Mechanical Properties and Accuracy of Additively Manufactured Silicone Soft Tissue Materials
Abstract
1. Introduction
- There is no statistically significant difference in the mechanical properties of conventional and different additively manufactured soft tissue materials in terms of their compressive strength, tensile strength, and Shore A hardness.
- There are no statistically significant differences in the accuracy of different additively manufactured soft tissue materials manufactured under different printing parameters.
2. Materials and Methods
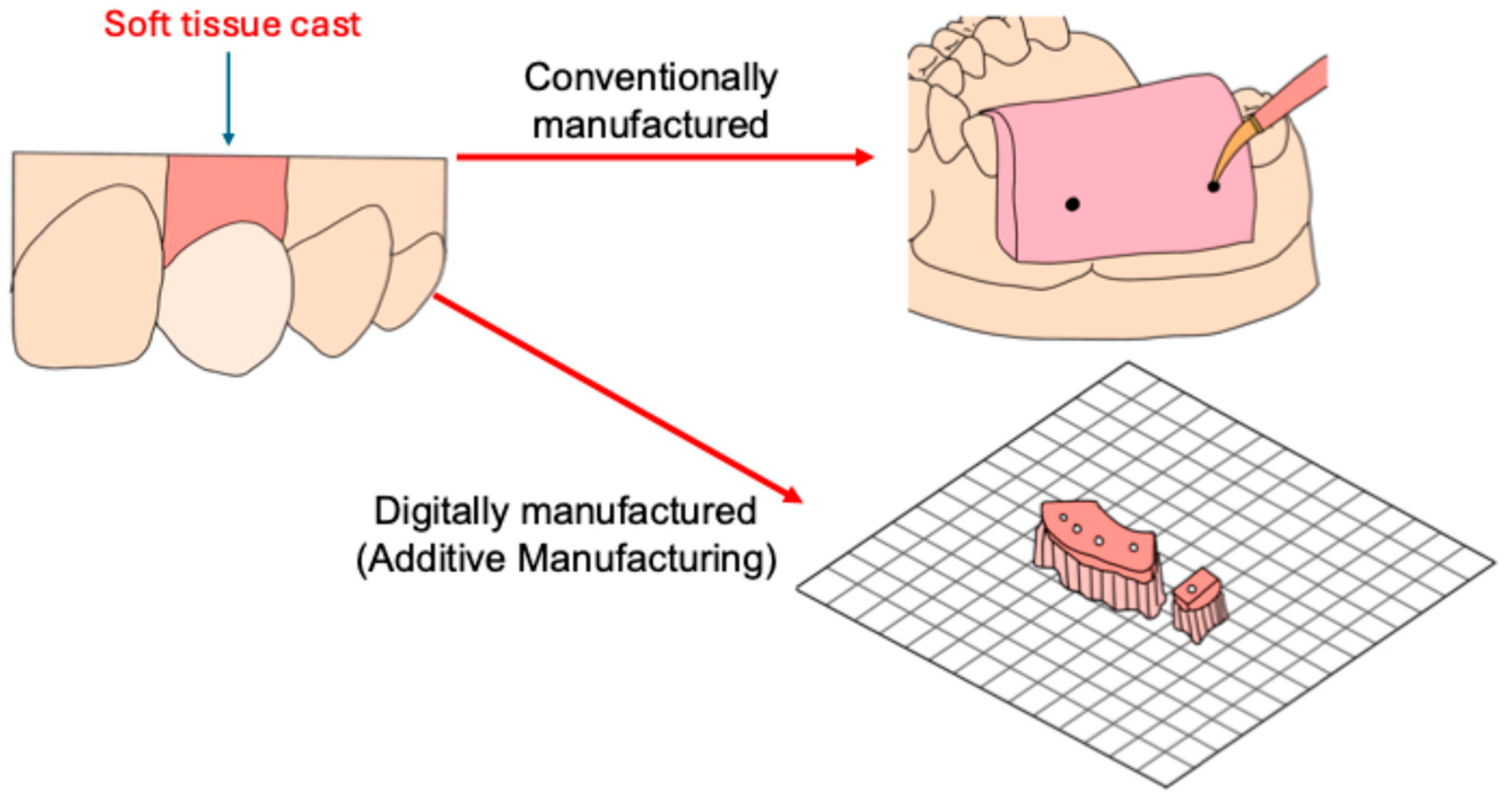
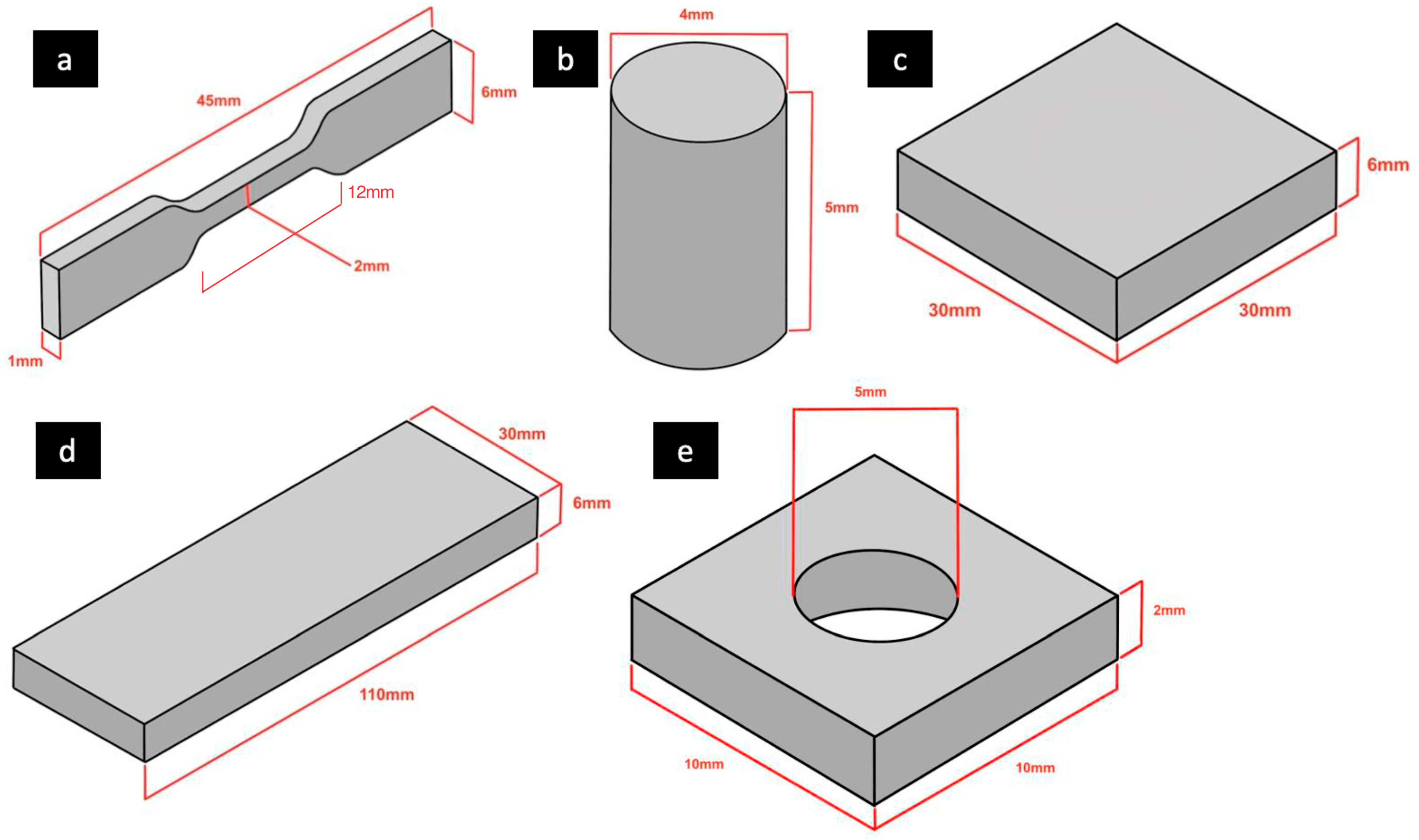
2.1. Specimen Fabrication
