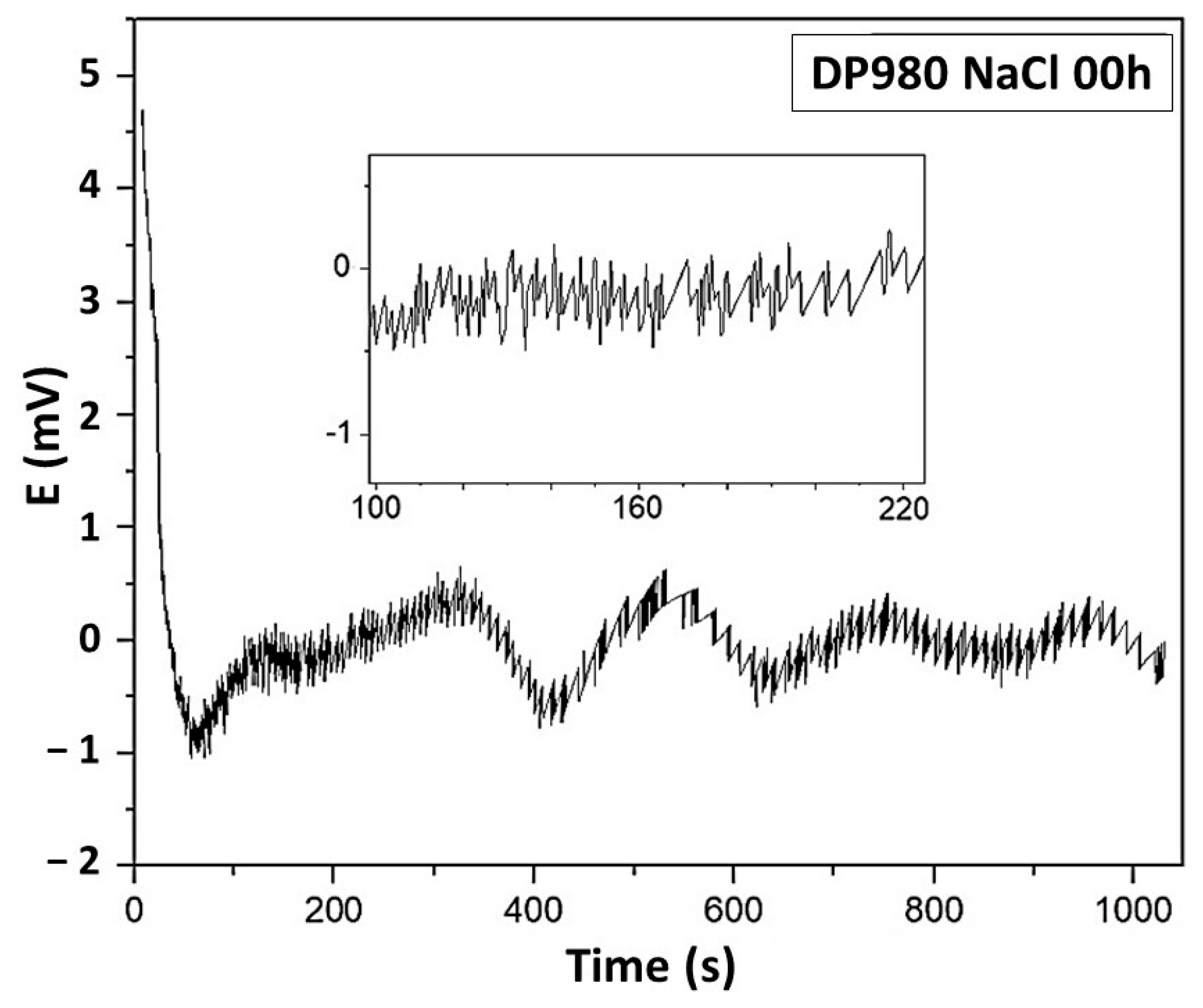
3.5. Electrochemical Noise Analysis
3.5.1. DC Trend Removal
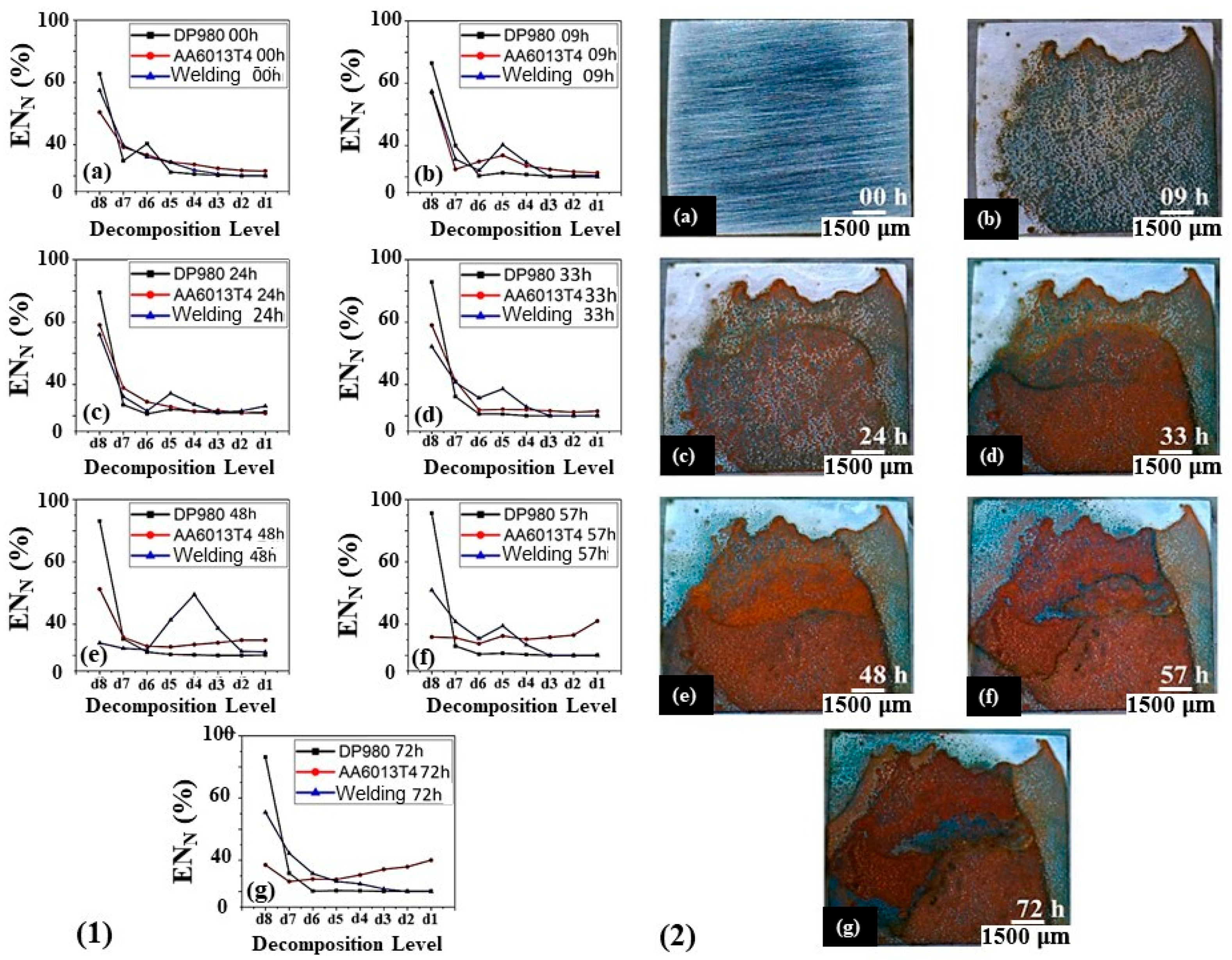
3.5.2. Power Diagrams
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marra, K.M. Aços Dual Phase da Usiminas: Características e Potencial de Aplicação em Veículos Automotores. In Proceedings of the Congresso Anual da ABM, São Paulo, Brazil, 31 July–3 August 2008; Volume 63, p. 2. [Google Scholar]
- Faria Neto, A.R.; Dias, E.X.; Fukugauchi, C.S.; Martins, M.S.; Pereira, M.S. Mechanical and microstructural characterization of class 800 complex phase steel before and after the laser welding process. J. Manuf. Mater. Process. 2024, 8, 232. [Google Scholar]
- Faria Neto, A.R.; Fukugauchi, C.S.; Pereira, M.S. Using design of experiments in the evaluation of the microstructural characterization parameters with the LePera reagent in a multiphase steel. Mater. Res. Express 2019, 8, 15. [Google Scholar]
- Kishida, K. High Strength Steel Sheets for Light Weight Vehicle; Nippon Steel technical Report; Nippon Steel Corporation: Tokyo, Japan, 2000; Volume 81, pp. 12–16. [Google Scholar]
- DIAS, E.X.; Faria Neto, A.R.; Castro, T.S.; Martins, M.S.; Pereira, M.S. Laser Welding Applied to Complex Phase Steel; SAE Technical Paper; SAE: Warrendale, PA, USA, 2024. [Google Scholar]
- Sebastiany, Y.; Faria, A.R.; Martins, M.S.; Barkey, M. Isothermal Fatigue Characterization of P91 Steel Under Load Control. Mater. Res. 2025, 28, e20250129. [Google Scholar]
- Cao, W.; Shi, J.; Wang, C.; Wang, C.; Xu, L.; Wang, M.; Weng, Y.; Dong, H. The 3rd generation automobile sheet steels presenting with ultrahigh strength and high ductility. In Advanced Steel—The Recent Scenario in Steel Science and Technology, 1st ed.; Weng, Y., Dong, H., Gan, Y., Eds.; Springer: Beijing, China, 2011; Volume 22, pp. 209–227. [Google Scholar]
- Dong, H.; Sun, X.; Cao, W.; Liu, Z.; Wang, M.; Weng, Y. On the performance improvement of steels through M3 structure control. In Advanced Steel: The Recent Scenario in Steel Science and Technology, 1st ed.; Weng, Y., Dong, H., Gan, Y., Eds.; Springer: Beijing, China, 2011; Volume 6, pp. 35–57. [Google Scholar]
- Han, H.N.; Oh, C.S.; Kim, G.; Kwon, O. Design method for TRIP-aided steel based on a microstructure-based modelling for transformation-induced plasticity and mechanically induced martensitic transformation. Mater. Sci. Eng. A 2009, 499, 462–468. [Google Scholar]
- Matlock, D.K.; Speer, J.G.; De Moor, E.; Gibbs, P.J. Recent developments in advanced high strength sheet steels for automotive applications: An overview. Jestech 2012, 15, 1–12. [Google Scholar]
- Radwański, K.; Wrożyna, A.; Kuziak, R. Role of the advanced microstructures characterization in modeling of mechanical properties of AHSS steels. Mater. Sci. Eng. A 2015, 639, 567–574. [Google Scholar]
- Sun, S.; Pugh, M. Properties of thermomechanically processed dual-phase steels containing fibrous martensite. Mater. Sci. Eng. A 2002, 335, 298–308. [Google Scholar]
- Silva, E.A. Study of the Correlation Between Work Hardening Models and Crystallographic Characteristics in Advanced High-Strength Steels Subjected to the Springback Effect. Ph.D. Thesis, Paulista State University, Guaratinguetá, Brazil, 2016. [Google Scholar]
- Fukugauchi, C.S.; Faria Neto, A.R.; Ribeiro, R.B.; Pereira, M.S. Microstructural characterization of dual and multiphase steels applied to automotive industry. Mater. Sci. Forum 2014, 775–776, 146–150. [Google Scholar]
- Cheng, N.; Yang, S.; Li, Y.; Zhao, X.; Bi, J.; Tian, J. Analysis of fatigue fracture mechanism of laser spiral welding of dissimilar metals of 6082 high strength aluminum alloy and DP980 high strength dual phase steel. Mater. Today Commun. 2024, 41, 110665. [Google Scholar]
