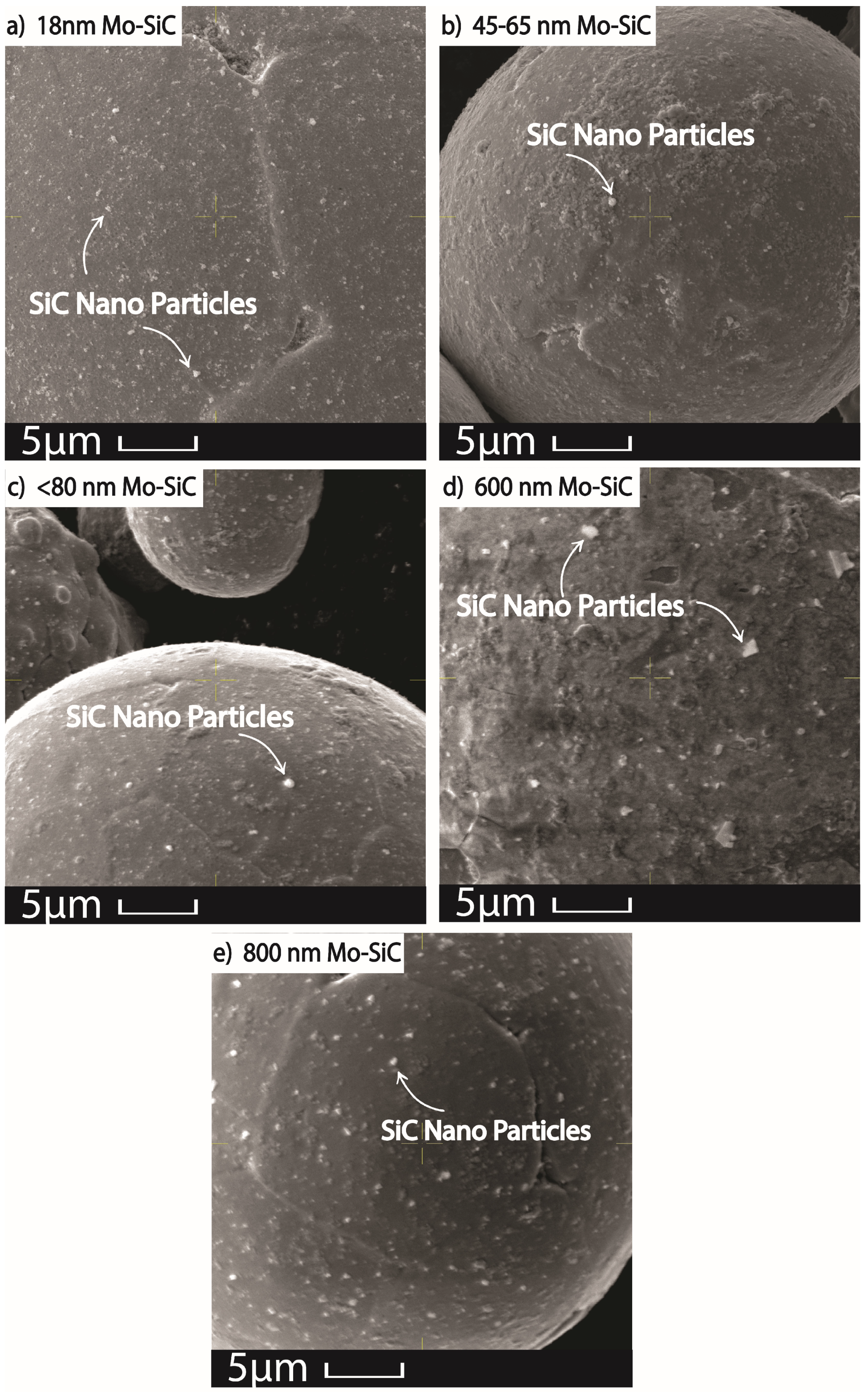
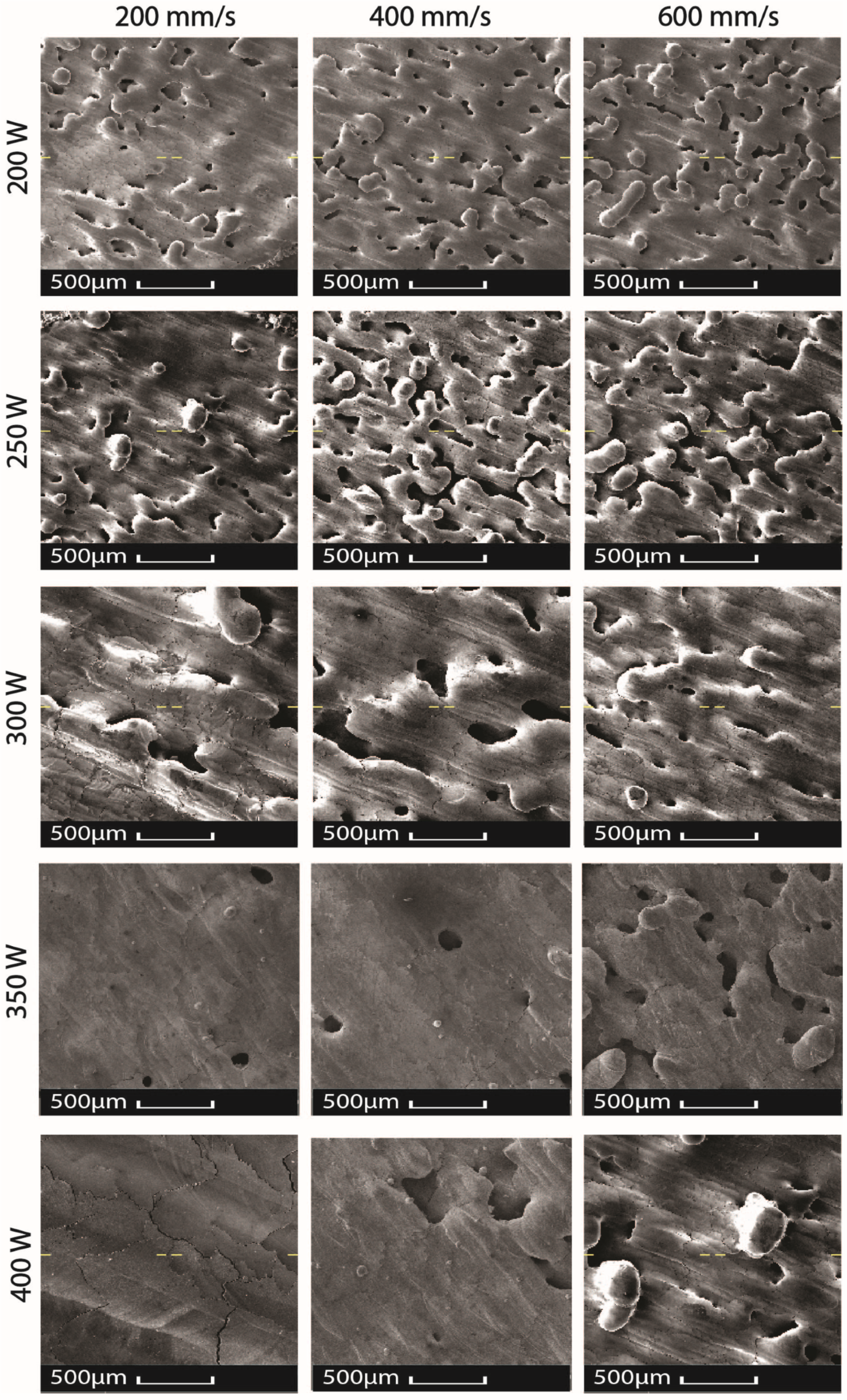
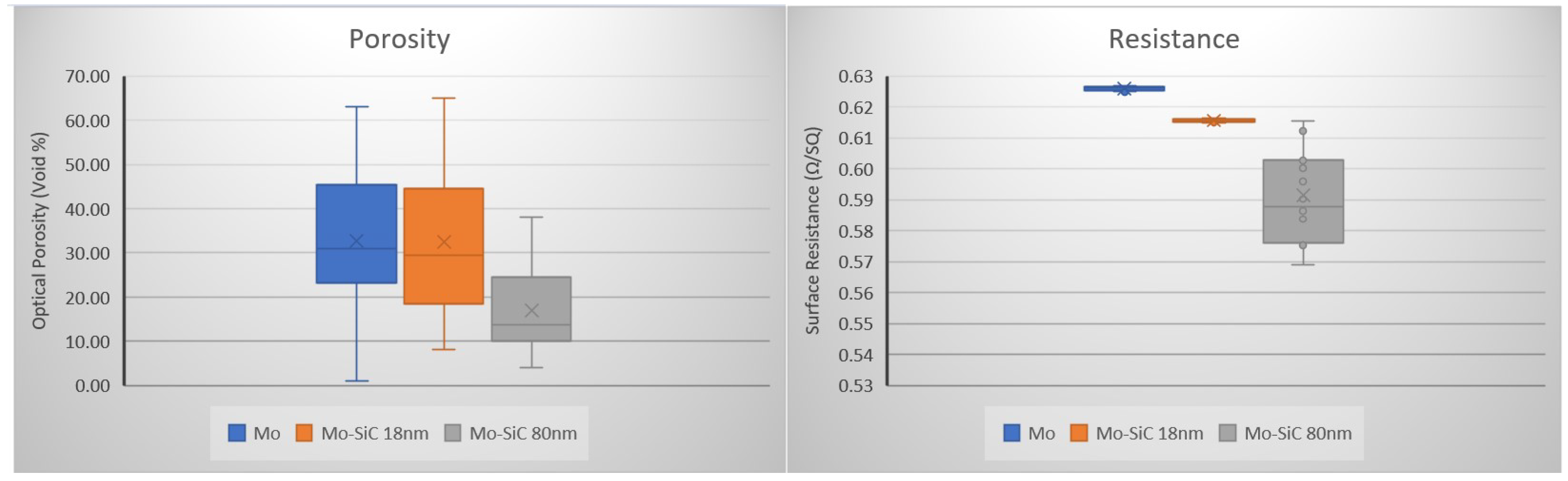
2.1. Oxidation in Laser Powder Bed Fusion
SiC is thermodynamically stable under Standard Normal Temperature and Pressure (STP) conditions and at much lower partial oxygen pressures due to rate limitations. The stability of SiC at lower temperatures is due to the large activation energies, causing the oxidation reaction rates to be slow. Moreover, continuous silicate coating formation of
can act as an oxygen diffusion barrier to slow oxidation. At high temperatures, the activation energy is more readily surmounted. Possible reactions of oxidation of SiC with oxygen that can contribute to the mechanism are shown in
Table 1 below from the report by Ervin et al. (1958) [
14]. All of these reactions are thermodynamically allowed to occur at high temperatures. Thermodynamically favored processes with large negative free energy changes but which are not elementary reactions and have large activation entropies can be kinetically disfavored even at high temperatures [
14].
Despite being the most favored reaction, the first reaction listed in
Table 1 fails to satisfy both criteria. The complex rearrangement of atoms involved suggests that it cannot be an elementary reaction, and the fact that there are more moles of gas on the reactant side than the product side indicates a negative change in the entropy of activation, leading to the reaction being disfavored.
The primary high-temperature reaction at low oxygen partial pressures is
This reaction is favored both thermodynamically and kinetically, resulting in silica distribution in the matrix and reduced mass. At very high temperatures, where the influence of the activation energy is insignificant, the reaction rate depends on the diffusion rate of oxygen molecules to the surface through the sample porosity and any oxide coating [
