1. Introduction
Adhesives are critical to a wide spectrum of industrial and commercial applications, including automotive, aerospace, construction, electronics, and packaging. The global adhesives market was valued at USD
$59.79 billion in 2022 and is projected to grow to USD
$87.04 billion by 2028. This represents an expanding demand for reliable and high-performance bonding solutions across sectors [
1]. As performance requirements become increasingly rigorous and sustainability gains importance, the development of cost-effective, durable, and environmentally responsible adhesives has become a critical area of materials research.
Thermosetting resins, including epoxy, phenolic, vinyl ester, and unsaturated polyester resin (UPR), dominate the adhesive landscape due to their excellent structural integrity and chemical resistance [
2]. Among these, epoxy resins are most commonly used because of their superior mechanical strength, strong adhesion to diverse substrates, and low shrinkage after curing [
3]. However, their higher cost and longer curing times limit their use in cost-sensitive or high-throughput manufacturing environments such as the automotive sector [
4]. While epoxy remains essential for high-performance applications like aerospace engineering [
5,
6], alternative resins are increasingly sought for in less demanding but high-volume applications.
UPRs, in particular, offer several advantages such as low density, corrosion resistance, design flexibility, shorter curing time, and cost-effectiveness. These properties make it suitable for applications in transportation, consumer electronics, and infrastructure [
7,
8,
9,
10,
11]. Moreover, UPR adhesives are formaldehyde-free and can be processed at lower curing temperatures and shorter cycle times, making them attractive from both economic and environmental perspectives [
12]. Nevertheless, UPRs are often relegated to low-stress or low-performance applications due to their relatively lower mechanical properties. For instance, cured UPR adhesives typically exhibit a tensile strength of 33 MPa [
13] and a compressive strength of 109 MPa [
14], which are inferior to epoxy’s properties (i.e., tensile strength of 73 MPa) [
15].
To narrow this performance gap, researchers have explored the integration of nanoparticles into UPR matrices as a means of enhancing mechanical and thermal properties. This approach has yielded promising results. For example, Rahman et al. demonstrated that the incorporation of ferric oxide, titanium oxide, and nickel ferrite nanoparticles into UPR improved tensile strength by 21.62% and Young’s modulus by 6.56% [
16]. Similarly, Chu et al. reported a 27.3 MPa increase in tensile strength through reinforcement with ramie fabric [
17]. These enhancements suggest that with appropriate modification, UPRs may become a more viable alternative to epoxy in structurally demanding adhesive applications.
In parallel with these efforts, the use of environmentally sustainable and renewable nanoparticles as reinforcement fillers is gaining attention in both research and real-world applications. Among these, chitin, the second most abundant biopolymer after cellulose, is a promising candidate for bio-derived nanocomposite development [
18,
19,
20,
21]. Chitin is a nontoxic, biodegradable, and biocompatible polymer that also exhibits thermal stability, antimicrobial activity, and antioxidative properties [
18]. It is primarily extracted from crustacean shell waste, with annual global availability estimated at over 2 million tonnes [
22], making it an abundant and renewable resource for sustainable material development.
In its nanowhisker form, chitin exhibits a rod-like morphology, typically measuring 200–500 nm in length and 10–20 nm in width [
23,
24]. These chitin nanowhiskers (CNWs) possess an exceptional Young’s modulus of approximately 200 GPa, offering excellent stiffness and mechanical reinforcement capabilities. The combination of nanoscale dimensions and outstanding mechanical properties makes CNWs highly suitable for incorporation into polymer matrices. While carbon nanomaterials such as graphene, carbon nanotubes, or fullerenes (buckyballs) have been extensively studied for their reinforcement potential in polymer nanocomposites, CNWs represent a sustainable alternative with comparable multifunctional benefits [
25,
26]. In this context, several studies have explored the use of CNWs as reinforcing fillers in polymer nanocomposites. Pend and Chen, for example, prefabricated a poly(vinyl alcohol) (PVA)-CNW nanocomposite film through heat treatment, resulting in substantial increase in Young’s modulus (i.e., from 207.41 MPa to 311.23 MPa); however, this compromised the elongation at break by over 40% [
27]. Huang et al. developed a soy protein fibrils-CNW complex gel with enhanced rigidity [
28]. Similarly, Midhun et al. revealed that dispersing CNWs into acrylonitrile-butadiene rubber significantly improved the material’s tensile and tear strengths by 116% and 54%, respectively [
29]. Despite these promising findings, there has been limited research focused on leveraging CNWs to enhance the performance of polymer adhesives.
Despite promising evidence supporting the mechanical reinforcement potential of CNWs in polymer systems, their integration into adhesive-grade UPRs has not been adequately studied. This work aims to fill this gap by investigating how different CNW incorporation strategies, specifically slurry compounding with various suspension media and direct mechanical mixing with dry powders, affect the mechanical, thermal, and adhesive performance of UPR-based nanocomposites. Direct dispersion of CNWs typically relies on aqueous or ethanol suspensions, surface modification, or sonication to disperse CNWs. Unlike traditional CNW dispersion routes, this study includes the examination of varying suspension media, including a hybrid dispersion strategy in which the CNWs are partially suspended in a solvent-modified fraction of the polyester resin itself. By matching the solvent environment, part of this work evaluates whether the approach may enhance compatibility, improve dispersion quality, and support the incorporation of CNWs during the manufacturing stage of the UPR. The examination of varying suspension mediums also reduces the risk of hydrolysis in the UPR, which is a limitation of traditional water- and ethanol-based CNW systems. Special attention is also given to the impact of processing conditions on polyester chain integrity, including degradation effects caused by thermal history and solvent interactions. By identifying processing–property relationships, this study aims to establish a practical route for developing high-performance, bio-reinforced adhesives suitable for scalable manufacturing applications.
2. Materials and Methods
2.1. Materials
Commercial-grade UPR (3M, Milton, ON, Canada, Bondo
® Fibreglass Resin 401C) was used as the base material in this study (the manufacturer’s provided safety data sheet indicated the UPR consists of dimethyl phthalate (o-phthalic acid ester), methyl ethyl ketone peroxide, 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate, hydrogen peroxide, methyl ethyl ketone, and water). It must be noted that this is a neat UPR without any reinforcement filler. Methyl ethyl ketone peroxide (MEKP, 3M, Milton, ON, Canada, Bondo
® Liquid Hardener 912C) served as the curing agent. Both the UPR and MEKP have a density of 1.1 g/mL. Acetone (Solvable
® 53-260) and methyl ethyl ketone (MEK, Solvable
® 53-361) were used as suspension media for dispersing CNWs. The boiling points of acetone and MEK are 56.2 °C and 79.6 °C, respectively. CNWs, supplied either as a 5 wt.% ethanol suspension or in dry powder, were obtained from Neptune Nanotechnologies Inc. (Markham, ON, Canada). These bio-derived nanoparticles were employed as reinforcing additives for the UPR-based adhesive formulations. The CNWs used in this study had whisker-structures with 200–500 nm in length and 10–20 nm in width. They originated from the same source reported by Wang et al. [
24].
2.2. Preparation of CNW Suspensions in Different Solvents
CNWs were suspended in pure ethanol and pure acetone using a rotary evaporation technique, following a standardized two-cycle protocol to promote dispersion while minimizing aggregation. For ethanol-based suspensions (denoted as CE), 300 g of ethanol was added to the CNW powder and subjected to rotary evaporation at 80 °C. Evaporation was continued until CNWs began adhering to the inner wall of the flask. This indicated partial solvent removal and increased viscosity. At this point, a second 300 g portion of ethanol was added, and the evaporation process was repeated under the same conditions. Acetone-based suspensions (denoted as CA) were fabricated following an identical procedure, with rotary evaporation performed at 60 °C for each of the two 300 g cycles. This two-step addition approach was critical to avoid excessive initial solvent volume, which can hinder evaporation efficiency and promote CNW aggregation due to their polar nature. The staged evaporation also prevented overheating, ensured controlled solvent removal, and minimized the risk of condenser flooding or solvent boil-over.