- Dong, W.; Zhang, Y.; Bao, L.; Shin, K. Effects of laser scanning strategy on bending behavior and microstructure of DP980 steel. Materials 2024, 17, 2415. [Google Scholar] [PubMed]
- Liu, H.; Wang, Y.; Zhang, J.; Zhu, Z.; Yang, S. Effect of laser spot offset on microstructure and properties of laser welded joint of medium Mn/DP980 dissimilar steel. Mater. Lett. 2024, 363, 136331. [Google Scholar]
- Pan, H.; Ya, X.; Yu, W.; Tao, W.; Zhang, S.; Zhou, W. The Effect of Laser Power on Microstructure and Mechanical Properties between Medium Mn Steel and DP980 Joint by Laser Welding. J. Mater. Eng. Perform. 2024, 34, 10568–10576. [Google Scholar]
- Djukanovic, G. European aluminium recycling industry improves. Alum. Int. Today 2016, 28, 28. [Google Scholar]
- Derbiszewski, B.; Obraniak, A.; Rylski, A.; Siczek, K.; Wozniak, M. Studies on the quality of joints and phenomena therein for welded automotive components made of aluminum alloy—A review. Coatings 2024, 14, 601. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Sinke, J.; Benedictus, R. Mechanics of tailor welded blanks: An overview. Key Eng. Mater. 2007, 344, 373–382. [Google Scholar]
- Shakeri, H.R.; Buste, A.; Worswick, M.J.; Clarke, J.A. Study of damage initiation and fracture in aluminum tailor welded blanks made via different welding techniques. J. Light Met. 2002, 2, 95–110. [Google Scholar]
- Rahman, A.B.M.M.; Kumar, S.; Gerson, A.R. Galvanic corrosion of laser weldments of AA6061 aluminium alloy. Corros. Sci. 2007, 49, 4339–4351. [Google Scholar]
- Usiminas. Cold Rolled. Available online: https://www.usiminas.com/en/pages/markets-and-products/cold-rolled/ (accessed on 23 March 2025).
- The Aluminum Association. International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys, 2nd ed.; The Aluminum Association: Arlington, TX, USA, 2015. [Google Scholar]
- Siqueira, R.H.M. Mechanical and Microstructural Characterization of Laser Welded 6013 T4 Aluminum Joints. Master’s Thesis, Paulista State University, Guaratinguetá, Brazil, 2012. [Google Scholar]
- ASTM E 3-11; Standard Guide for Preparation of Metallographic Specimens. American Society for Testing and Materials: Conshohocken, PA, USA, 2017.
- ASTM E 384; Standard Test Method for Microindentation Hardness of Materials. American Society for Testing and Materials: Conshohocken, PA, USA, 2017.
- Çam, G.; Koçak, M. Progress in joining of advanced materials. Int. Mater. Rev. 1998, 43, 1–44. [Google Scholar]
- Cao, X.; Wallace, W.; Poon, C.; Immarigeon, J.P. Research and progress in laser welding of wrought aluminum alloys. I. Laser welding processes. Mater. Manuf. Process. 2003, 18, 23–49. [Google Scholar]
- Kou, S. Solidification and liquation cracking issues in welding. JOM 2003, 55, 37–42. [Google Scholar]
- Cui, X.; Chen, B.; Chen, B.; He, D. Dual beam laser keyhole welding of steel/aluminum lapped joints. J. Mater. Process. Technol. 2018, 256, 87–97. [Google Scholar]
- Qi, Y.; Song, G. Interfacial structure of the joints between magnesium alloy and mild steel with nickel as interlayer by hybrid laser-TIG welding. Mater. Des. 2010, 31, 605–609. [Google Scholar]
- Wei, Y.H.; Dong, Z.B.; Liu, R.P.; Dong, Z.J. Three-dimensional numerical simulation of weld solidification cracking. Model. Simul. Mater. Sci. Eng. 2005, 13, 437. [Google Scholar]
- Wang, X.; Lu, F.; Wang, H.P.; Cui, H.; Tang, X.; Wu, Y. Mechanical constraint intensity effects on solidification cracking during laser welding of aluminum alloys. J. Mater. Process. Technol. 2015, 218, 62–70. [Google Scholar]
- Calliste, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering an Introduction, 6th ed.; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Dawes, C.T. Laser Welding: A Practical Guide; Woodhead Publishing: Cambridge, UK, 1992. [Google Scholar]
- Walsh, C.A. Laser Welding–Literature Review. Available online: https://www.phase-trans.msm.cam.ac.uk/ (accessed on 7 July 2025).