17]. On the other hand, at high oxygen partial pressures, the dominant reaction is
This reaction leads to an increase in mass. It involves a complex reaction mechanism, with its initiating activated step likely similar to the low-pressure reaction. The first reaction, which produces SiO(g), is the only reaction that needs to be considered for SiC oxidation in LB-PBF processing of Mo-SiC.
The size of the SiC particles also affects the reaction rate. According to Yin et al. (2021), oxidation in air follows a weight increase function of the parabolic rate [
18]. They observed that the reaction initiates at a lower temperature, 783 °C, for nanoscale particles than for microscale particles, which begin at 843 °C [
18]. As a result, it was determined that the activation energy for oxidation was lower for nanoscale particles, at
than for microscale particles, at
[
18].
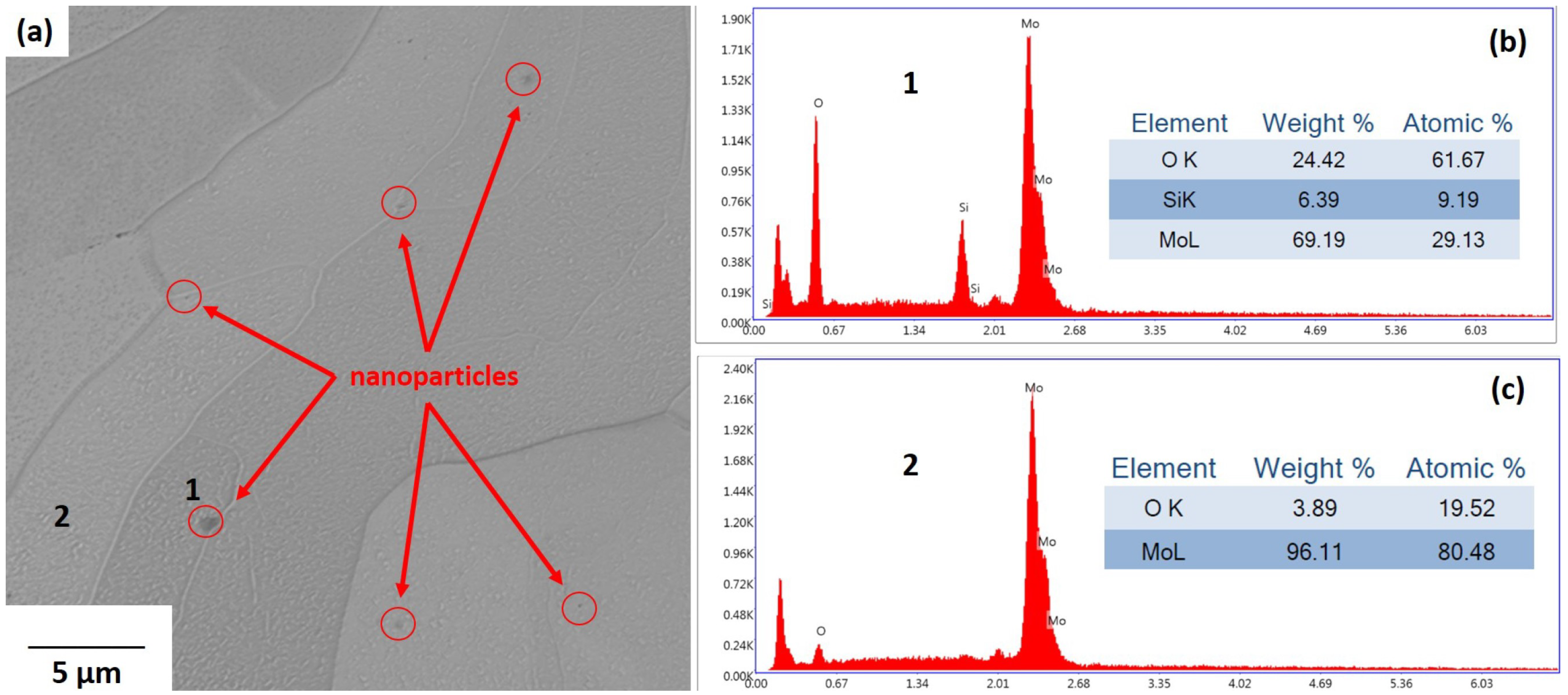
The source of most oxygen in the LB-PBF printing was oxide on the surface of the
powder particles. When exposed to air,
surfaces undergo oxidation to form various oxides including
,
, and intermediate stoichiometric phases such as
and non-stoichiometric
phases. At different equilibrium oxygen partial pressures, the surface oxides have different stabilities. For instance,
on the metal surface remains stable even at high temperatures of up to 772 °C under low oxygen partial pressures below
bar [
19,
20]. Conversely,
is unstable at low oxygen partial pressures and high temperatures, where surface
can either dissociate or volatilize [
21,
22]. SiC juxtaposed to the oxide surface reacts with the
oxide, forming carbon monoxide. Whereas in the absence of the oxide layer, carbon diffuses into molybdenum and
atoms diffuse into defective SiC to form a phase-separated silicide above 1200 °C [
21,
22,
23].