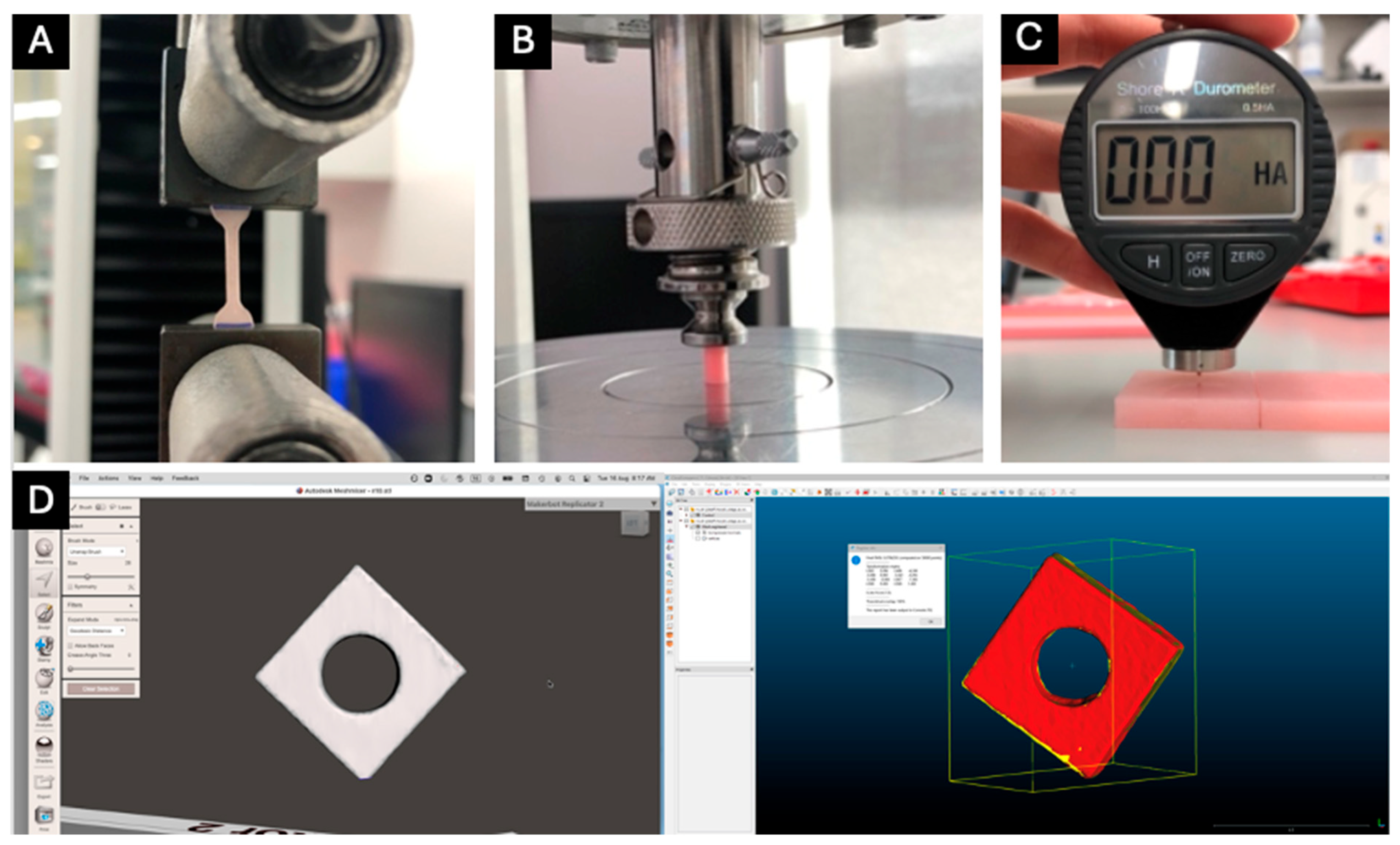
2.2. Tensile Strength Testing
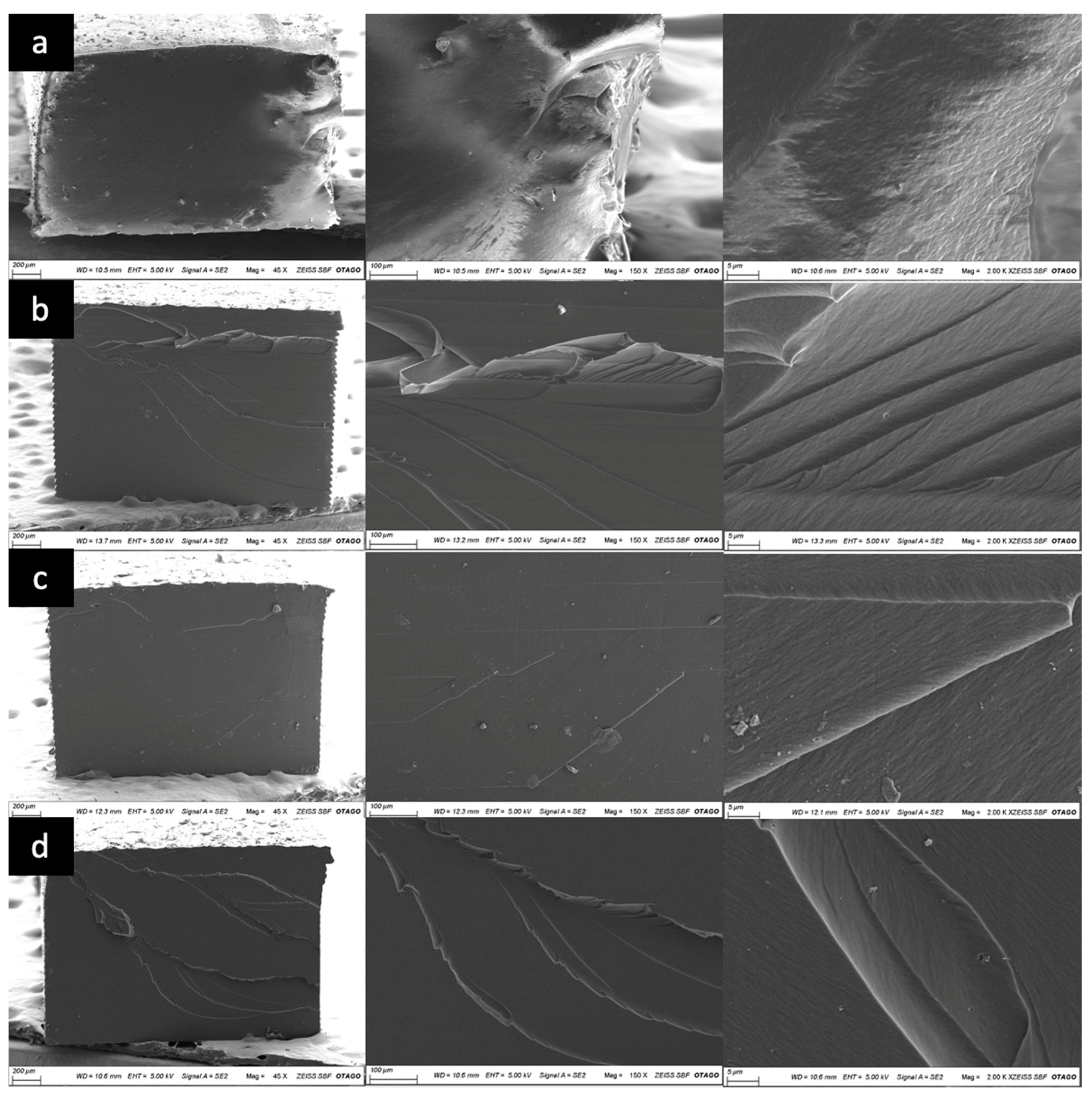
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Compressive Strength Testing
2.5. Shore A Hardness Testing
2.6. Accuracy Analysis