For CNW samples in a combination of ethanol/acetone (denoted as CEA), a stepwise protocol designed to promote dispersion and minimize aggregation was used. Initially, the CNW-ethanol suspension was heated to 60 °C until a visible increase in viscosity indicated partial solvent removal and enhanced particle interaction. Acetone was then introduced at a 2:1 wt. ratio (acetone:CNW) under continued stirring and heating. The resulting mixture was ultrasonicated at 25 Hz for less than 5 min to disrupt agglomerates, followed by mild heating at 40 °C. After a 5 min decanting period, acetone was added again at a 10:1 wt. ratio relative to CNW under stirring at 60 °C. A second ultrasonication step was conducted under the same conditions, followed by another round of mild heating and decanting. A final acetone addition (10:1 wt. ratio) was carried out under continued heating and stirring at 60 °C until a final CNW concentration of 5 wt.% in the suspension medium was achieved.
For CNW samples in a combination of ethanol/acetone/MEK suspensions (denoted as CEAM), the CEA procedure outlined above was followed with the addition of two final steps. After the last acetone addition and heating cycle, the suspension was further heated to evaporate approximately 10 wt.% of the solvent mixture. Subsequently, MEK was added at 15 wt.% relative to the solvent mass, and the suspension was again heated to allow partial evaporation until the CNW concentration returned to 5 wt.% in the final ternary mixture. This modification aimed to leverage the volatility and polarity balance of MEK to improve CNW dispersion stability and solvent compatibility with polyester resin systems.
2.3. Preparation of UPR-CNW