The stability of
coatings on Mo particles is limited at low oxygen pressures and high temperatures exceeding 750 °C [
19]. Under such conditions,
can volatilize and dissociate to form non-stoichiometric
and reduced Mo oxides. Heating at 1500 °C in
Pa of oxygen can completely remove absorbed oxygen from the metal surface [
20]. The oxidation of Mo is modeled using a two-interface onion-skin kinetic model, which involves the oxidation of
to
with an activation energy barrier of 77.691
[
23]. Interface diffusion controls the oxidation of
to
, which has an activation energy of 158.664
[
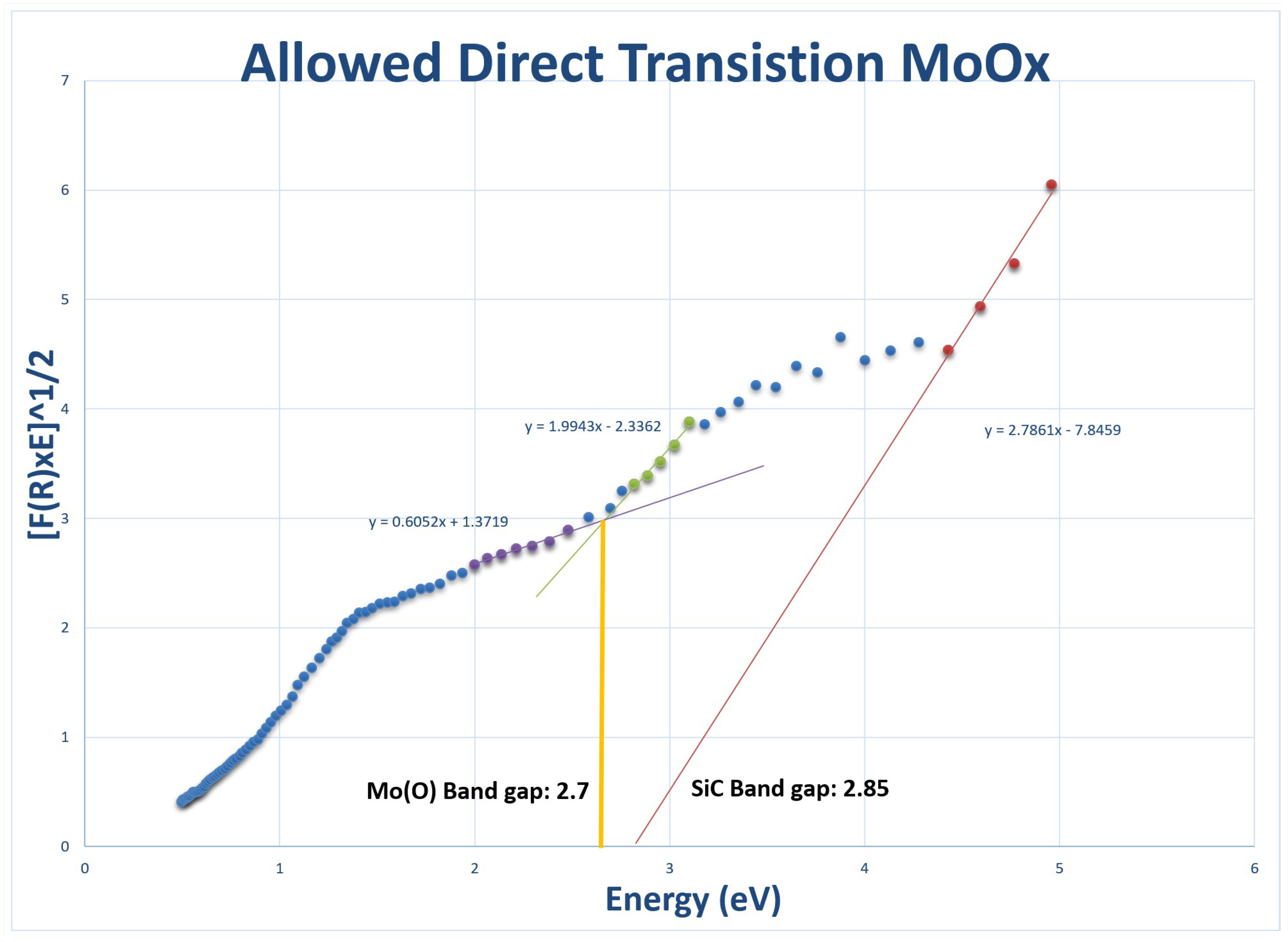
24]. Additionally, Mo oxide thin films that were prepared by electron beam evaporation and heated in the air exhibited an orthorhombic
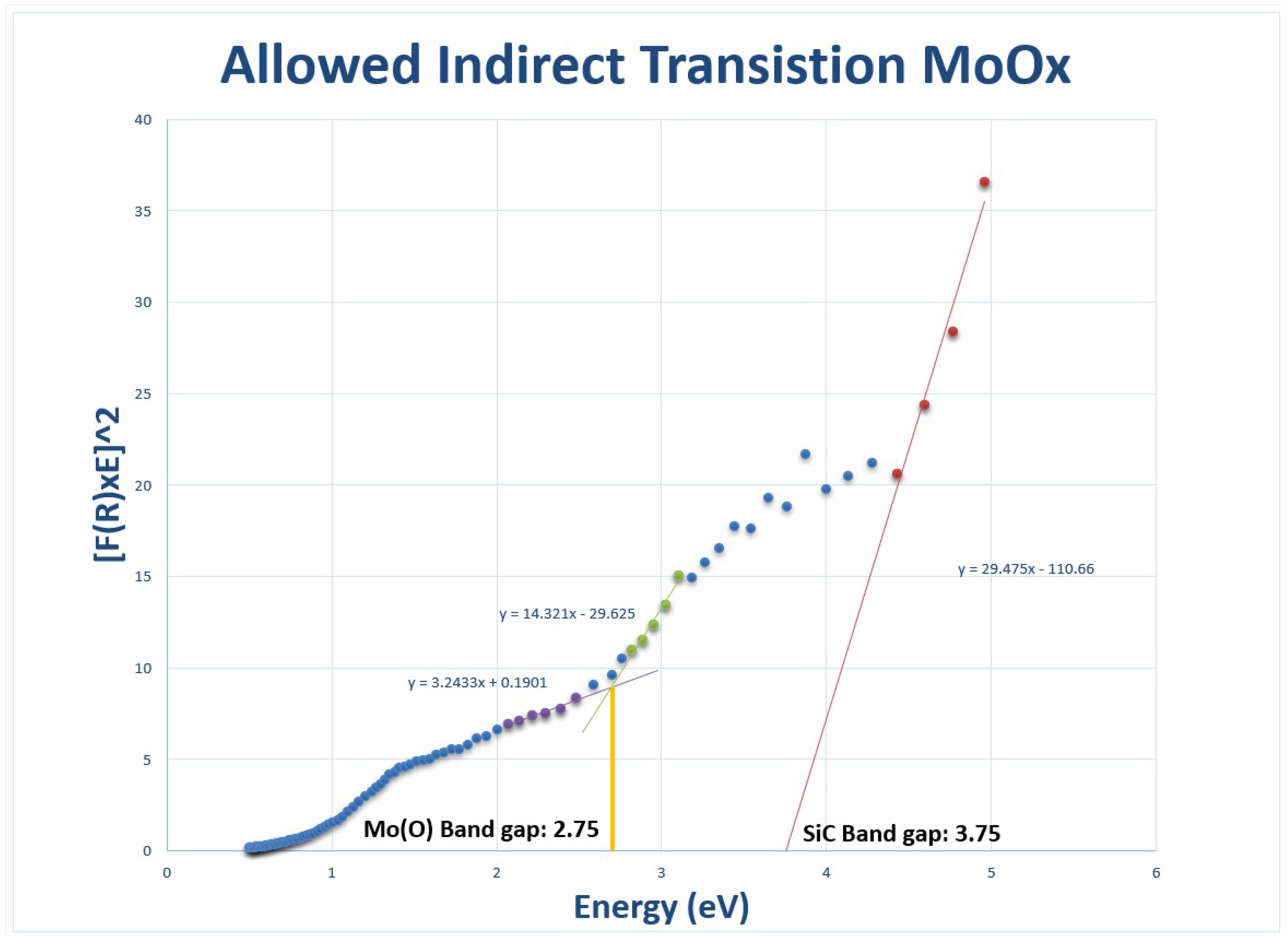
-phase with an optical band gap of
eV [
21,
22].
According to Dukstiene et al. [
25],
films exhibit a high Absorption Coefficient (k) for a direct band-to-band transition. The k is reported to be
, with an extinction of 2.1 times the refractive index as seen in
Table 2. The band gap energy ranges from 2 to 2.4 eV and varies depending on the film thickness, density, and morphology. Rabalais et al. proposed a simple qualitative band model to account for the variability of the electrical and optical properties of
films as described in Equation (
1) [
26]. In this equation describing k as the extinction coefficient,
is the absorption coefficient and
is the wavelength.
Assuming the bonding in the
surface oxide is mostly ionic, the valence band orbital consists of electrons from the oxygen
and the vacant
and
orbitals. In substoichiometric