2.7. Statistical Analyses
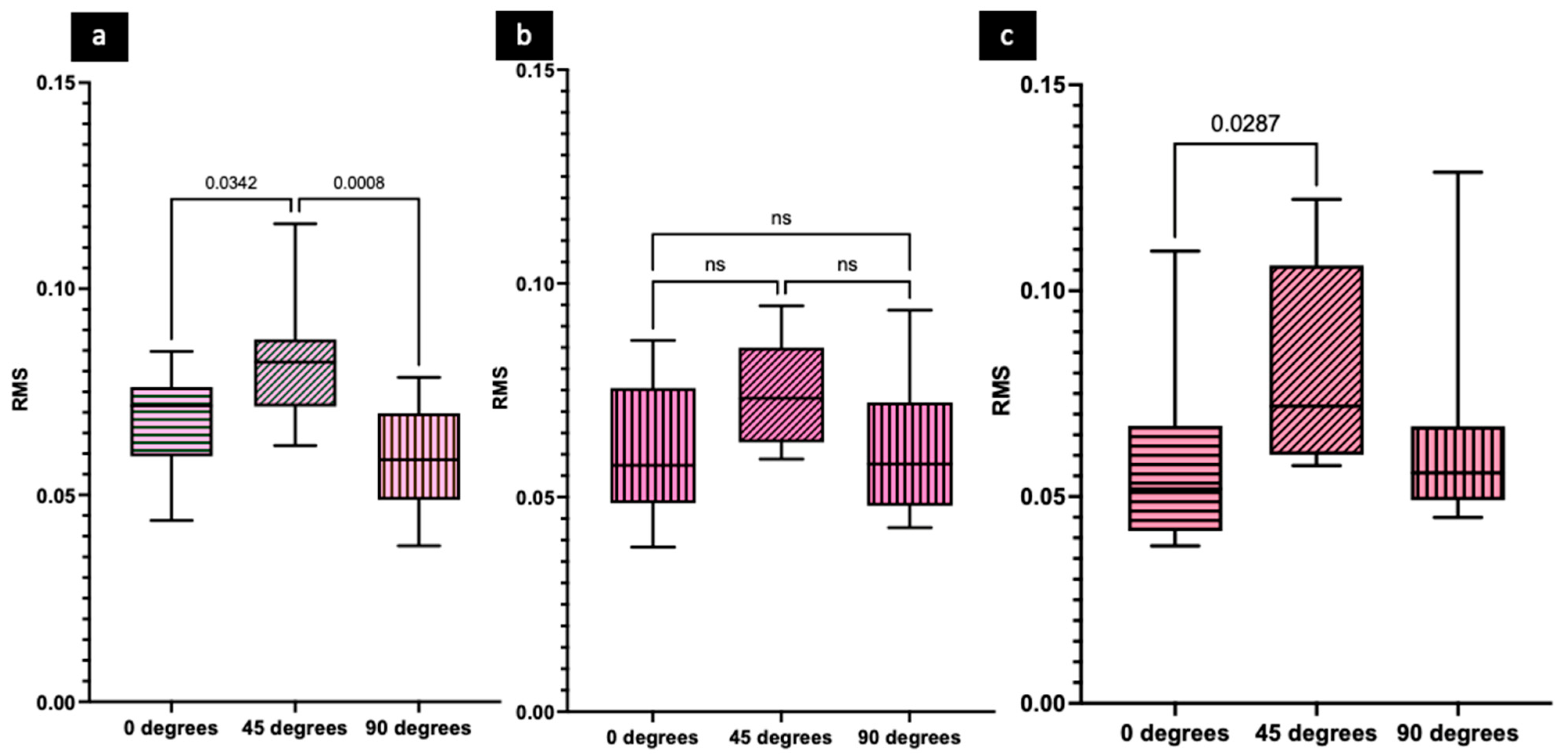
3. Results
4. Discussion
5. Conclusions
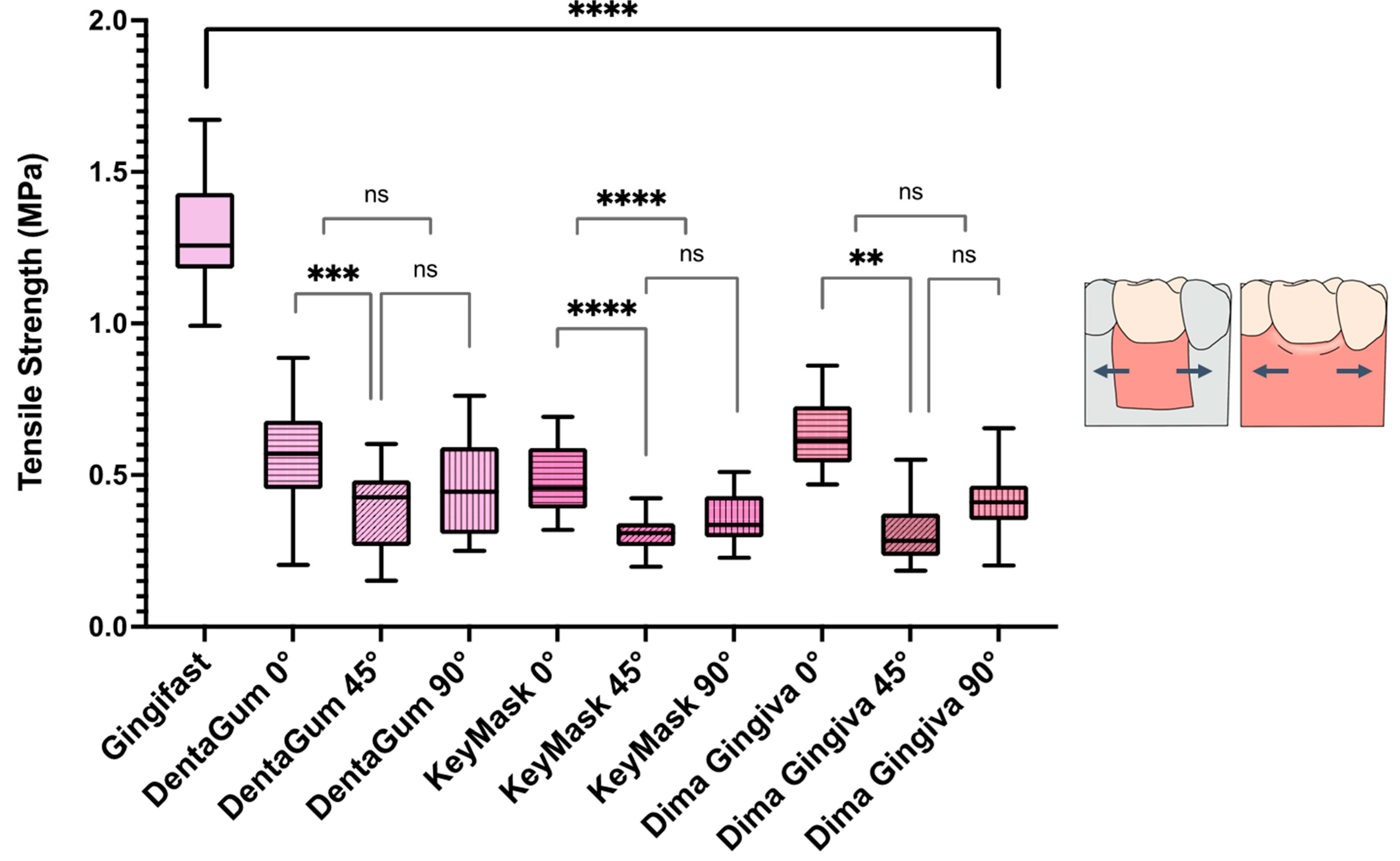
- The conventional injected soft tissue material had a significantly higher tensile strength than all the additively manufactured soft tissue materials. All the additively manufactured soft tissue materials within all build angles studied showed a statistically significantly lower tensile strength.