- Matsunawa, A. Defects formation mechanisms in laser welding and their suppression methods. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Orlando, FL, USA, 17–20 October 1994; Laser Institute of America: Orlando, FL, USA, 1994; pp. 203–209. [Google Scholar]
- Freitas, F.H.C. Study of the Influence of Laser Welding Parameters on Overlapping Joints of DP980 and TRIP780 Steels Using Statistical Tools. Ph.D. Thesis, Paulista State University, Guaratinguetá, Brazil, 2022. [Google Scholar]
- Taylor, M.D.; Choi, K.S.; Sun, X.; Matlock, D.K.; Packard, C.E.; Xu, L.; Barlat, F. Correlations between nanoindentation hardness and macroscopic mechanical properties in DP980 steels. Mater. Sci. Eng. A 2014, 597, 431–439. [Google Scholar]
- He, H.; Forouzan, F.; Volpp, L.; Robertson, S.M.; Vuorinen, E. Microstructure and mechanical properties of laser-welded DP steels used in the automotive industry. Materials 2021, 14, 456. [Google Scholar]
- Ordoñez, J.H.; Ambriz, R.R.; García, C.; Plascencia, G.; Jaramillo, D. Overloading effect on the fatigue strength in resistance spot welding joints of a DP980 steel. Int. J. Fatigue 2019, 121, 163–171. [Google Scholar]
- Xia, M.; Sreenivasan, N.; Lawson, S.; Zhou, Y.; Tian, Z. A comparative study of formability of diode laser welds in DP980 and HSLA steels. J. Eng. Mater. Technol. 2007, 129, 446–452. [Google Scholar]