, an oxygen ion is electron deficient, resulting in defect orbitals that act as gap electronic states between the valence and conduction bands. These positively charged oxygen defects, which are not detected by positron spectroscopy, can increase carrier concentration. The
sites can be reduced to
with a
eV energy change, which moves an electron into the delocalized conduction band [
27]. This process is responsible for the increased conductivity of
oxide. While
is a semiconductor with a band gap of
eV, when the oxide is reduced, its conductivity can increase by at least an order of magnitude by partial reduction to
. Reduction produces shallow oxygen vacancies which transfer electrons to
ions, forming
or
holes in distorted lattice positions, yielding a lowered band gap. The band gap for
is
eV, and the oxide band gap approaches zero for
, at which the oxide exhibits metal-like properties. The electrical and optical properties of the
film depend on its thickness and the equilibrated redox activity.
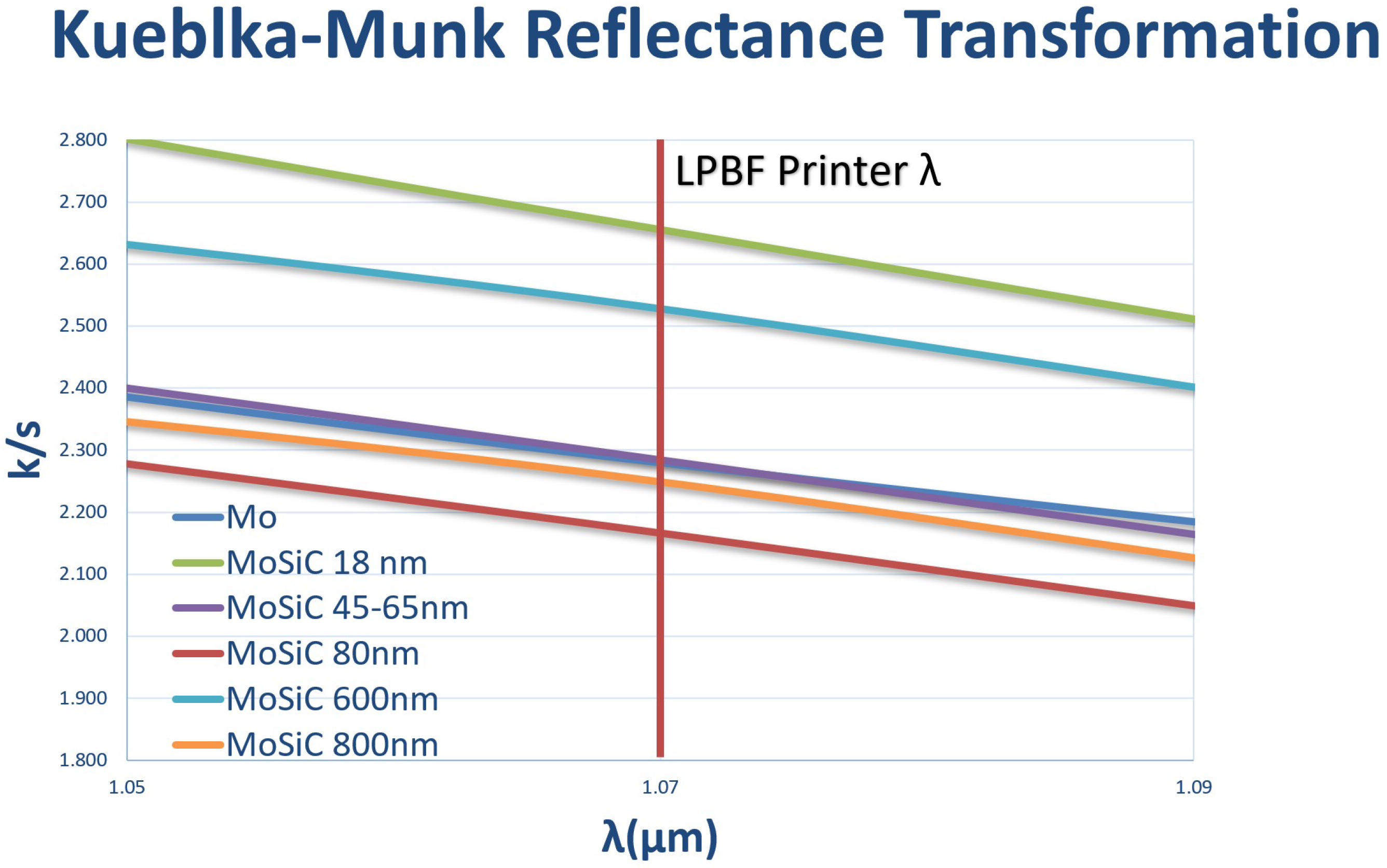
The minimum thickness required for the resonance condition of interference of light between the two interfaces of a
coating on Mo at a wavelength of 1070 nm is 260 nm. When oxide coatings are applied on micron-sized Mo metal particles, resonance reflection coupling is expected to occur for the LB-PBF laser wavelength at thicknesses equal to small integer multiples of approximately 260 nm. Reflectance analysis is a useful tool to understand the behavior of such coatings. It has been observed in previous studies that the addition of small quantities of SiC particles can enhance the performance of LB-PBF parts under certain operational conditions. The presence of SiC may also increase the absorption of laser energy for laser powder-bed metal manufacturing by modifying melt pool dimensions and surface morphology [
29].