A 5 wt.% CNW suspension was ultrasonicated at 24.45 kHz for 10 min to reduce agglomeration and promote uniform dispersion. Precise amounts of the CNW suspension were then incorporated into UPR and stirred thoroughly. The resulting mixtures were heated to evaporate the suspension medium. Systems containing ethanol and/or MEK were heated at 80 °C for approximately 45 min, while suspensions containing only ethanol were heated at 60 °C. Solvent removal was monitored by periodic weighing of the mixtures. Following solvent evaporation, the mixtures were degassed, cured using methyl ethyl ketone peroxide (MEKP), and cast into moulds for further testing.
2.4. Morphological Characterization of UPR and CNW-UPR Nanocomposites
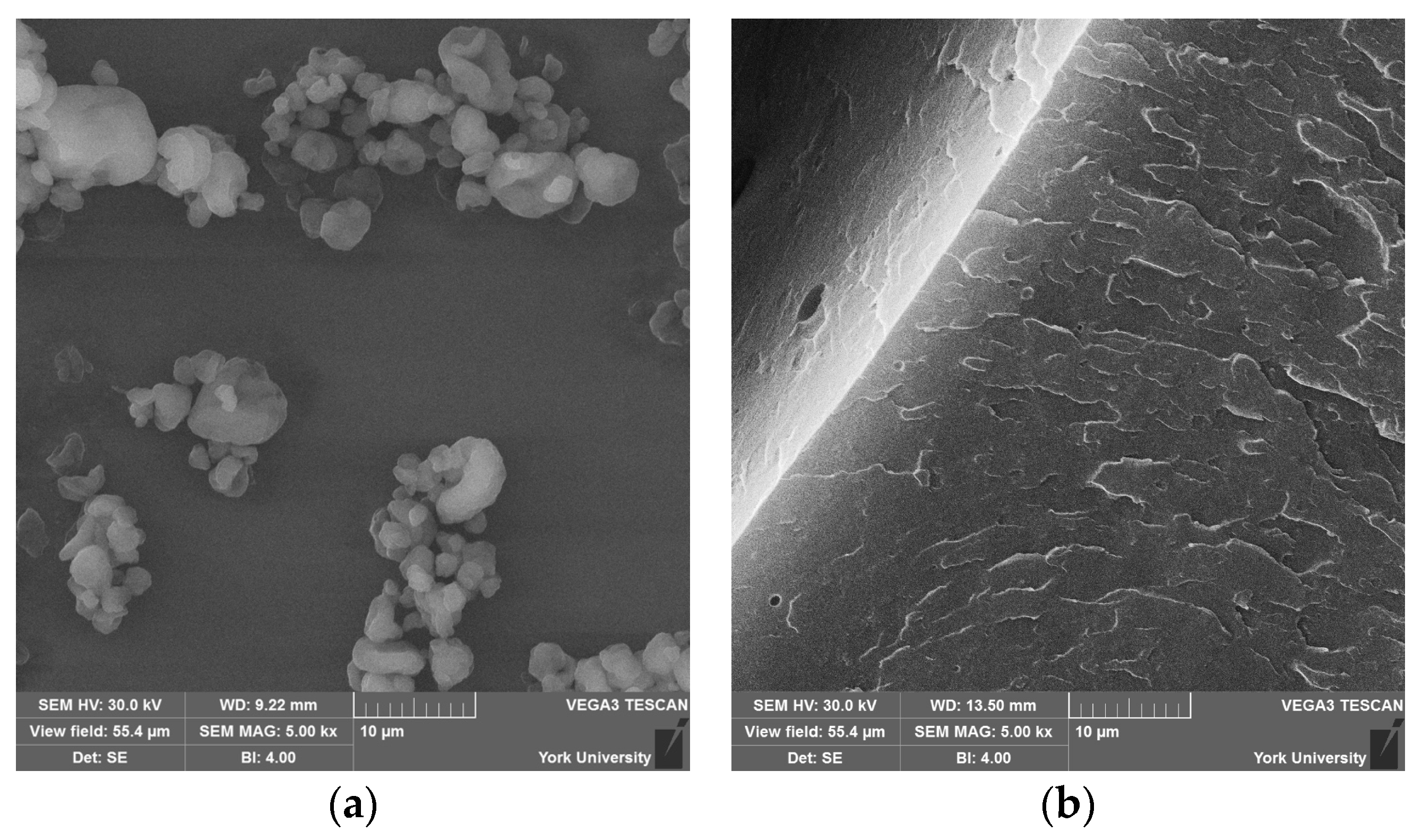
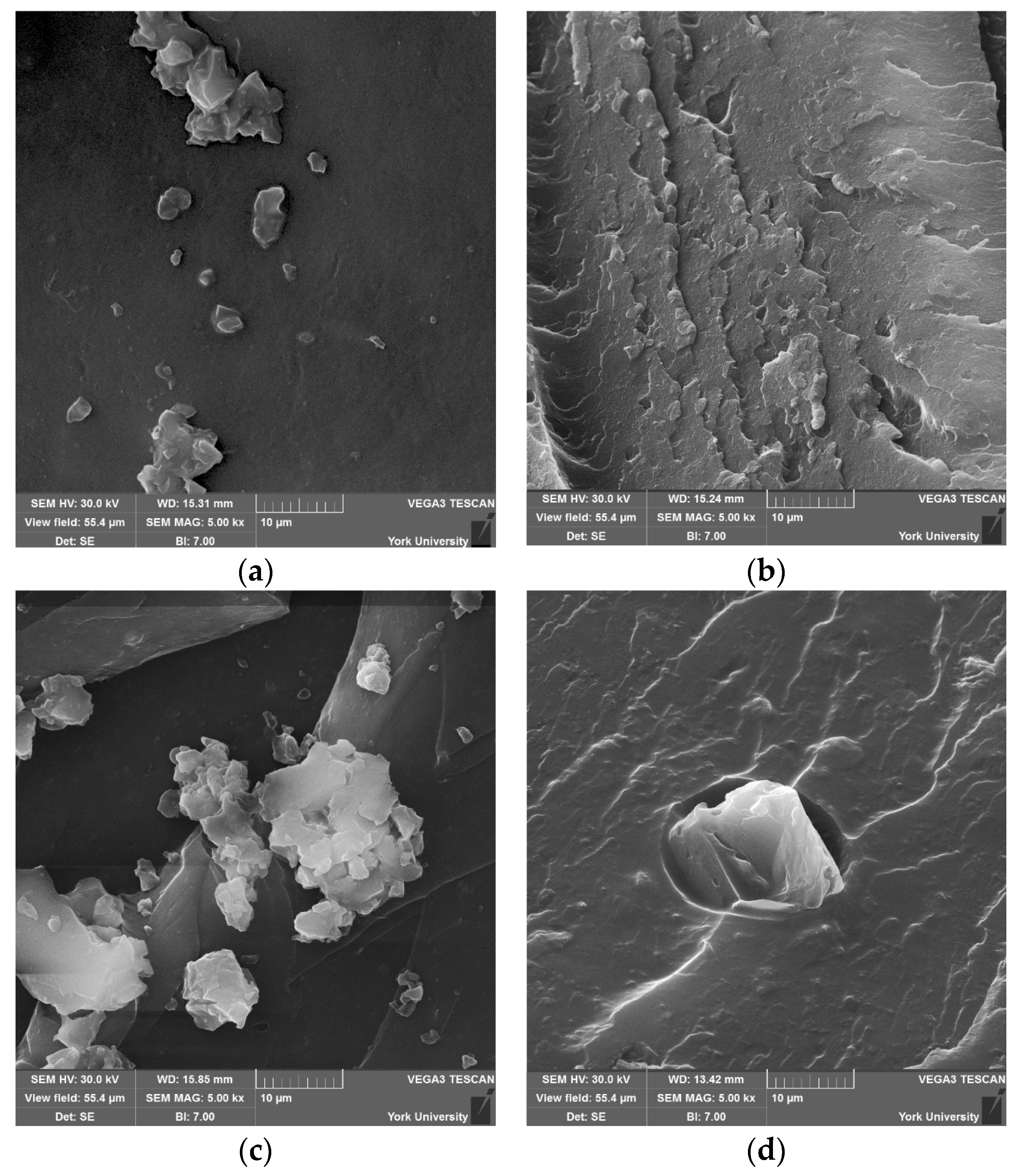
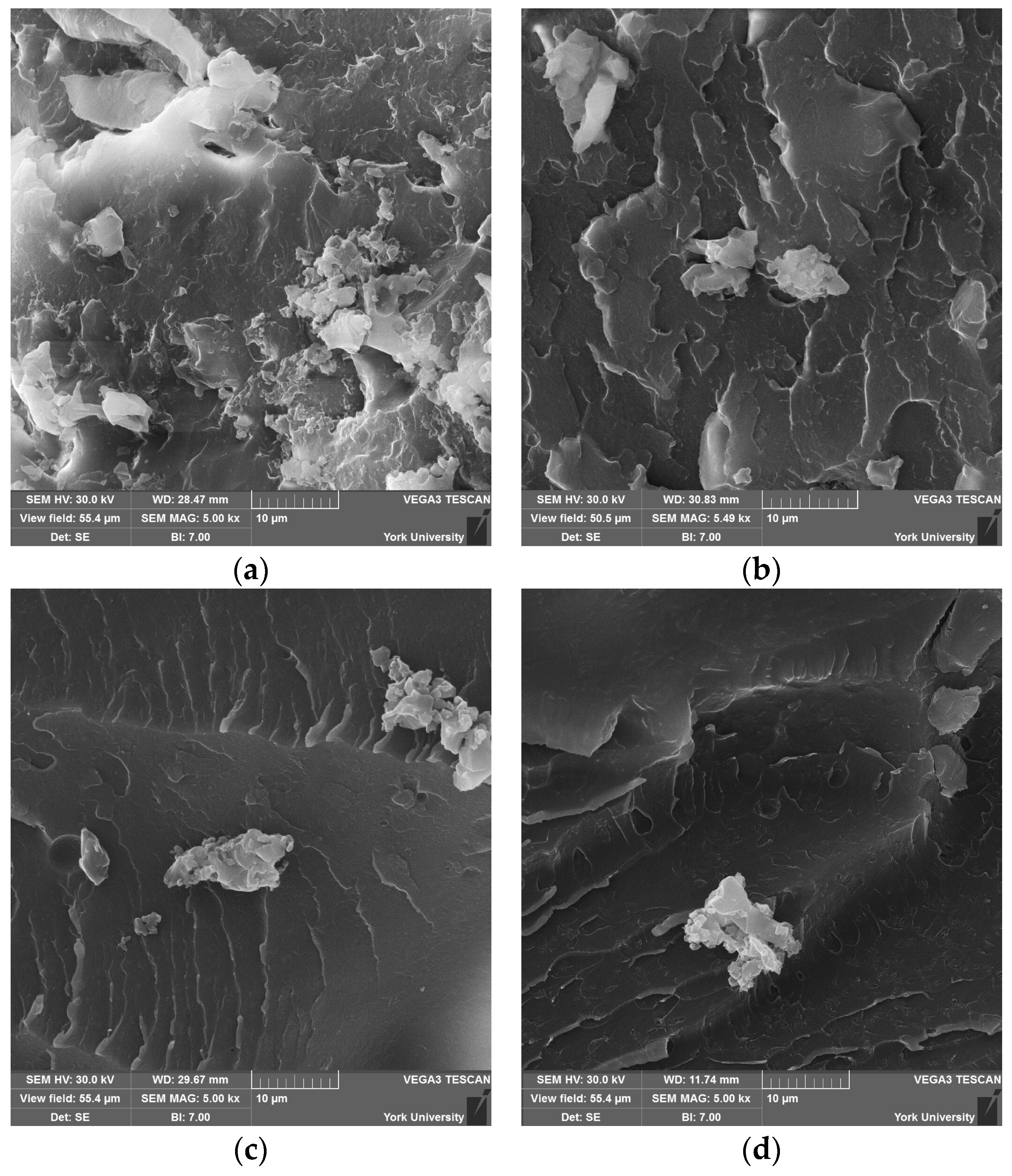
Scanning electron microscopy (SEM) (Vega 3, from Tescan, Brno, Czech Republic) was used to examine the surface morphology, nanoparticle dispersion, and fracture characteristics of the commercial UPR and CNW samples. The samples were cold fractured and mounted on aluminum stubs using conductive carbon tape and subsequently sputter-coated with a thin layer of gold (Desk V, from Denton, Moorestown, NJ, USA) using plasma deposition under an argon gas atmosphere. A thin layer of conductive carbon tape was applied along the sides of the specimens. The samples were viewed at 30.0 kV.
2.5. Thermal Analyses of UPR and CNW-UPR Nanocomposites
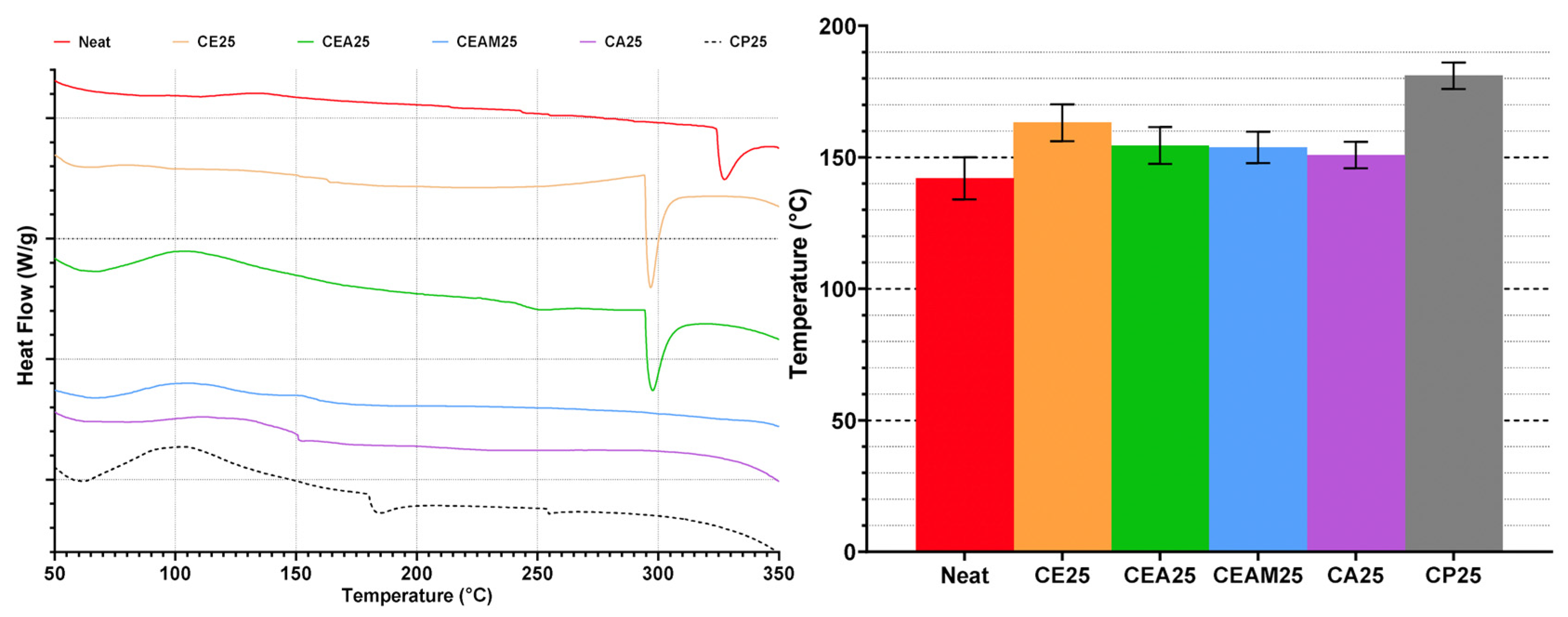
Differential scanning calorimetry (DSC) (Discovery DSC 250, from TA Instruments, New Castle, DE, USA) was used to characterize the glass transition and characteristic temperatures related to other endothermic reactions. This was done by analyzing the heat flow through the samples as a function of temperature. A total of five replicate samples were prepared and tested for each condition. The samples were ground into powders and sieved using an 80-grade mesh and were placed in a Tzero® (TA Instruments) aluminum pan that was loaded into the DSC chamber. The samples were equilibrated at 35 °C before heating to 400 °C at a rate of 10 °C/min.