- Atílio, I.; Braga, V.; de Siqueira, R.H.M.; de Carvalho, S.M.; de Lima, M.S.F. Comparing the weldability of AA6013-T4 aluminium alloy on DP600 dual-phase steel with laser welding and resistance spot welding. J. Braz. Soc. Mech. Sci. 2020, 42, 71. [Google Scholar]
- Indhu, R.; Divya, S.; Tak, M.; Soundarapandian, S. Microstructure development in pulsed laser welding of dual phase steel to aluminium alloy. Procedia Manuf. 2018, 26, 495–502. [Google Scholar]
- Tian, L.I.; Yan, Y.; Ping, P.; Liu, J. First-principles and experimental investigations on ductility/brittleness of intermetallic compounds and joint properties in steel/aluminum laser welding. Trans. Nonferrous Met. Soc. China 2021, 31, 2962–2977. [Google Scholar]
- Dinda, S.K.; Jana, S.; Roy, G.G.; Srirangam, P. Effect of beam oscillation on porosity and intermetallics of electron beam welded DP600-steel to Al 5754-alloy. J. Mater. Process. Technol. 2019, 265, 191–200. [Google Scholar]
- Smulko, J. Methods of electrochemical noise analysis for investigation of corrosion processes. FNL 2006, 6, R1–R9. [Google Scholar]
- Rios, E.C.; Zimer, A.M.; Pereira, E.C.; Mascaro, L.H. Analysis of AISI 1020 steel corrosion in seawater by coupling electrochemical noise and optical microscopy. Electrochim. Acta 2014, 124, 211–217. [Google Scholar]
- Xia, D.H.; Song, S.Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar]