2.2. Van der Pauw Resistivity
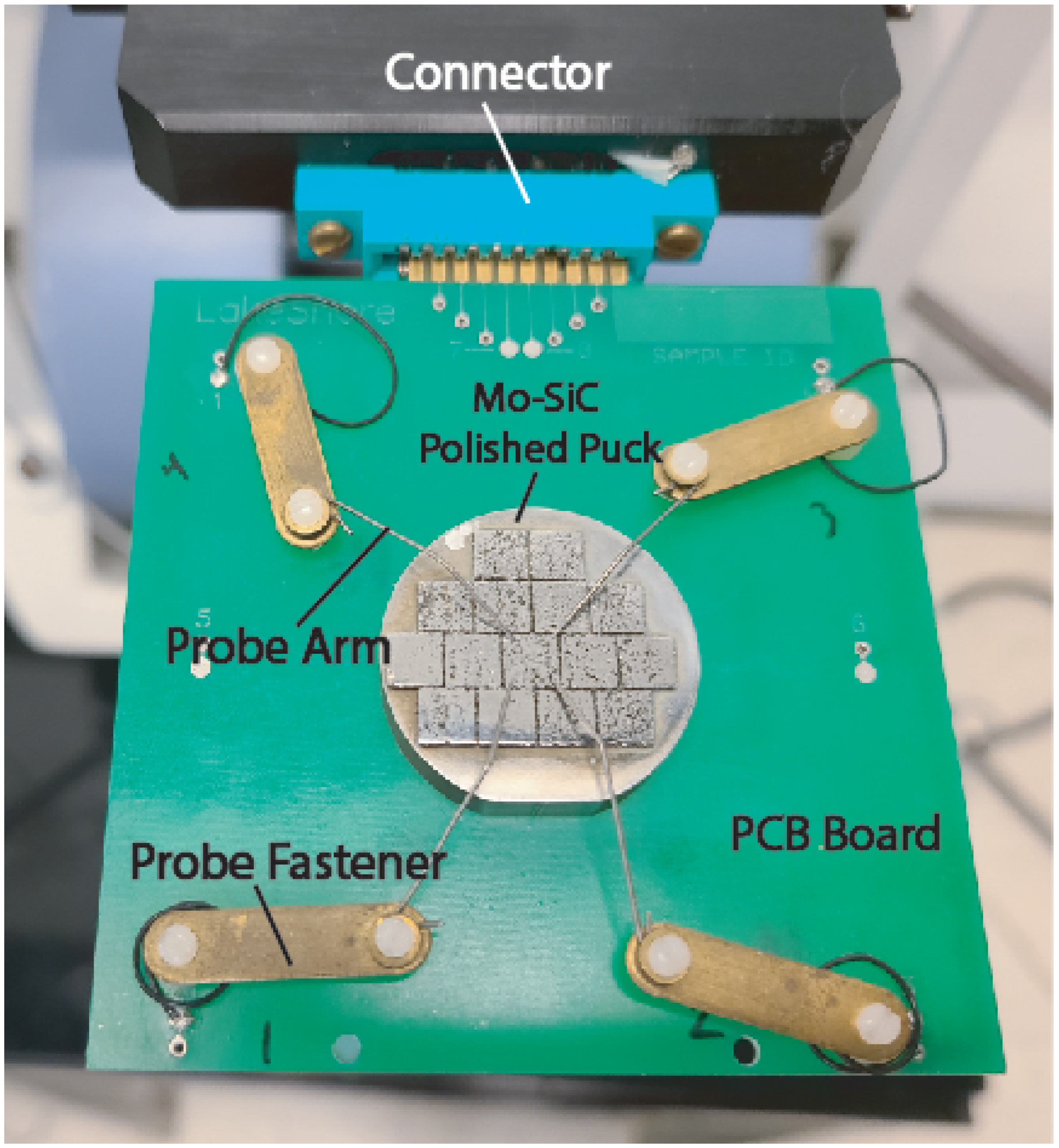
The Van der Pauw method is one of the most effective and widely used four-probe methods of measuring resistivity in thin film materials [
30]. Some experimental setups can be indirectly measured using a non-contact eddy-current-based testing device. A schematic of the four-probe experimental setup for a square geometry can be seen in
Figure 1. The Van der Pauw technique derives sheet resistance across a Two-Dimensional (2D) area. Sheet resistance normalizes a material’s resistance, making it invariant to scaling. Therefore, sheet resistance can be used to compare the electrical properties of materials with different compositions or sizes. The equation for sheet resistance is shown in Equation (
2).
Linear point-to-point resistance, which is variable to scaling, must be used to measure sheet resistance. As shown, Equation (
2) depends upon paired probe arm contacts in the four-terminal contact array.
and
are shown in Equations (
4) and (
5) to be dependent on Voltage (V) and Current (I) between probes, where the subscripts indicate probe contact pairs. These referenced probe positions are shown in
Figure 1.
Resistance measurements are intrinsically geometry-dependent and sensitive to the boundary and surficial conditions. Consequently, correction factors for fitting data to models are empirically derived and indicated by the function
. These correction factors have been determined for a multitude of complex probe geometries, and the general equation is shown in Equation (
3). In this equation, the ratio of
to
is dependent on relative probe positioning or probe position outline. In the case of a perfect square probe array, the left-hand side of Equation (
3) reduces to two, which correspondingly makes the function one. Luckily, this probe setup is common and a correction factor of one is widely used across experiments [
30,
31].