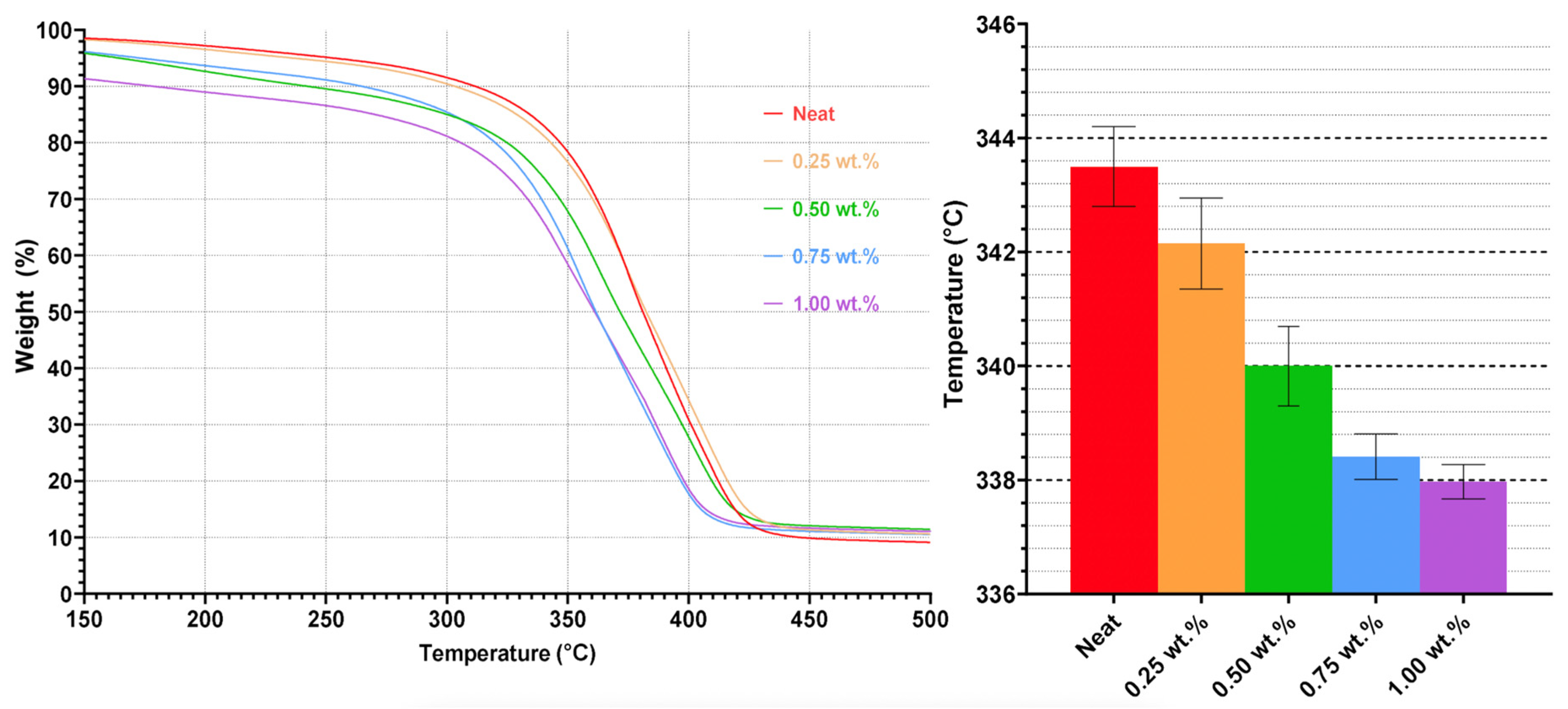
Thermogravimetric analysis (TGA) (TGA 55, from TA Instruments) was used to characterize the composition and thermal stability of the synthesized and commercial UPRs. This was done by analyzing the weight difference and change in the samples as a function of temperature. A total of five replicate samples were prepared and tested for each condition. The samples were ground into powders and sieved using an 80-grade mesh and were placed on an aluminum pan. The samples were equilibrated at 35 °C before a heating rate of 20 °C/min was applied to heat the samples to 600 °C while the furnace was purged under a nitrogen medium.
2.6. Mechanical Testing of UPR and CNW-UPR Nanocomposites
Tensile testing of the samples was performed in accordance with ASTM D638, Standard Test Method for Tensile Properties of Plastics [
30], using Type IV specimens designed for small plastic samples. Platinum silicone moulds conforming to the ASTM D638 Type IV geometry were procured from MB Prototyping Ltd., and the samples were cast in the moulds. following the manufacturer’s specifications. After curing, the specimens were carefully sanded with 400-grit sandpaper in accordance with specifications and conditioned under standard room conditions (i.e., 23 °C) for 72 h as per ASTM D618 [
31]. Mechanical testing was conducted on a linear-torsion dynamic test instrument (ElectroPuls E3000, from INSTRON, Norwood, MA, USA) at a crosshead speed of 5 mm/min, with eight specimens tested for each composition.
Adhesive strength and failure mode were evaluated in accordance with ASTM D1002, Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal) [
32]. Samples were prepared and cured following the specifications and applied to the bonding area under standard laboratory conditions. The samples were then allowed to cure under laboratory conditions in accordance with ASTM D618 [
31]. Cold-rolled 2024 aluminum alloy sheets with T3 temper treatment, procured from McMaster-Carr, served as the substrates and were cut to the required dimensions. The substrates were cleaned of debris using precision wipes before bonding. The adhesive tests were conducted using a linear-torsion dynamic test instrument (ElectroPuls E3000, from INSTRON, Norwood, MA, USA). Shims were added to the substrates in the grip area to compensate for grip misalignment, thereby suppressing bending moments caused by eccentric loading and promoting a more uniform shear stress distribution across the bonded area.
4. Discussion
The results presented in the previous section are discussed to evaluate the role of CNWs and suspension media on the performance of PE nanocomposites. The findings reveal a complex balance between chemical interactions, dispersion, thermal stability, and mechanical performance. Ethanol, employed as the baseline suspension medium due to its established use in CNW-epoxy systems, which enabled direct comparison of CNW compatibility with polyester matrices. Subsequent evaluation of thermal processing conditions and alternative suspension media further clarified the mechanisms governing performance and identified both opportunities and limitations of CNW incorporation in polyester resin.