- Sasaki, K.; Isaacs, H.S. Origins of electrochemical noise during pitting corrosion of aluminum. J. Electrochem. Soc. 2004, 151, B124. [Google Scholar]
- De Faria Neto, A.R.; Souza, A.N.; Passos, R.R.; Pereira, M.S.; Pocrifka, L.A.; Brambilla de Souza, R.F.; Rios, E.C. Evaluation of Paullinia Cupana as a green corrosion inhibitor for carbon steel utilizing gravimetric and electrochemical noise techniques. Mater. Res. Express 2019, 6, 076522. [Google Scholar]
- Elfergani, H.; Abdalla, A. Effect of Chloride Concentration on the Corrosion Rate in Carbon Steel. In Proceedings of the 2nd Libyan Conference on Chemistry and its Applications, Benghazi, Libya, 9–11 May 2017. [Google Scholar]
- Natishan, P.M.; O’grady, W.E. Chloride ion interactions with oxide-covered aluminum leading to pitting corrosion: A review. J. Electrochem. Soc. 2014, 161, C421. [Google Scholar]
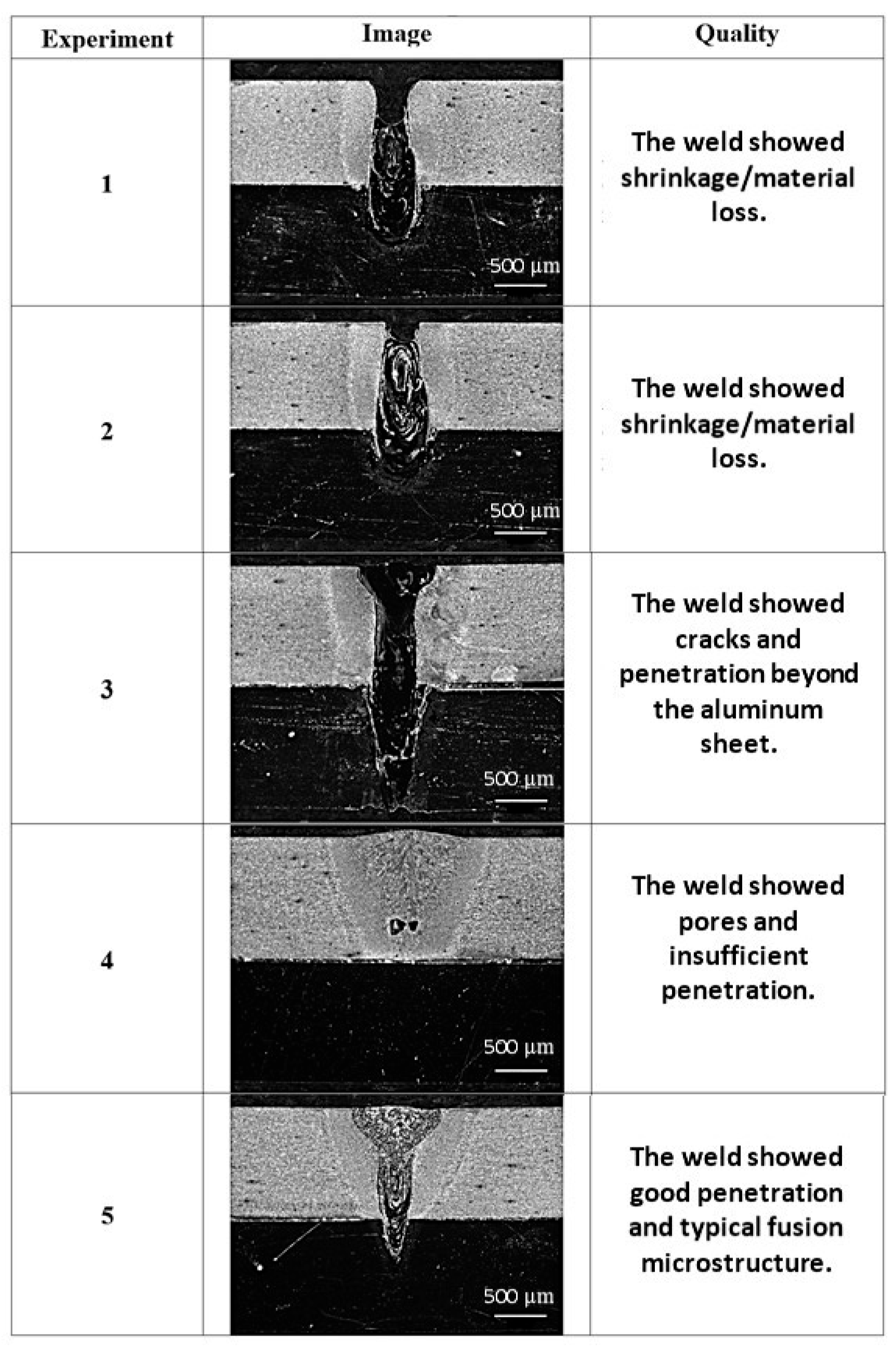
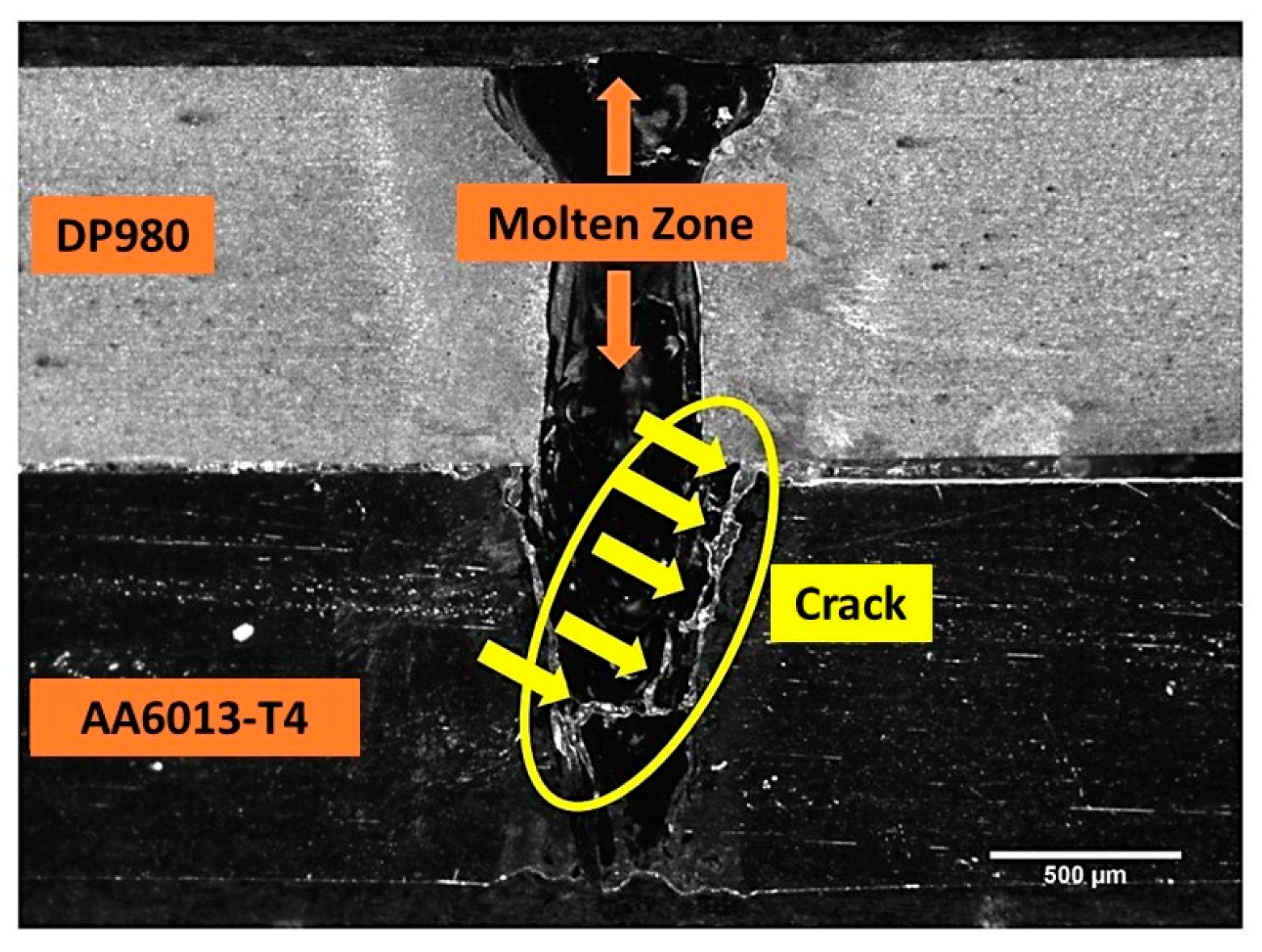
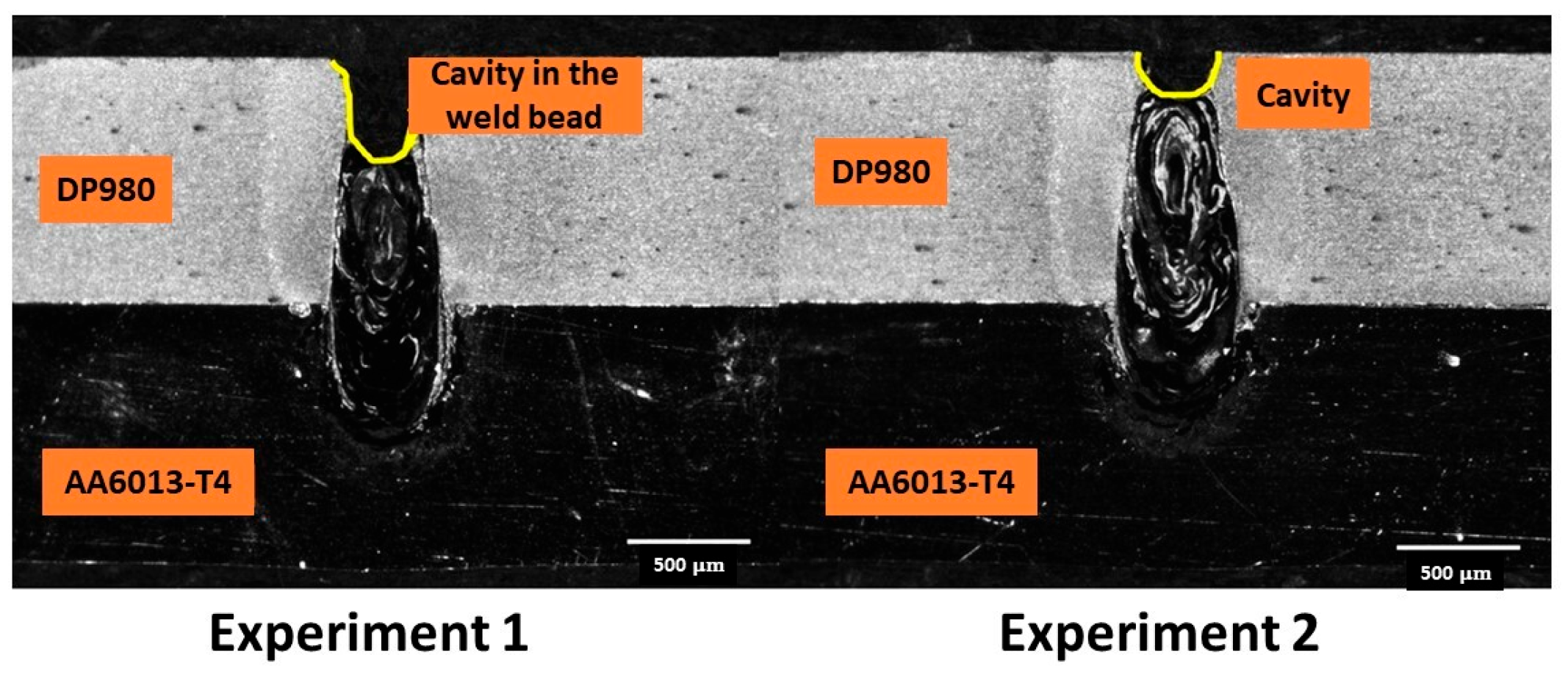
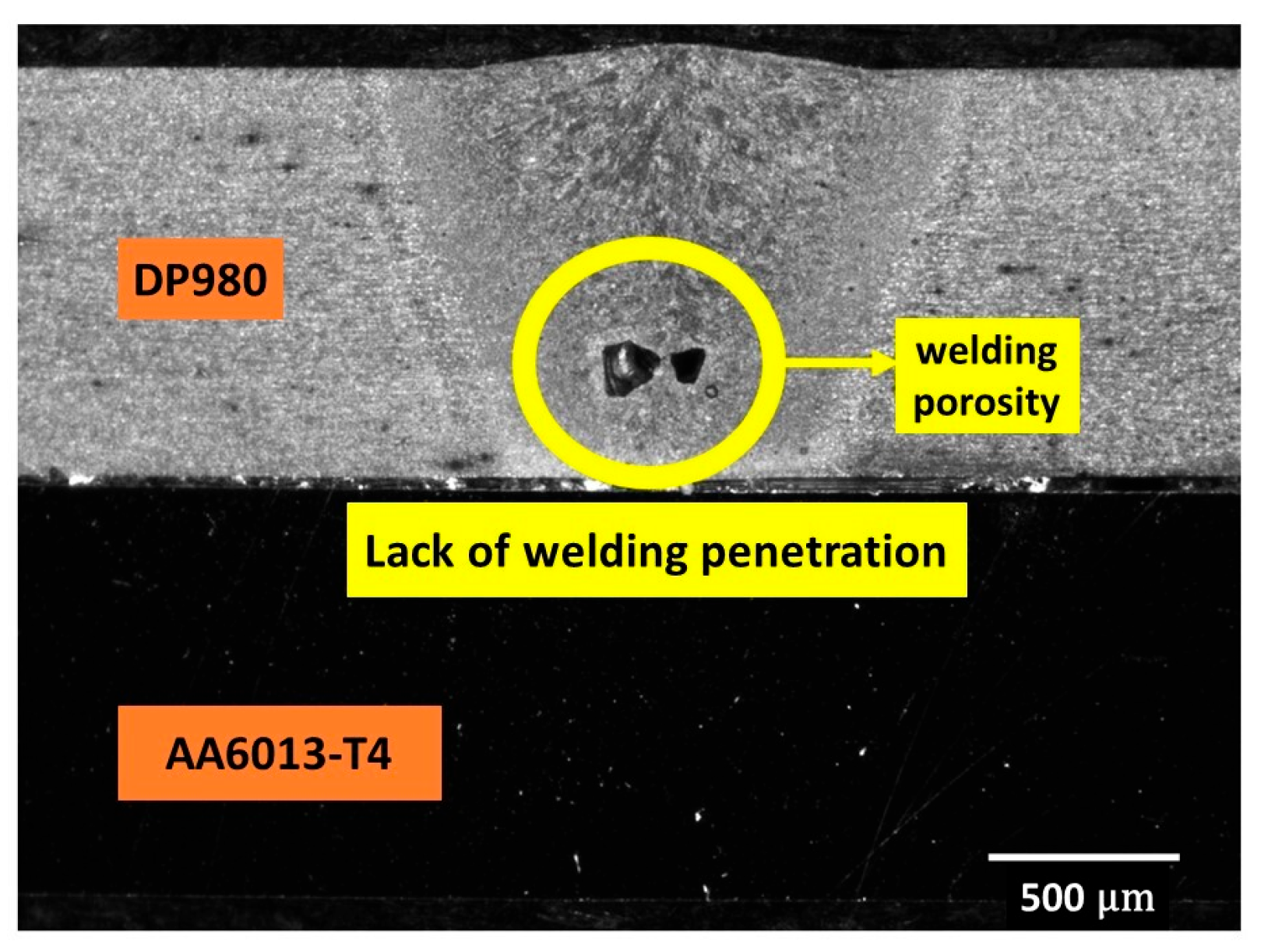
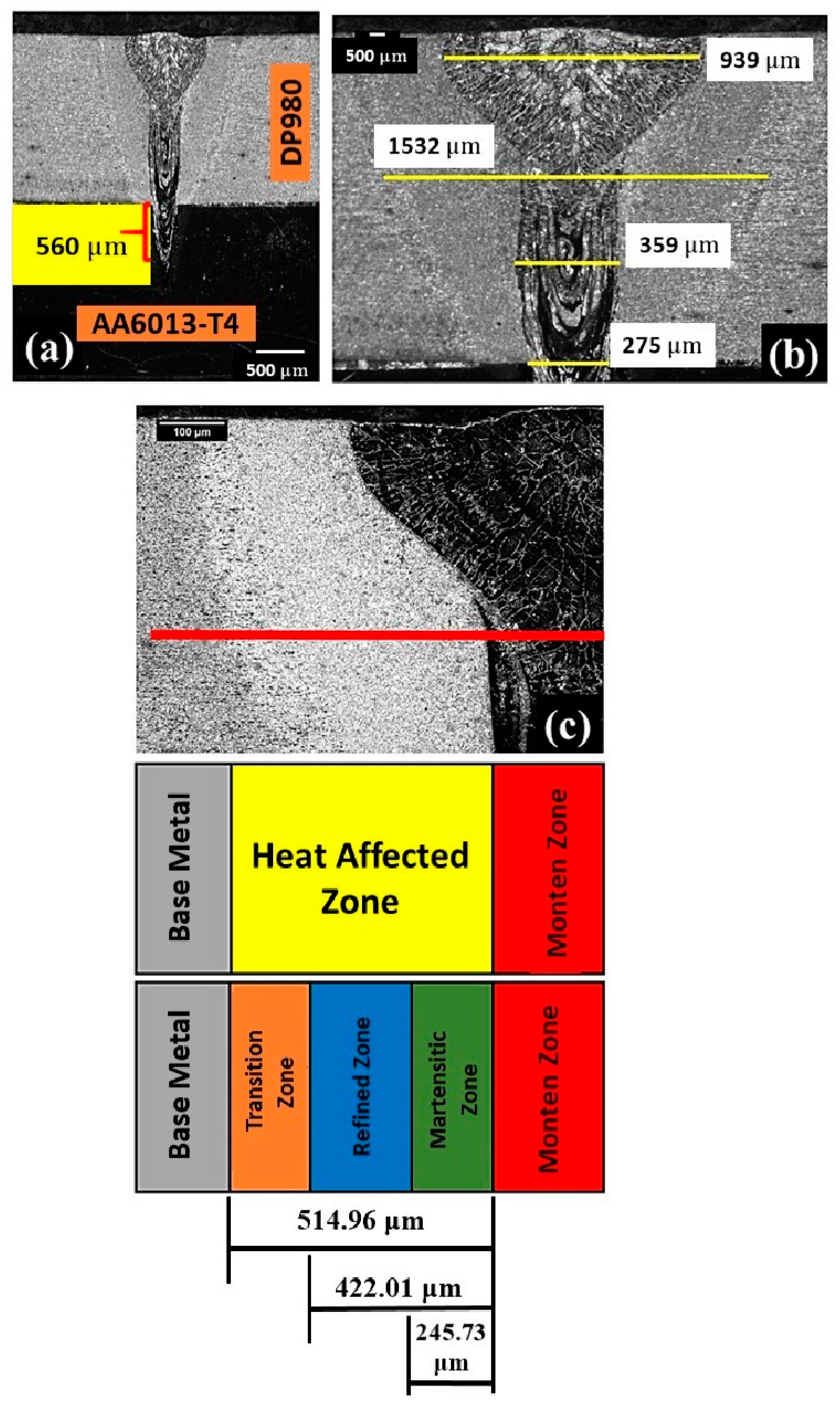
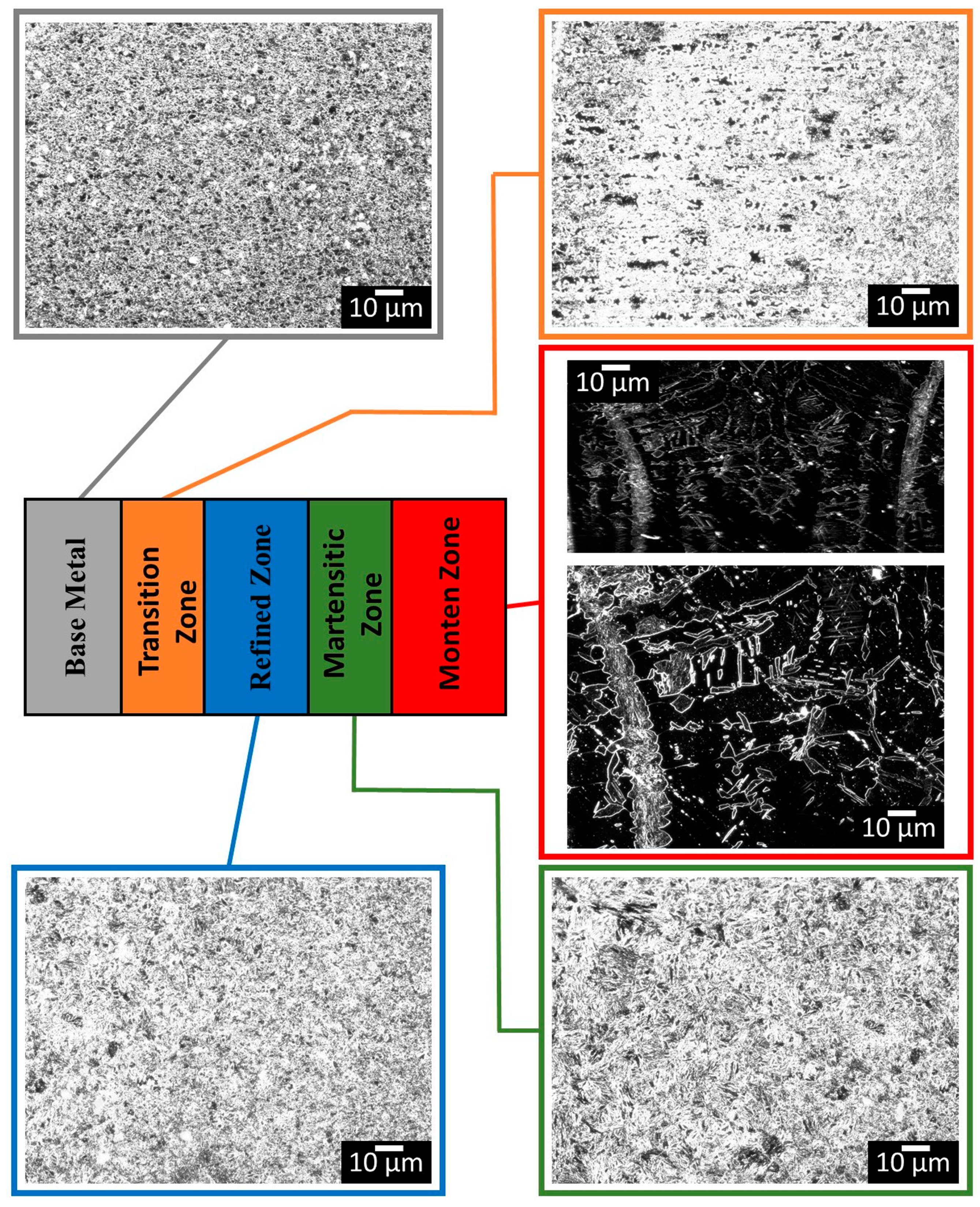
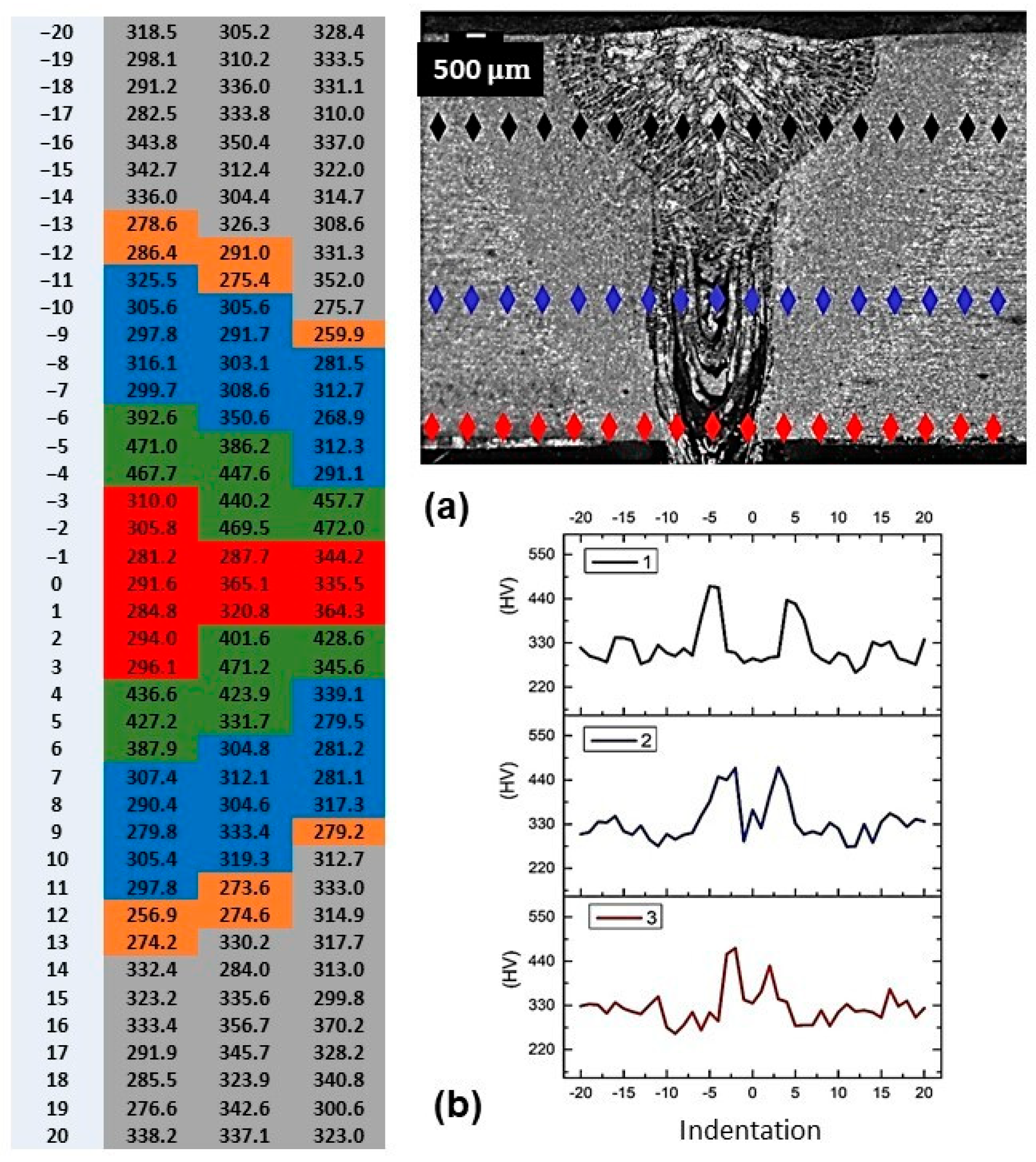
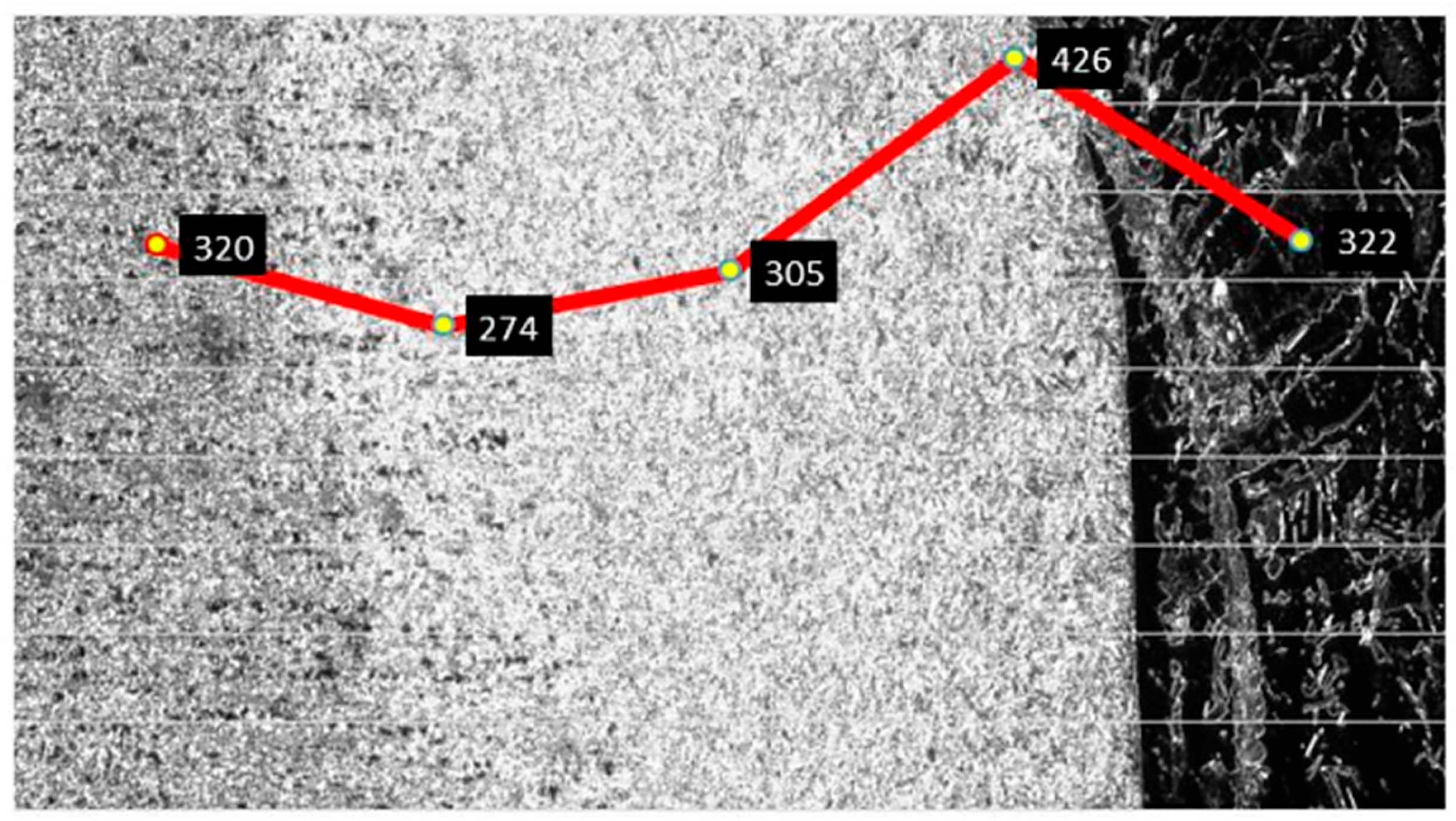

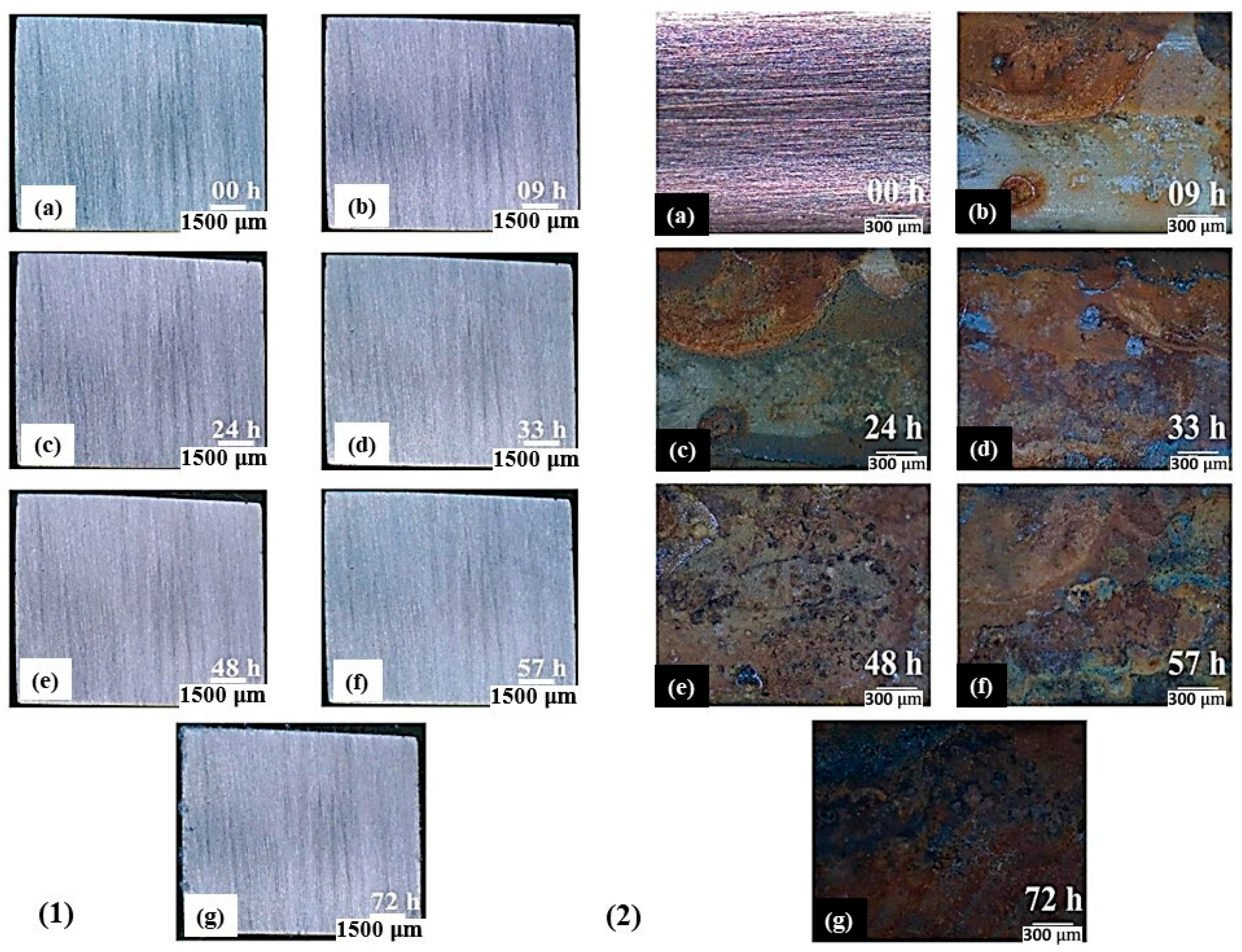
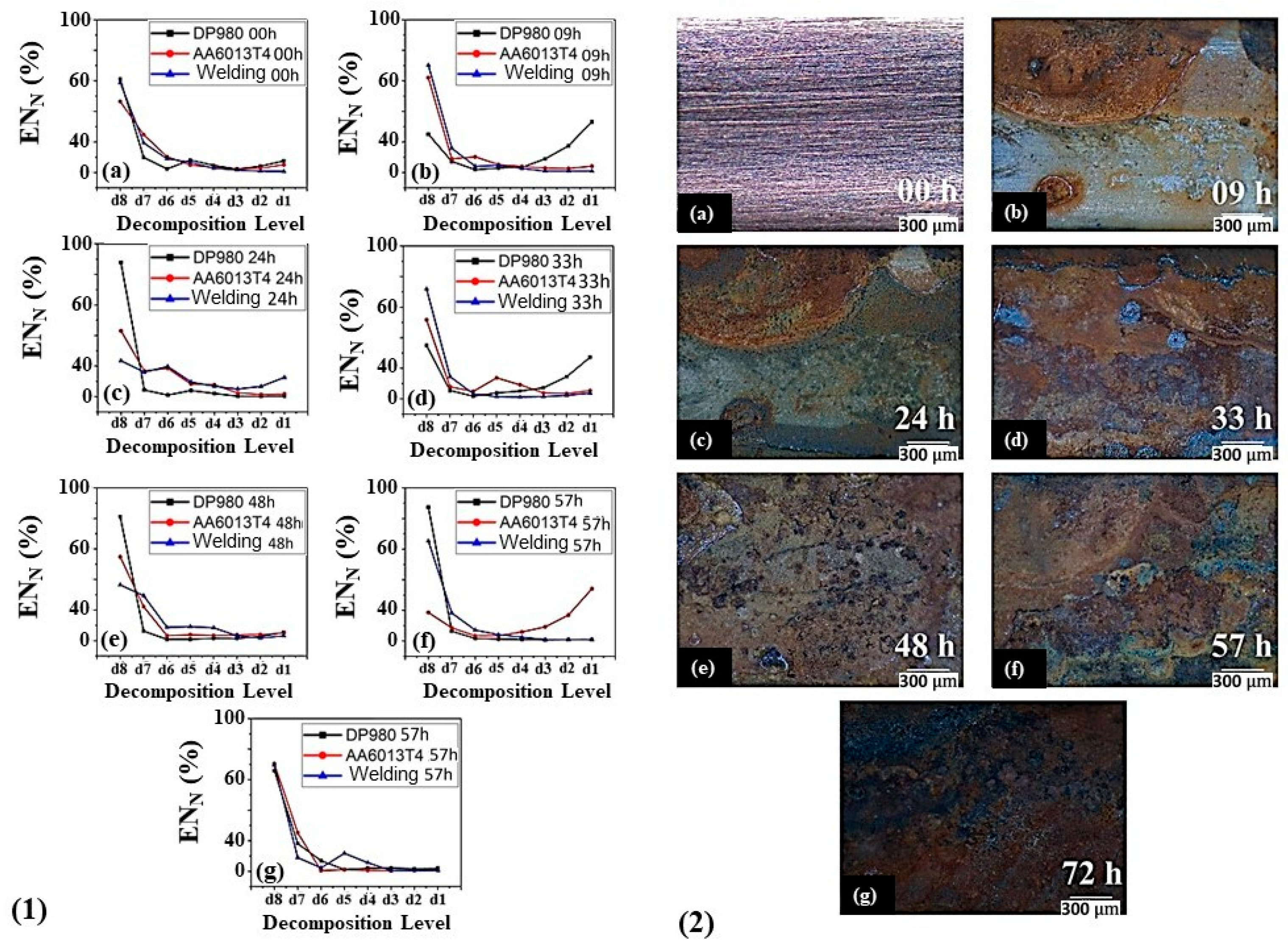


| Alloy Element | DP980 Standard | DP980 Measured | A6013-T4 Standard | A6013-T4 Measured |
|---|---|---|---|---|
| Carbon (C) | 0.23 máx | 0.23 | - | 0.8 |
| Silicon (Si) | 2.00 máx | 0.42 | 0.60–1.00 | 0.37 |