2.3. Kubelka-Munk Model
Light interactions can be described in simplified terms using three wavelength-dependent mechanisms [
32]. Firstly, the transmission of light through a guidance structure like optical fibers or light-shaping lenses [
32]. Then the mechanism of either absorption, reflectance, or transmittance occurs. In the process of absorption, photons transfer all of their kinetic energy to electrons within the material and thus are converted into phonons [
32]. The result is the heating of the matter. The process of transmission and reflection occurs at interfaces, where a light ray either penetrates the material or scatters off particles, atoms, or nuclei. These three mechanisms are strongly dependent on the wavelength of the electromagnetic radiation [
32].
According to the Kubelka-Munk theory of diffuse reflectance, the energy of incident light in each layer of material can be expressed as
, where
R denotes macroscopic reflectance,
A denotes absorption, and
T denotes transmittance. By integrating this formula over a semi-infinite opaque sample, Equation (
6) can be derived. Rearranging this equation leads to Equation (
7), which shows the ratio between total absorption and total incident light energy for opaque materials. Since there is no transmittance of light in opaque materials, the ratios of
and
are equivalent to
A and
R, respectively.
The extinction coefficient of solid materials can be expressed as the sum of the k and the Scattering Coefficient (s), i.e.,
. In the K-M, an increase in the s is associated with a decrease in particle size and results in an increase in reflectance. While for particle sizes below
m, the s-parameter decreases in proportion to the inverse of the scattering length,
, as noted in Dai et al. [
33] for larger mixed particle sizes the s-parameter is approximately constant and for large k/s, the ratio is proportional to absorbance. On the other hand, as a light ray encounters more scattering interfaces, the gls s increases, leading to a reduction in the penetration depth of radiation [
33]. This reduction in penetration depth, combined with a decrease in particle size, causes an increase in the fraction of diffusely reflected light and a decrease in the absorbed fraction of radiation. Meanwhile, the average depth of penetration decreases with an increase in the k.
The representative layer theory is employed for mixtures of particles of different sizes. Dahm et al. demonstrated that the contribution of a particle type to absorption is proportional to its volume fraction, while its contribution to reflectance is proportional to the fraction of the cross-sectional area in the layer [