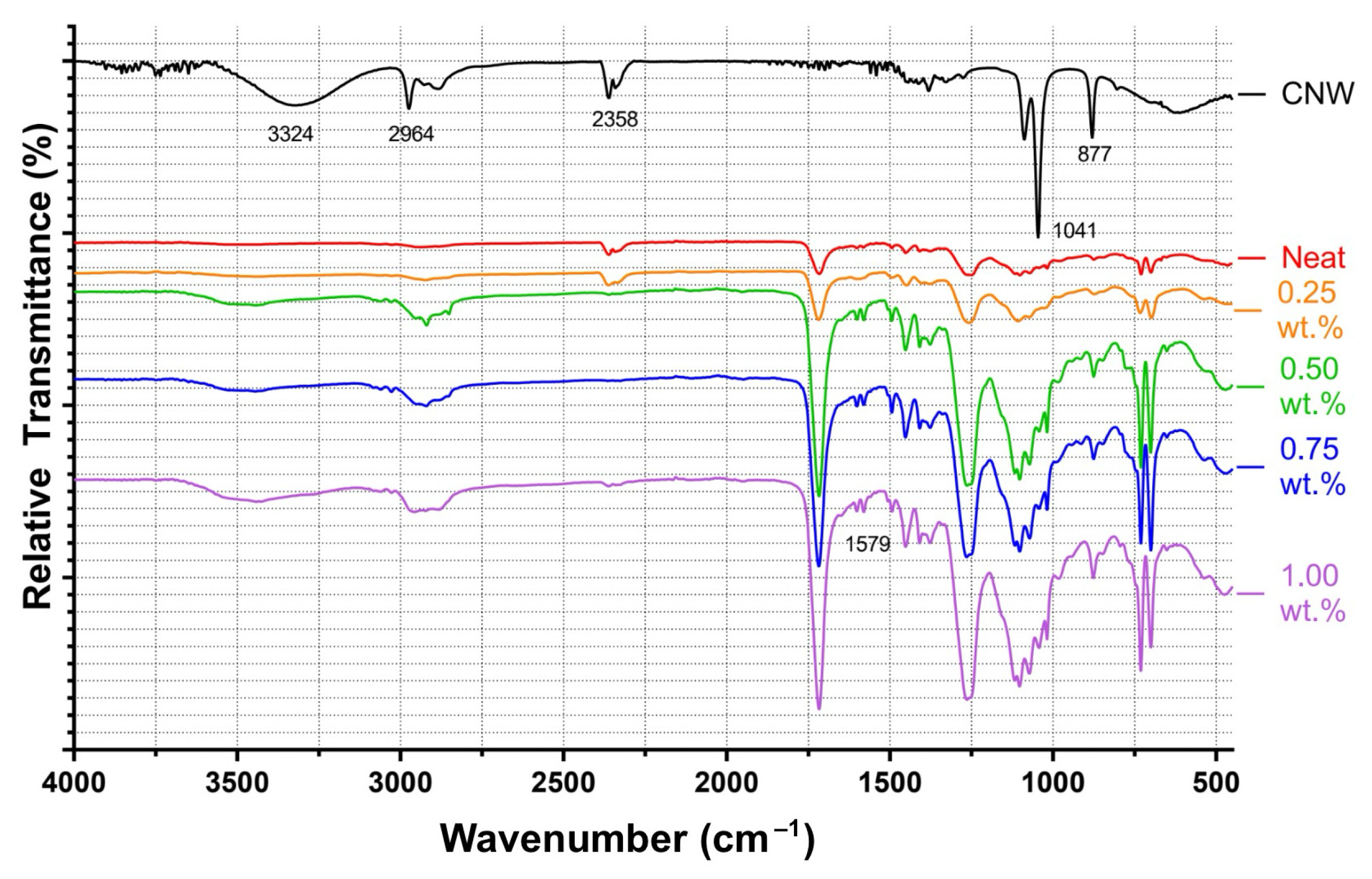
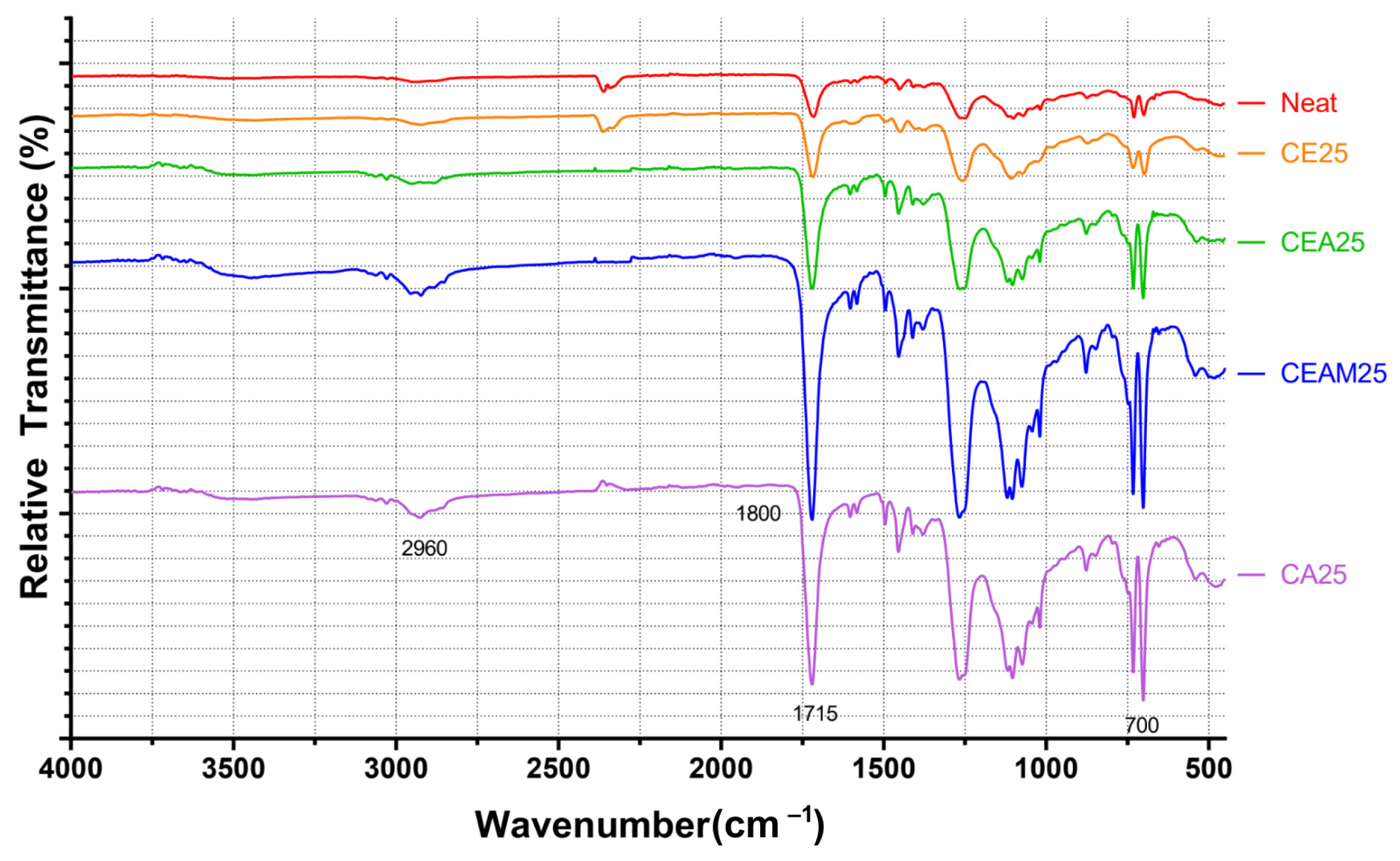
4.1. Chemical Interactions and FTIR Analysis
FTIR results suggest that CNWs introduce both reinforcing and potentially degradative effects depending on their concentration and the associated suspension medium. Low to moderate CNW loadings promote interactions between the hydroxyl- and amine-rich surfaces of CNWs and the UPR matrix, restricting chain mobility and improving composite rigidity. However, increasing CNW content also increases the amount of ethanol introduced, which promotes hydrolysis of ester bonds in the polymer network, as indicated by the growth of carboxylate-related absorption bands. This trend implies a balance exists between hydrogen bonding at the filler-matrix interface and solvent-mediated degradation. Agglomeration observed in SEM micrographs reinforces this conclusion, as clustered CNWs reduce effective interfacial contact, potentially acting as stress concentrators and limiting chemical reinforcement.
The choice of suspension medium further modulates chemical interactions. Ethanol-based systems provided relatively uniform dispersion with minor clustering, preserving matrix–filler interactions. Mixed solvents (CEA25 and CEAM25) altered FTIR spectra, suggesting residual solvent interactions or incomplete removal, which may modify bonding between the CNWs and PE resin. Direct incorporation without a solvent enhanced agglomeration, confirming that solvent-assisted dispersion is critical for maintaining interfacial compatibility. Overall, the chemical data indicate that CNW reinforcement is optimized at low CNW loadings with carefully selected suspension media to minimize solvent-induced hydrolysis while maximizing hydrogen-bond interactions.