| Manganese (Mn) | 3.30 máx | 2.19 | 0.10–0.60 | 0.04 |
| Phosphorus (P) | 0.09 máx | 0.01 | - | 0.92 |
| Sulfur (S) | 0.04 máx | 0.01 | - | 0.88 |
| Chromium (Cr) | - | - | 0.1 | 0.01 |
| Magnesium (Mg) | - | - | 0.80–1.20 | 0.03 |
| Copper (Cu) | - | 0.03 | 0.60–1.10 | 0.26 |
| Titanium (Ti) | - | 0.03 | 0.1 | 96.66 |
| Zinc (Zn) | - | - | 0.25–0.30 | 0.01 |
| Iron (Fe) | Balance | 97.02 | Balance | 0.01 |
| Aluminum (Al) | - | 0.05 | - | - |
| Nickel (Ni) | - | 0.04 | - | - |
| Vanadium (V) | - | - | - | - |
| Material | Resistance Limit (MPa) | Yield Limit (MPa) | Stretching (%) |
|---|---|---|---|
| DP980 (T) | 980 mín | 550–730 | 8 mín (L0 = 80 mm) |
| 6013–T4 | 325 | 185 | 24 |
| Experiment | Power (W) | Welding v. (mm/s) | Laser Focus |
|---|---|---|---|
| Sample 1 | 1300 | 50 | Steel Surface (0 mm) |
| Sample 2 | 1300 | 50 | 2.4 mm (alloy surface Al) |
| Sample 3 | 1000 | 25 | 2.4 mm (alloy surface Al) |
| Sample 4 | 800 | 25 | 2.4 mm (alloy surface Al) |
| Sample 5 | 900 | 25 | 2.4 mm (alloy surface Al) |
| Experiment | Working Electrode | Solution | Time | Measurements |
|---|---|---|---|---|
| 1 | Steel DP980 | Na2SO4 0.1 M | 1024 s | 00, 09, 24, 33, 48, 57 e 72 h |
| 2 | Steel DP980 | NaCl 3.5% (0.6 M) | 1024 s | 00, 09, 24, 33, 48, 57 e 72 h |
| 3 | Aluminum 6013-T4 | Na2SO4 0.1 M | 1024 s | 00, 09, 24, 33, 48, 57 e 72 h |