- A statistically significantly higher compressive strength was found in all the additively manufactured soft tissue materials compared to the control group. Similar to their compressive strengths, the additively manufactured soft tissue materials all had significantly higher Shore A hardness values than the control group.
- Additively manufactured soft tissue materials printed at 45° were found to have significantly higher mean RMS values (less accuracy) than specimens printed at 0° and 90° for both DentaGum and Dima Gingiva.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Janner, S.F.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.; Starr, J.; Da Silva, J.; Gallucci, G. Trends in Dental Implant Use in the US, 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [PubMed]
- Jemt, T. Implant Survival in the Edentulous Jaw—30 Years of Experience. Part I: A Retro-Prospective Multivariate Regression Analysis of Overall Implant Failure in 4585 Consecutively Treated Arches. Int. J. Prosthodont. 2018, 31, 425–435. [Google Scholar] [PubMed]
- Tarlow, J.L. Procedure for Obtaining Proper Contour of an Implant-Supported Crown: A Clinical Report. J. Prosthet. Dent. 2002, 87, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Beyak, B.L.; Chee, W.W. Compatibility of elastomeric impression materials for use as soft tissue casts. J. Prosthet. Dent. 1996, 76, 510–514. [Google Scholar] [PubMed]
- Nayyar, A.; Moskowitz, M.; Pollard, B.L. Improving the Emergence Profile of Dental Restorations with Accurate Reproduction of Soft Tissue Topography. J. Esthet. Restor. Dent. 1995, 7, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Schoenbaum, T.R. Implants in the Aesthetic Zone: A Guide for Treatment of the Partially Edentulous Patient; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Patil, P.G. Modified Soft Tissue Cast for Fixed Partial Denture: A Technique. J. Adv. Prosthodont. 2011, 3, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Neale, D.; Chee, W.W. Development of Implant Soft Tissue Emergence Profile: A Technique. J. Prosthet. Dent. 1994, 71, 364–368. [Google Scholar] [PubMed]
- Kurtzman, G.M.; Schneider, A.L. Implantology—Implant Prosthetic Soft Tissue Model Fabrication. Oral Health 2003, 93, 29–33. [Google Scholar]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern Implant Dentistry Based on Osseointegration: 50 Years of Progress, Current Trends and Open Questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [PubMed]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.-S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; Alharbi, N.; Wismeijer, D. Build Angle: Does It Influence the Accuracy of 3D-Printed Dental Restorations Using Digital Light-Processing Technology? Int. J. Prosthodont. 2017, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.E.; Zwirner, J.; Ramani, R.S.; Ma, S.; Hussaini, H.M.; Waddell, J.N.; Hammer, N. Mechanical Properties of Human Oral Mucosa Tissues Are Site Dependent: A Combined Biomechanical, Histological and Ultrastructural Approach. Clin. Exp. Dent. Res. 2020, 6, 602–611. [Google Scholar] [PubMed]
- Goktas, S.; Dmytryk, J.J.; McFetridge, P.S. Biomechanical Behavior of Oral Soft Tissues. J. Periodontol. 2011, 82, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Kydd, W.L.; Daly, C.H. The Biologic and Mechanical Effects of Stress on Oral Mucosa. J. Prosthet. Dent. 1982, 47, 317–329. [Google Scholar] [PubMed]