34]. Thus, the mass fraction determines the contribution of a particle type to light absorption.
The efficiency of LB-PBF processes is largely determined by the ratio of absorbance to reflectance, which depends on the optimal wavelength range for a material. Surface-to-volume ratios, packing structure, oxidation level, and particle size distribution affect the interaction volumes and thus influence the optimal wavelength range [
35]. This ratio plays a crucial role in optimizing LB-PBF systems and increasing part production efficiency [
32]. Therefore, several models, such as the K-M model, have been developed to accurately predict and describe this ratio.
The K-M model is used to describe how light interactions differ between homogeneous and heterogeneous powder mixtures, with a focus on differences in diffuse reflectance. Diffuse reflectance was first developed to analyze powders under standard temperature and pressure conditions [
36]. The propagation of light can vary significantly between homogeneous and heterogeneous media due to scattering off points in its path [
36]. The K-M is widely used to describe diffuse reflection due to its simplifying assumptions in semi-infinite samples [
36]. In the K-M model, all geometric details of a heterogeneous sample are summarized in a single parameter known as the s [
36]. The s was introduced to account for internal scattering processes and is a semi-empirical parameter that depends mainly on the particle size and refractive index of the sample [
36]. Unlike the k, the s is weakly dependent on wavelength and treated as a constant for a given sample, but it does change significantly with packing density [
36]. The diffuse reflectance
is given by Equation (
8), where the k is shown in Equation (
9), and it depends on the incident light wavelength
and an arbitrary fitting coefficient
that is usually taken to be 1 [
37]. The K-M transformation, as shown in Equation (
10), expresses the proportion between absorption concentration and scattering concentration about a unit volume of powder. Many researchers focus on accurately describing and predicting this proportion because it is crucial for optimizing LB-PBF systems and increasing part production efficiency [
32].
Since
, this gives the absorption spectrum offset by a scattering term displaced by
. While the s might change with particle size, the absorption, A, for near-infrared diffuse reflectance is given by Equation (
11).