4.2. Thermal Behaviour
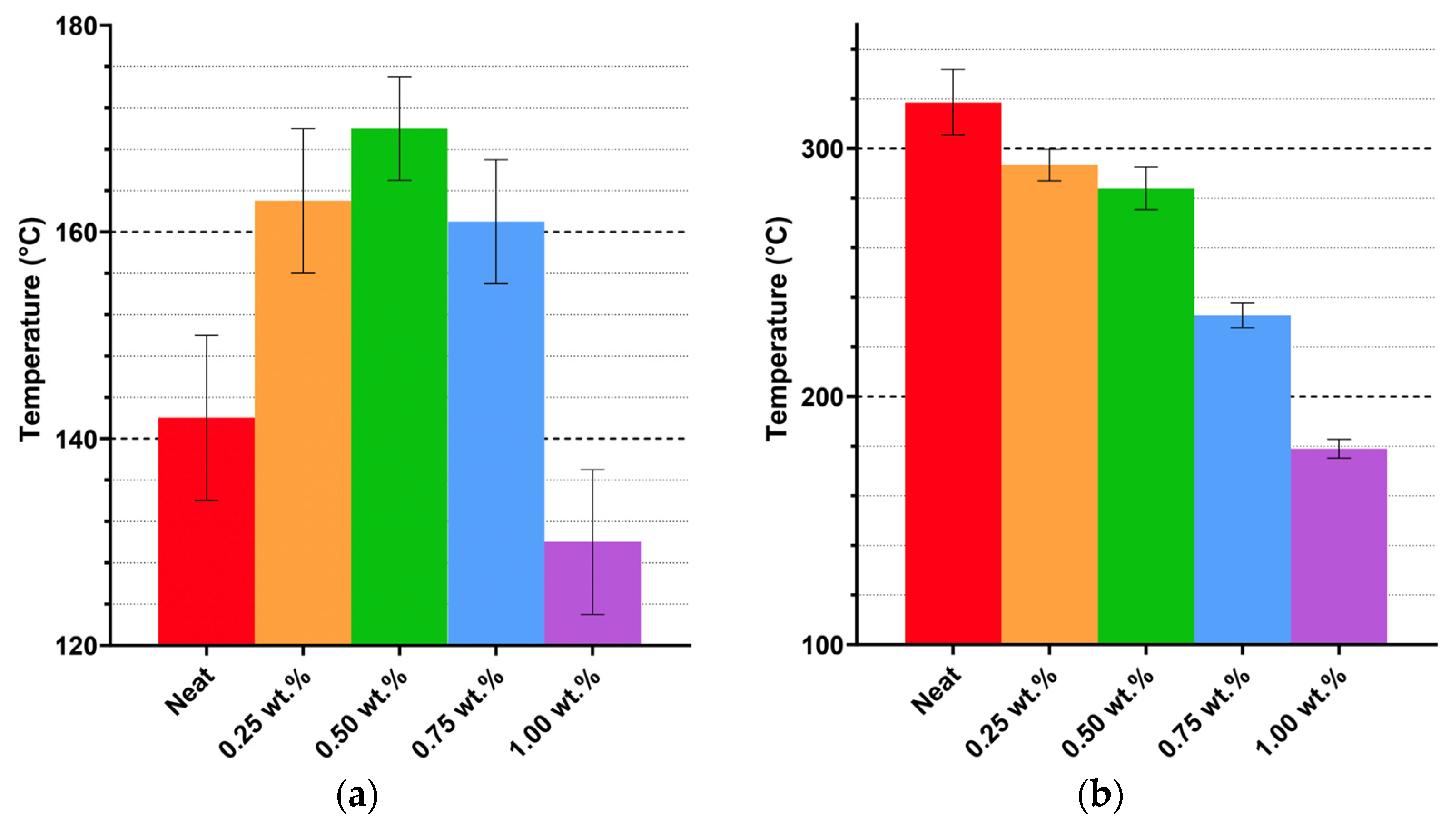
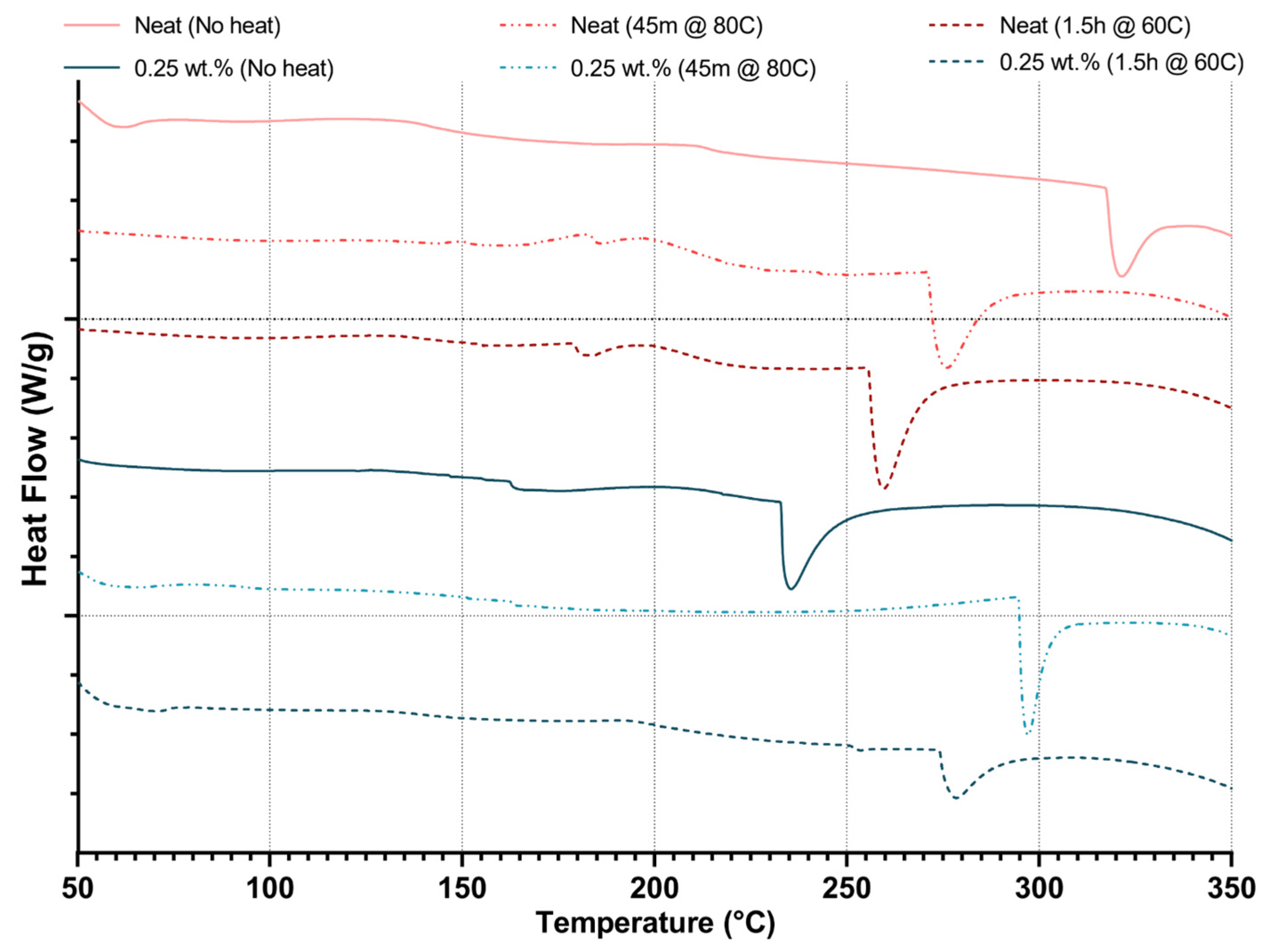
Thermal analysis demonstrates that CNWs can enhance polymer rigidity and Tg at moderate loadings but introduce structural weaknesses at higher concentrations. DSC data indicate that Tg increases with CNW additions up to 0.50–0.75 wt.%, reflecting restricted chain mobility from hydrogen bonding. Beyond this threshold, Tg declines, suggesting that excessive CNWs cause agglomeration and disrupt network homogeneity, which diminishes thermal reinforcement. The emergence of earlier and more prominent endothermic peaks with increasing CNW loading, along with TGA results showing reduced degradation onset, confirms that hydrolysis and molecular fragmentation occur in ethanol-containing systems, compromising stability. At higher CNW loadings, the reduction in degradation onset can be attributed to the formation of CNW agglomerates that introduce thermally weak regions within the matrix. These clusters create localized regions that heat more rapidly and initiate chain scission earlier when compared to the surrounding polymer network. Moreover, the increased amount of hydroxyl groups of the CNWs promotes moisture retention and accelerates the hydrolysis of ester bonds, contributing to the accelerated thermal breakdown.