- Picton, D.; Wills, D. Viscoelastic Properties of the Periodontal Ligament and Mucous Membrane. J. Prosthet. Dent. 1978, 40, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Darvell, B.W. Materials Science for Dentistry; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Goldstein, G.R. Making Clinical Sense of 3-Dimensional Metrology Software Programs. J. Prosthet. Dent. 2024, 131, 925–932. [Google Scholar] [PubMed]


| Material | Name | Manufacturer | Composition |
|---|---|---|---|
| Conventional injection silicone material | Gingifast | Zhermack | ≥80% to <90% vinylpolysiloxane |
| Additively manufactured silicone material | DentaGUM | Asiga | 7,7,9 (or 7,9,9)-trimethyly-4,13-dioxo-3,14-dioxa-5,12-diazahexadecane-1,16-diyl bi,methacrylate: 10–25% Tetrahydrofurfuryl methacrykate: 10–20% Diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide: 10–20% |
| Additively manufactured silicone material | KeyMask | KeyMask Industries | Urethane acrylate oligmomer: ≥25 to ≤50% Alphatic Urethane Acrylate Oligomer: ≤10% Proprietary ingredient #1: >1% Proprietary ingredient #2: ≤1% |
| Additively manufactured silicone material | Dima Print Gingiva Mask | Kulzer | Poly(oxy-1,4-butanediyl), alpha.-hydro-omega.-hydroxy-, polymer with 5-isocyanto-1-(isocyanatomethyl)-1,3,3-trimethylcyclohexane, 2-hydroxyethyl acrylate-blocked: ≥25 to ≤50% Phenoxy polyethylene glycol acrylate: ≥25 to ≤50% Ethoxylated o-phenylphenol acrylate: ≥25 to ≤50% Diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide: ≥1 to ≤2.5% |
| Method | Materials | Build Angle | Tensile Strength (MPa ± SD) | Compressive Strength (MPa ± SD) | Hardness (HA ± SD) | Accuracy |
|---|---|---|---|---|---|---|
| Conventional | Gingifast | N/A | 1.30 ± 0.19 | 5.35 ± 0.44 | 42.00 ± 0.41 | N/A |
| Additively manufactured | DentaGum | 0 | 0.57 ± 0.17 | 3.56 ± 0.37 | 47.30 ± 0.63 | 0.13 ± 0.20 |
| 45 | 0.39 ± 0.13 | 5.29 ± 0.55 | 61.50 ± 0.81 | 0.08 ± 0.01 | ||
| 90 | 0.46 ± 0.16 | 7.47 ± 1.33 | 71.97 ± 2.14 | 0.06 ± 0.01 | ||
| KeyMask | 0 | 0.48 ± 0.18 | 8.53 ± 2.64 | 61.60 ± 0.46 | 0.06 ± 0.02 | |
| 45 | 0.31 ± 0.05 | 8.00 ± 2.46 | 57.75 ± 1.27 | 0.07 ± 0.01 | ||
| 90 | 0.36 ± 0.08 | 10.86 ± 1.90 | 60.25 ± 0.92 | 0.06 ± 0.01 | ||
| Dima Gingiva | 0 | 0.63 ± 0.11 | 8.54 ± 1.14 | 57.55 ± 0.60 | 0.06 ± 0.02 | |
| 45 | 0.30 ± 0.09 | 6.68 ± 0.37 | 59.85 ± 1.11 | 0.08 ± 0.02 | ||
| 90 | 0.41 ± 0.1 | 9.12 ± 0.33 | 61.8 ± 0.82 | 0.06 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.X.; Aarts, J.M.; Choi, J.J.E. Mechanical Properties and Accuracy of Additively Manufactured Silicone Soft Tissue Materials. J. Manuf. Mater. Process. 2025, 9, 113. https://doi.org/10.3390/jmmp9040113
Chen PX, Aarts JM, Choi JJE. Mechanical Properties and Accuracy of Additively Manufactured Silicone Soft Tissue Materials. Journal of Manufacturing and Materials Processing. 2025; 9(4):113. https://doi.org/10.3390/jmmp9040113
Chicago/Turabian StyleChen, Pei Xin, John M. Aarts, and Joanne Jung Eun Choi. 2025. "Mechanical Properties and Accuracy of Additively Manufactured Silicone Soft Tissue Materials" Journal of Manufacturing and Materials Processing 9, no. 4: 113. https://doi.org/10.3390/jmmp9040113
APA StyleChen, P. X., Aarts, J. M., & Choi, J. J. E. (2025). Mechanical Properties and Accuracy of Additively Manufactured Silicone Soft Tissue Materials. Journal of Manufacturing and Materials Processing, 9(4), 113. https://doi.org/10.3390/jmmp9040113