The K-M transform of the measured spectroscopic reflectance is proportional to the k and hence is proportional to the concentration [
36]. Due to its simplicity, it has been widely used for diffuse reflectance transforms in standard infrared spectroscopy software of commercial Fourier Transform - Infrared (FT-IR) spectrometers. This model shows that smaller and heterogeneous mixtures will lead to greater absorption. Agreement with spectroscopy studies gives credence to applying the K-M model to oxidized metal powder mixtures.
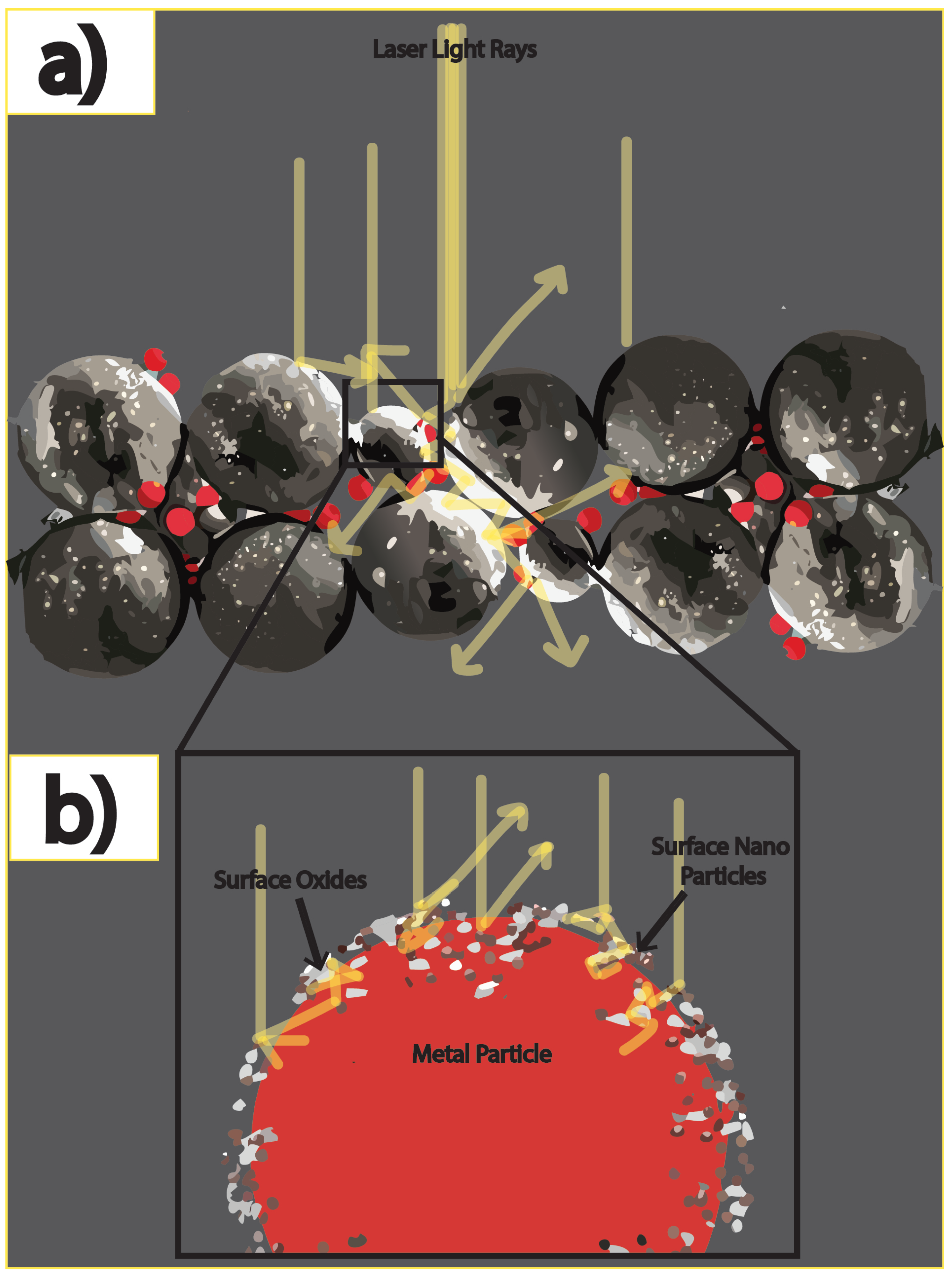
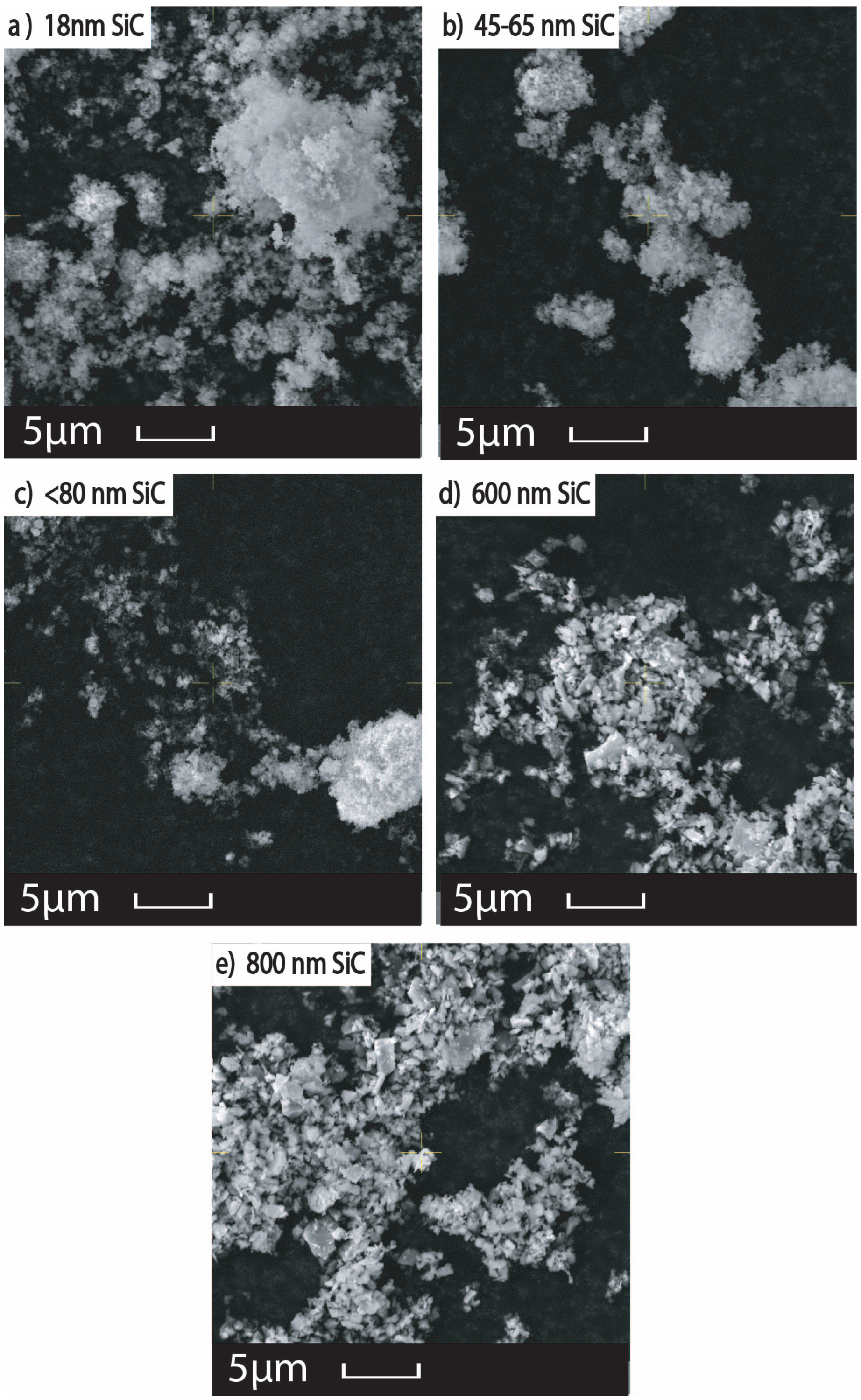
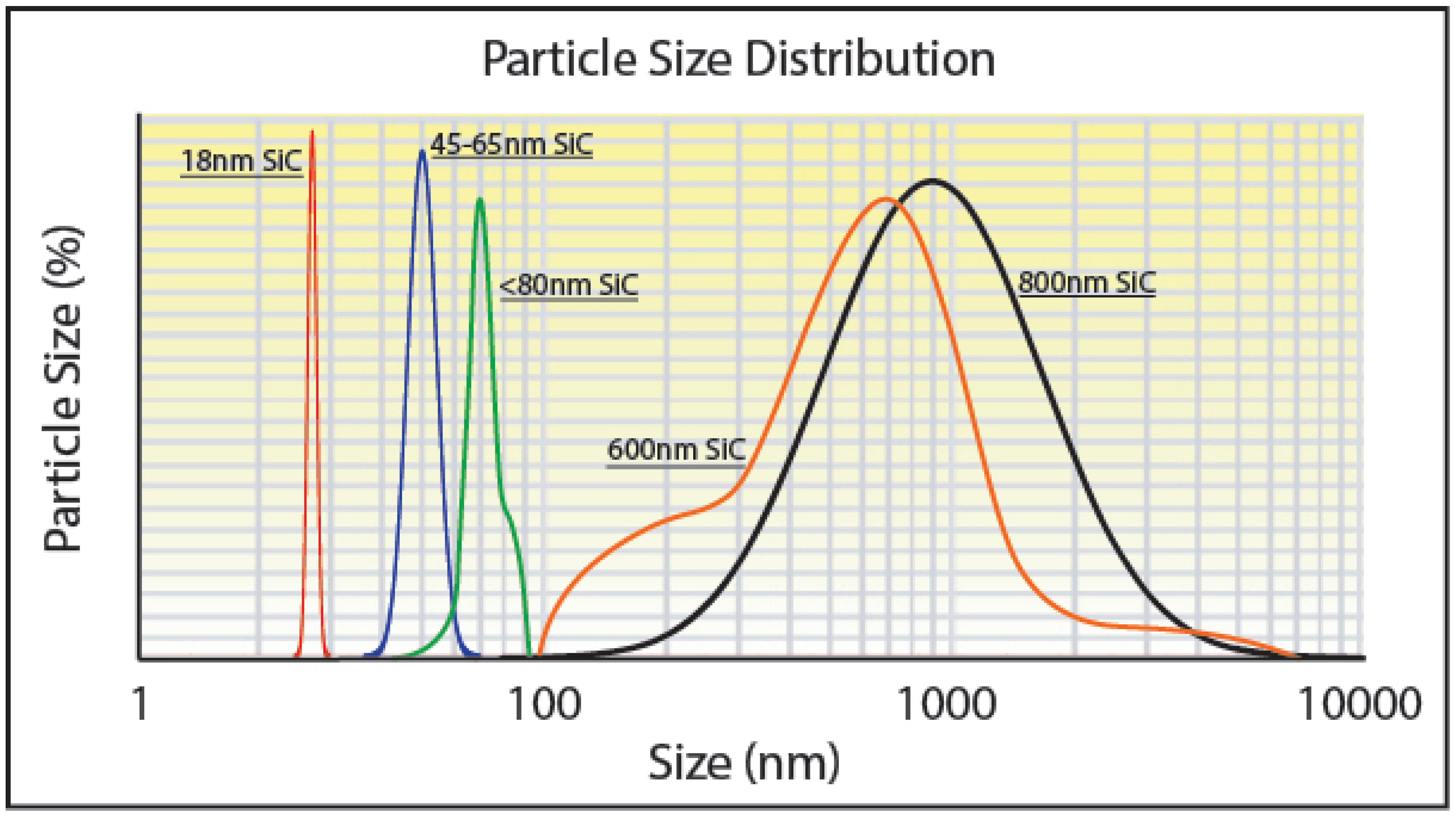
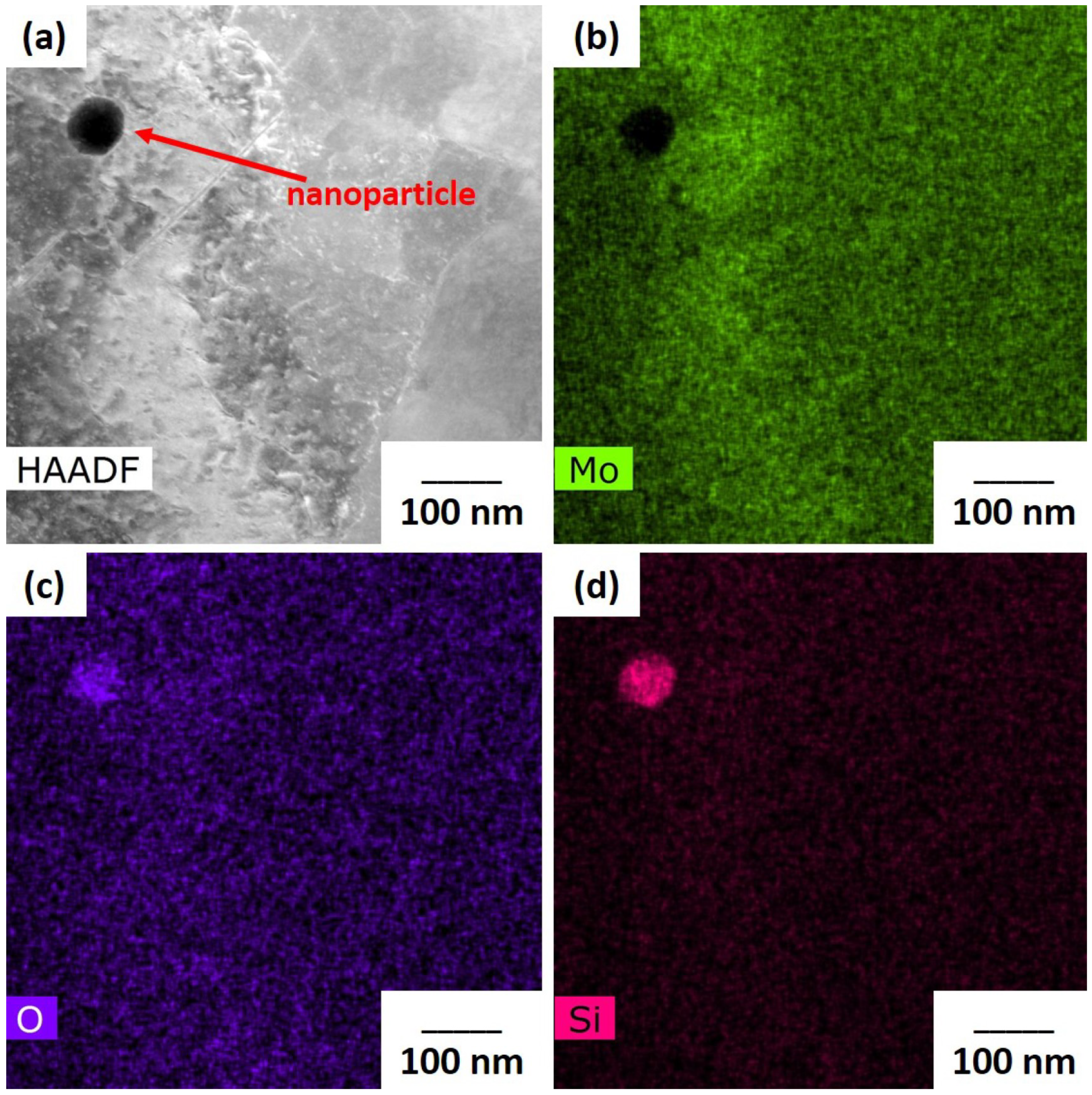
The addition of nanoparticles can increase the reflectivity via scattering as shown in
Figure 2. The scattering cross section is proportional to the 6
th power of the particle radius, producing scattering angles that increase with decreasing particle size. Enhanced scattering from smaller nanoparticles adsorbed on the oxide surface of the Mo particles enhances local light absorption in the
layer approximately in proportion with the inverse of the nanoparticle radius. A study investigated light absorbance in more than 35 metal powders utilized for LB-PBF processes, where laser wavelengths ranging between 330 and 1560 nm were employed [
32]. The powders displayed variations in grain size and degree of oxidation or degradation. The results showed that shorter wavelengths led to greater absorption across all powders. Furthermore, finer-grained powders exhibited higher absorption compared to coarse-grained and heavily oxidized powders, with a 2.4 to 3.3 times higher absorption factor across multiple wavelengths [
32]. The researchers explained that the probability of multiple reflections increased for particles smaller than the diameter of the incident beam, leading to an increase in absorbance. This conclusion is supported by single ray tracing experimental models conducted on homogeneous
by Yang et al. [
12]. The models demonstrated that if the particle size distribution was within the range of incident light wavelength, then the probability of multiple reflections decreased, resulting in Rayleigh and Mie scattering [
12,
32,
37].
Consequently, these powder surfaces optically behaved as fully dense bulk material. The study also found that the morphology of the powders could produce the same phenomenon of increased light scattering and absorption [
32]. Non-spherical or conglomerate particles with rougher surfaces can increase the probability of multiple reflections by providing a greater surface-to-volume ratio.