Thermal history interacts with CNW presence to further modulate behaviour. Short-duration, high-temperature heating removes residual ethanol, preserving matrix–filler interactions and improving thermal stability, as evidenced by increased degradation onset and higher Tg in CNW-reinforced samples. In contrast, prolonged low-temperature heating exacerbates hydrolytic effects, lowering both Tg and degradation onset. These findings indicate that thermal processing can either mitigate or amplify solvent-related degradation, highlighting the importance of controlled heating protocols to optimize CNW dispersion and thermal performance.
Suspension medium choice also influences thermal behaviour. Using ethanol as the suspension medium exhibit moderate Tg increases and the appearance of residual solvent-associated endothermic peaks, while direct incorporation without solvent preserves inherent resin stability. Mixed solvent systems show more complex interactions that can either enhance or compromise Tg, indicating that solvent selection must balance dispersion efficiency with the risk of thermal instability.
4.3. Mechanical Properties
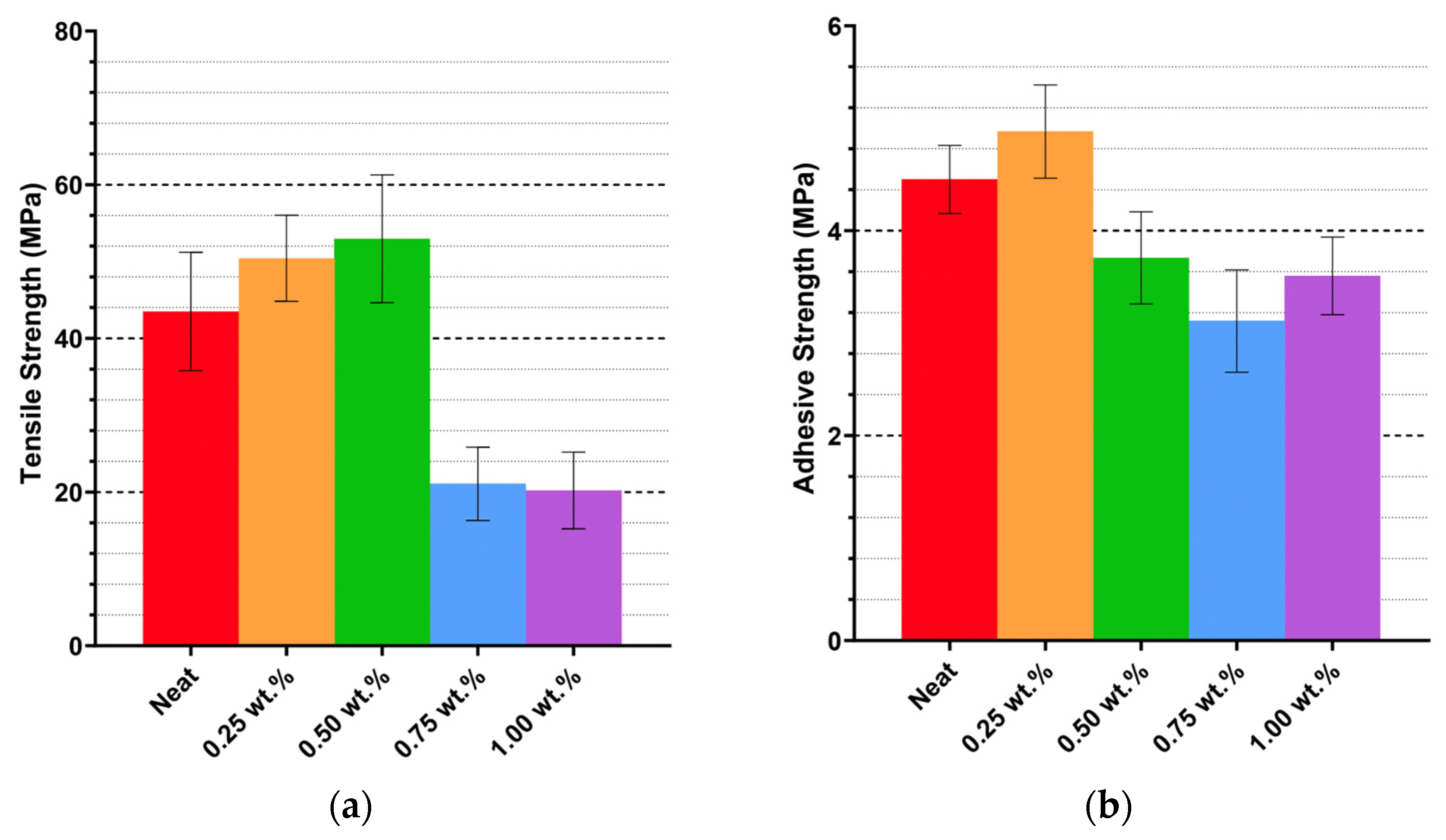
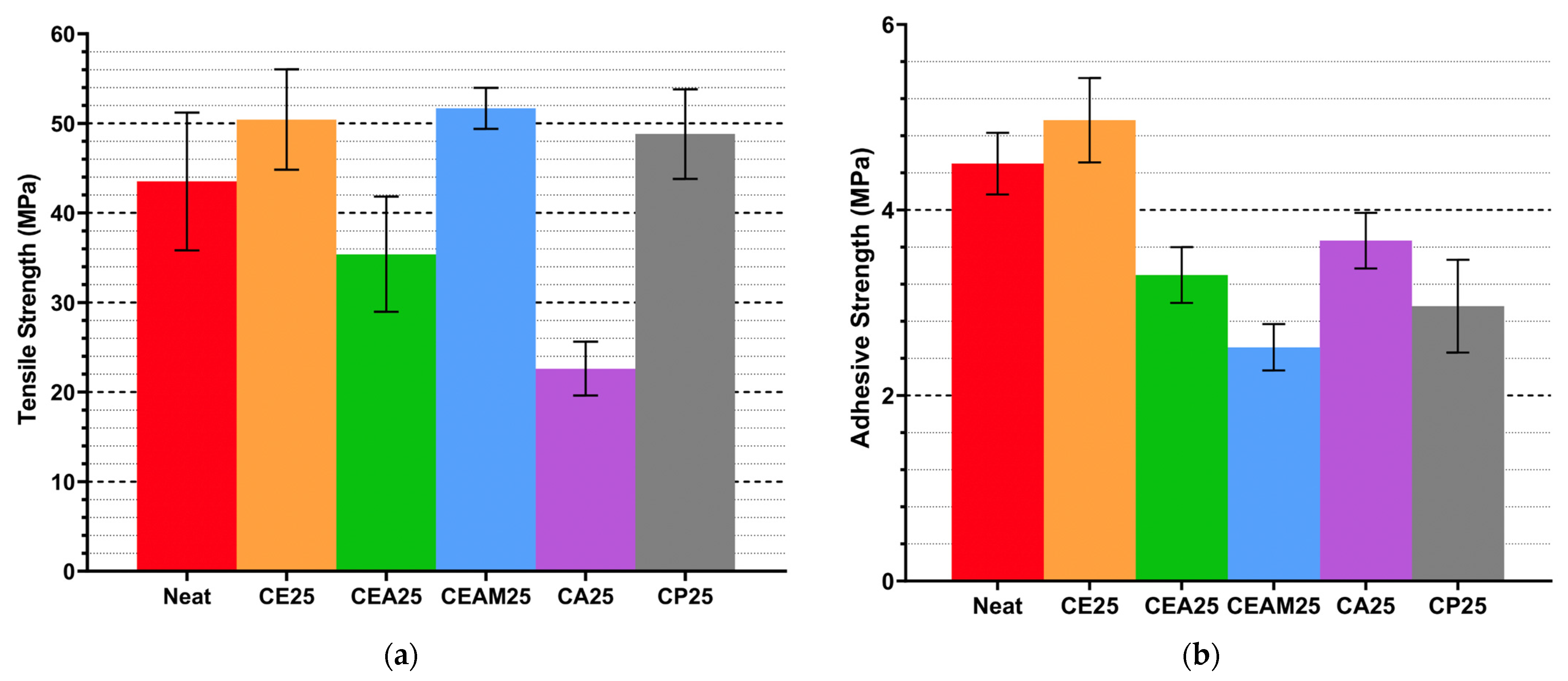
Mechanical testing reveals that CNWs improve tensile strength and ductility at optimal low-to-moderate CNW loadings, while higher concentrations lead to performance declines. Tensile strength peaked at 0.50 wt.% CNW, corresponding with SEM observations of relatively uniform dispersion and limited clustering, which facilitate efficient load transfer. Agglomeration at higher loadings introduces microvoids and stress concentrators, reducing reinforcement efficiency. Elongation at break similarly improved at moderate loadings, indicating that CNWs act as effective stress-transfer bridges, enhancing energy absorption and resilience under load. Beyond 0.50–0.75 wt.%, elongation plateaus, reflecting saturation of reinforcement potential.