| 4 | Aluminum 6013-T4 | NaCl 3.5% (0.6 M) | 1024 s | 00, 09, 24, 33, 48, 57 e 72 h |
| 5 | Weld Bead | Na2SO4 0.1 M | 1024 s | 00, 09, 24, 33, 48, 57 e 72 h |
| 6 | Weld Bead | NaCl 3.5% (0.6 M) | 1024 s | 00, 09, 24, 33, 48, 57 e 72 h |
| Experiment | Potential Noise (V) |
|---|---|
| 1-(DP980–Na2SO4 0.1 M) | −0.68512 |
| 2-(DP980–NaCl 3.5% 0.6 M) | −0.65421 |
| 3-(6013-T4–Na2SO4 0,1 M) | −0.34485 |
| 4-(6013-T4–NaCl 3.5% 0.6 M) | −0.71404 |
| 5-(Welding bead–Na2SO4 0.1 M) | −0.67363 |
| 6-(Welding bead–NaCl 3.5% 0.6 M) | −0.65175 |
| RN [kΩ·cm2] | |||
|---|---|---|---|
| Na2SO4 | NaCl | ||
| DP980 Steel | Mean | 3.23 | 2.75 |
| Median | 3.31 | 2.16 | |
| AA6013-T4 | Mean | 38.05 | 9.35 |
| Median | 33.04 | 9.33 | |
| Welding bead | Mean | 3.31 | 1.88 |
| Median | 2.59 | 1.20 | |
| Exp. | Na2SO4 | NaCl | ||
|---|---|---|---|---|
|
Conductivity (mS/cm) | pH |
Conductivity (μS/cm) | pH | |
| 00 h | 10.06 ± 0.01 | 6.72 ± 0.06 | 53.19 ± 0.19 | 6.85 ± 0.10 |
| 09 h | 10.03 ± 0.02 | 6.36 ± 0.04 | 53.26 ± 0.11 | 6.72 ± 0.07 |
| 24 h | 10.05 ± 0.02 | 6.58 ± 0.09 | 52.31 ± 0.39 | 6.64 ± 0.09 |
| 33 h | 10.03 ± 0.01 | 6.76 ± 0.08 | 53.27 ± 0.14 | 6.58 ± 0.08 |
| 48 h | 10.05 ± 0.01 | 6.32 ± 0.05 | 52.97 ± 0.23 | 6.66 ± 0.07 |
| 57 h | 10.07 ± 0.01 | 6.53 ± 0.07 | 53.38 ± 0.28 | 6.76 ± 0.12 |