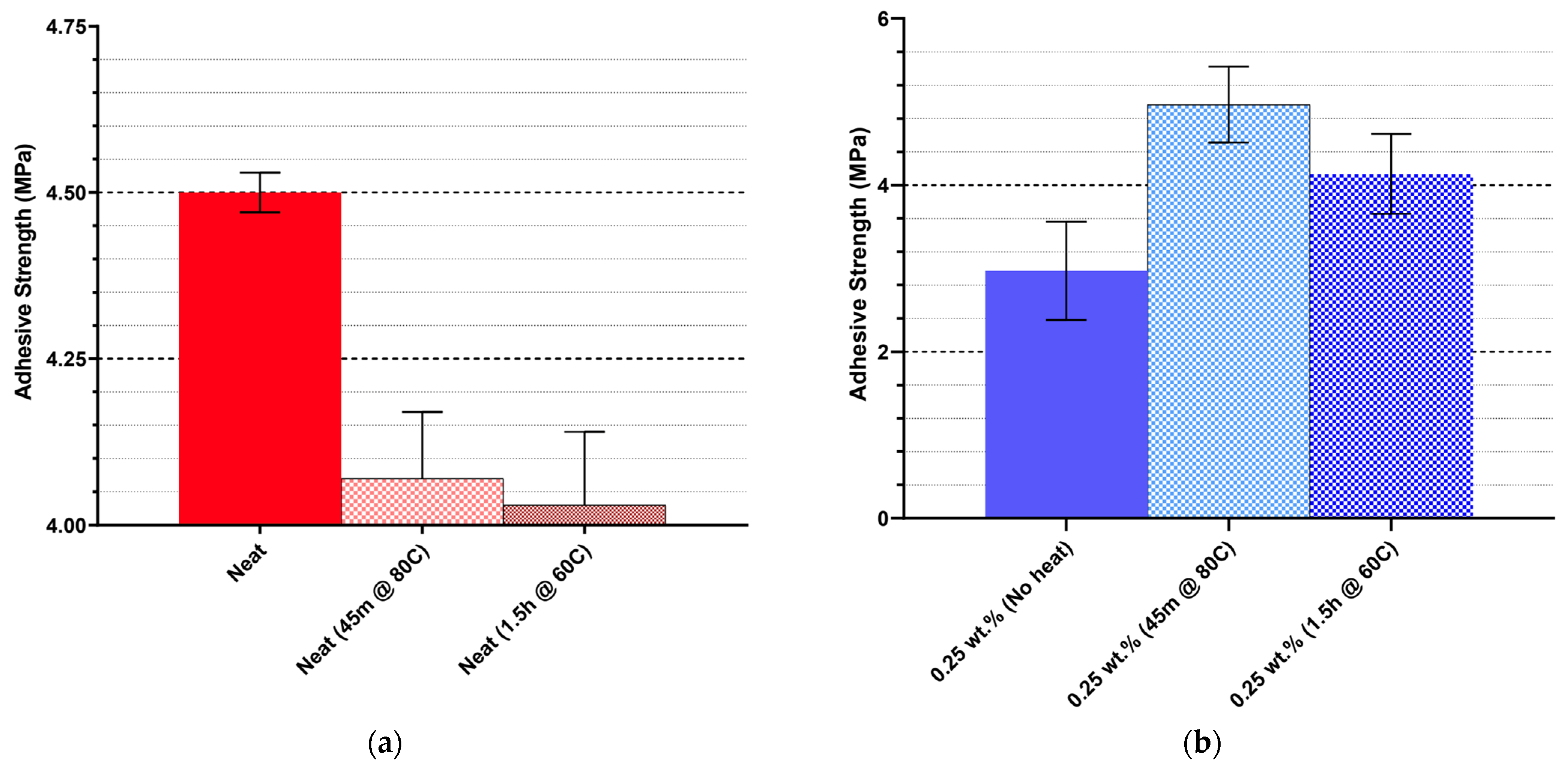
Adhesive performance is more sensitive to CNW dispersion and solvent effects. Ethanol-assisted incorporation (CE25) improved lap shear strength relative to neat resin, suggesting that well-dispersed CNWs enhance interfacial bonding. In contrast, mixed solvent systems and direct CNW incorporation generally reduced adhesive strength, indicating that bulk improvements in tensile properties may not translate to adhesive performance. Residual solvent and agglomeration appear to generate weak points within the adhesive layer, compromising cohesive integrity. Thermal processing further modulates mechanical outcomes: controlled short-duration heating improves CNW dispersion and tensile performance, whereas prolonged heating diminishes reinforcement due to hydrolysis and agglomeration effects.
Overall, the mechanical and adhesive data indicate that CNWs are effective reinforcements when dispersion is controlled, solvent choice is appropriate, and thermal processing is optimized. Ethanol-assisted dispersion at 0.25–0.50 wt.% loading, combined with brief high-temperature heating, provides the most balanced enhancement of both tensile and adhesive properties.
5. Conclusions
At low to moderate loadings (0.25–0.50 wt.%), CNWs effectively enhanced the polyester matrix by forming hydrogen-bonded interfacial networks that restricted chain mobility and improved tensile strength, ductility, and thermal stability. Optimal reinforcement was observed at 0.50 wt.% CNW, achieving a 19.4% increase in glass transition temperature and a 21.7% improvement in tensile strength compared to the neat resin. However, higher loadings (>0.75 wt.%) led to agglomeration, void formation, and diminished interfacial adhesion, which collectively reduced the thermal and mechanical stability of the composite.
The choice of suspension medium emerged as a critical factor in controlling dispersion and interfacial bonding. Ethanol proved to be the most effective medium, yielding well-dispersed CNWs and balanced improvements in both tensile and adhesive strength. In contrast, mixed solvent systems (ethanol/acetone and ethanol/acetone/MEK) introduced residual solvent interactions that modified the chemical and thermal behaviour of the resin, while direct incorporation without a solvent resulted in severe agglomeration and poor performance. These findings emphasize that solvent-assisted dispersion is essential to maintaining interfacial compatibility and minimizing structural defects. Thermal processing further influenced the properties of nanocomposite. Short-duration heating at 80 °C for 45 min enhanced mechanical strength and thermal stability by promoting solvent removal and improving CNW dispersion. In contrast, prolonged exposure at lower temperatures (60 °C for 1.5 h) accelerated hydrolysis and chain scission, reducing performance. This demonstrates that controlled heat treatment is necessary to optimize nanocomposite formation while preventing solvent-induced degradation.
Overall, this work establishes a framework for incorporating bio-based nanofillers into commercial polyester adhesives using solvent-assisted dispersion techniques. The results confirm that CNWs can reinforce polyester resins when properly dispersed and processed, offering a sustainable pathway to enhance performance in automotive and structural adhesive applications. Future work should explore solvent-free or surface-modified CNW systems to further improve compatibility and long-term stability while advancing the sustainable use of bio-derived nanoparticles in polymer composites.