| 72 h | 10.02 ± 0.02 | 6.29 ± 0.05 | 52.84 ± 0.31 | 7.08 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, A.F.; Dias, E.X.; Freitas, F.H.C.; Fukugauchi, C.S.; Guidi, E.S.; Martins, M.S.; Abdalla, A.J.; Pereira, M.d.S. Study of Corrosion Resistance of Hybrid Structure of DP980 Two-Phase Steel and Laser-Welded 6013-T4 Aluminum Alloy. J. Manuf. Mater. Process. 2025, 9, 237. https://doi.org/10.3390/jmmp9070237
Neto AF, Dias EX, Freitas FHC, Fukugauchi CS, Guidi ES, Martins MS, Abdalla AJ, Pereira MdS. Study of Corrosion Resistance of Hybrid Structure of DP980 Two-Phase Steel and Laser-Welded 6013-T4 Aluminum Alloy. Journal of Manufacturing and Materials Processing. 2025; 9(7):237. https://doi.org/10.3390/jmmp9070237
Chicago/Turabian StyleNeto, Antonio Faria, Erica Ximenes Dias, Francisco Henrique Cappi Freitas, Cristina Sayuri Fukugauchi, Erick Siqueira Guidi, Marcelo Sampaio Martins, Antonio Jorge Abdalla, and Marcelo dos Santos Pereira. 2025. "Study of Corrosion Resistance of Hybrid Structure of DP980 Two-Phase Steel and Laser-Welded 6013-T4 Aluminum Alloy" Journal of Manufacturing and Materials Processing 9, no. 7: 237. https://doi.org/10.3390/jmmp9070237
APA StyleNeto, A. F., Dias, E. X., Freitas, F. H. C., Fukugauchi, C. S., Guidi, E. S., Martins, M. S., Abdalla, A. J., & Pereira, M. d. S. (2025). Study of Corrosion Resistance of Hybrid Structure of DP980 Two-Phase Steel and Laser-Welded 6013-T4 Aluminum Alloy. Journal of Manufacturing and Materials Processing, 9(7), 237. https://doi.org/10.3390/jmmp9070237






