Hybrid GTAW–FCAW of 316L Stainless Steel Pipes: Influence of Oxygen Content in Baking Gas and Surface Preparation on Oxide Characteristics and Corrosion Behavior
Abstract
1. Introduction
2. Methodology
2.1. Materials and Sample Preparation
2.2. Surface Preparation and Roughness
2.3. Welding Procedures
2.4. Oxide Layer Analysis
2.5. Corrosion Testing
3. Results and Discussion
3.1. Oxide Layer Discoloration Analysis
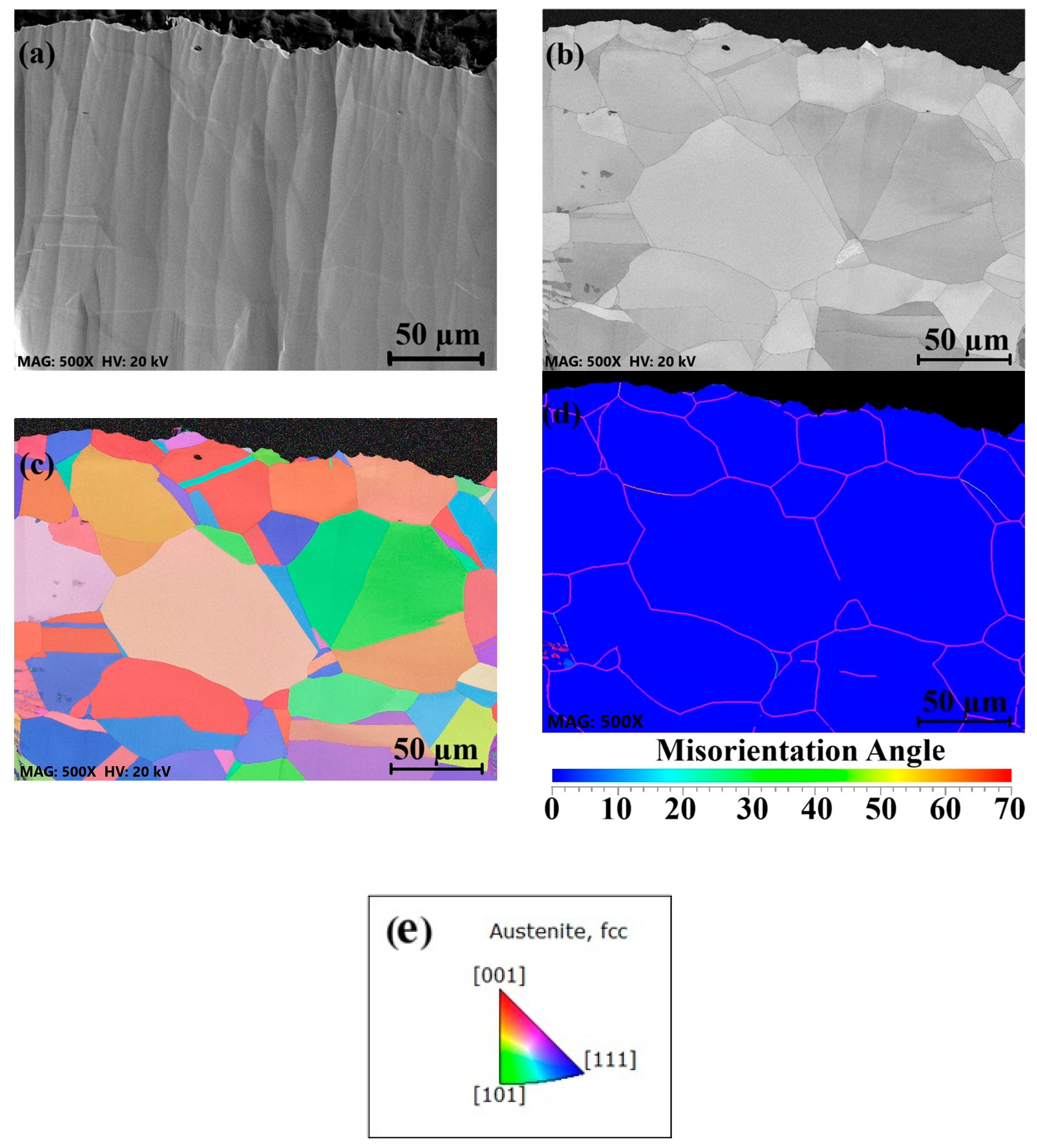
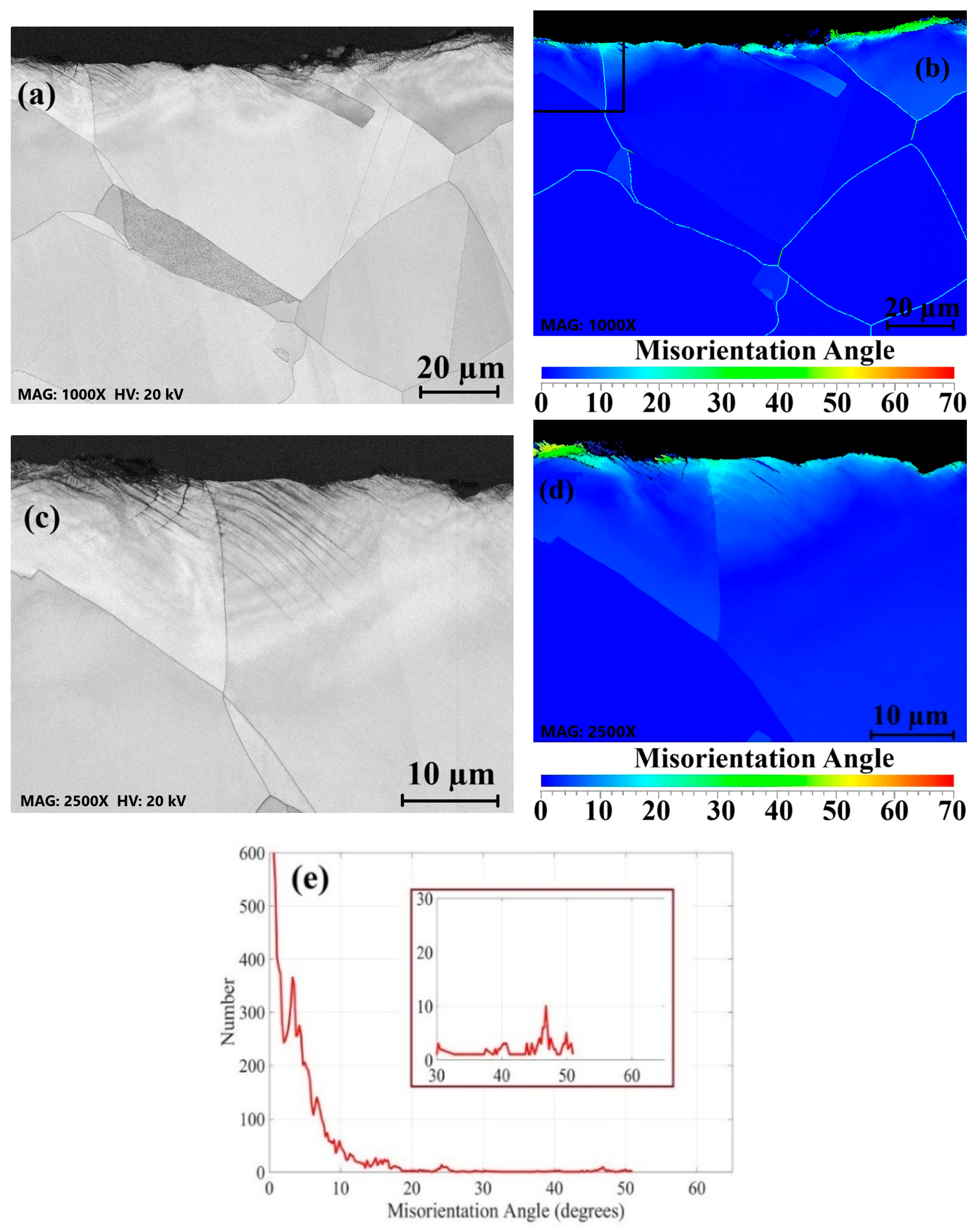
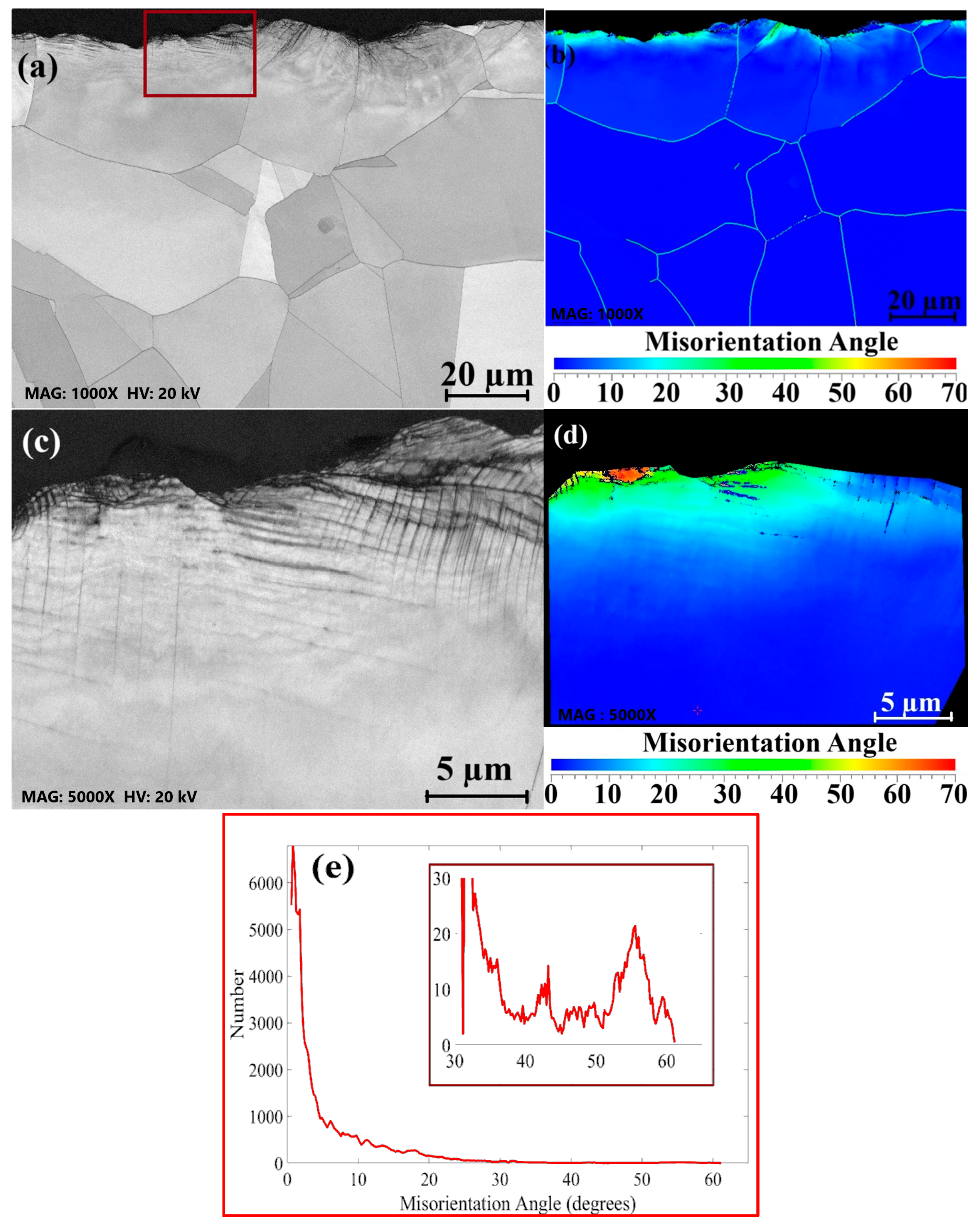
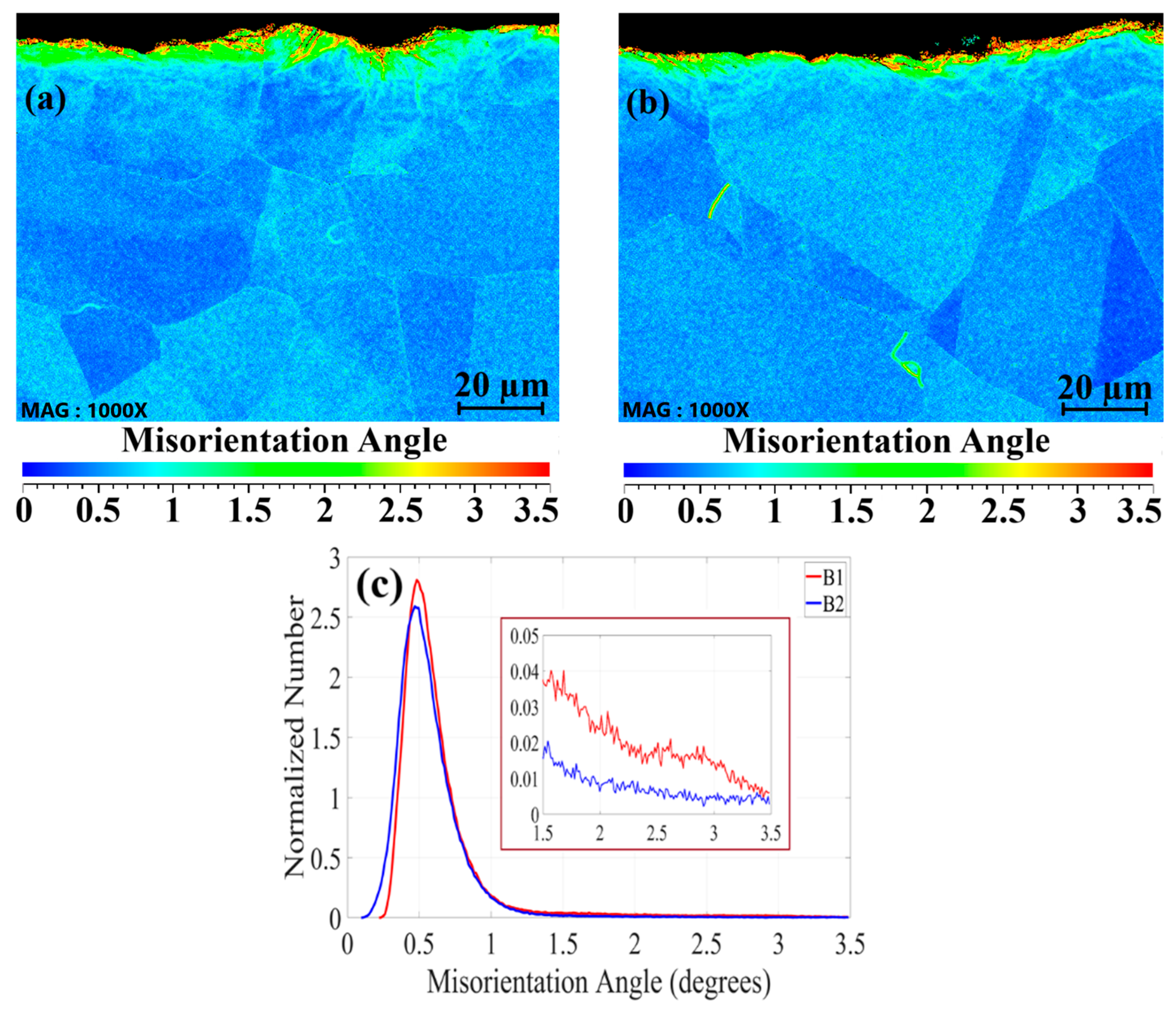
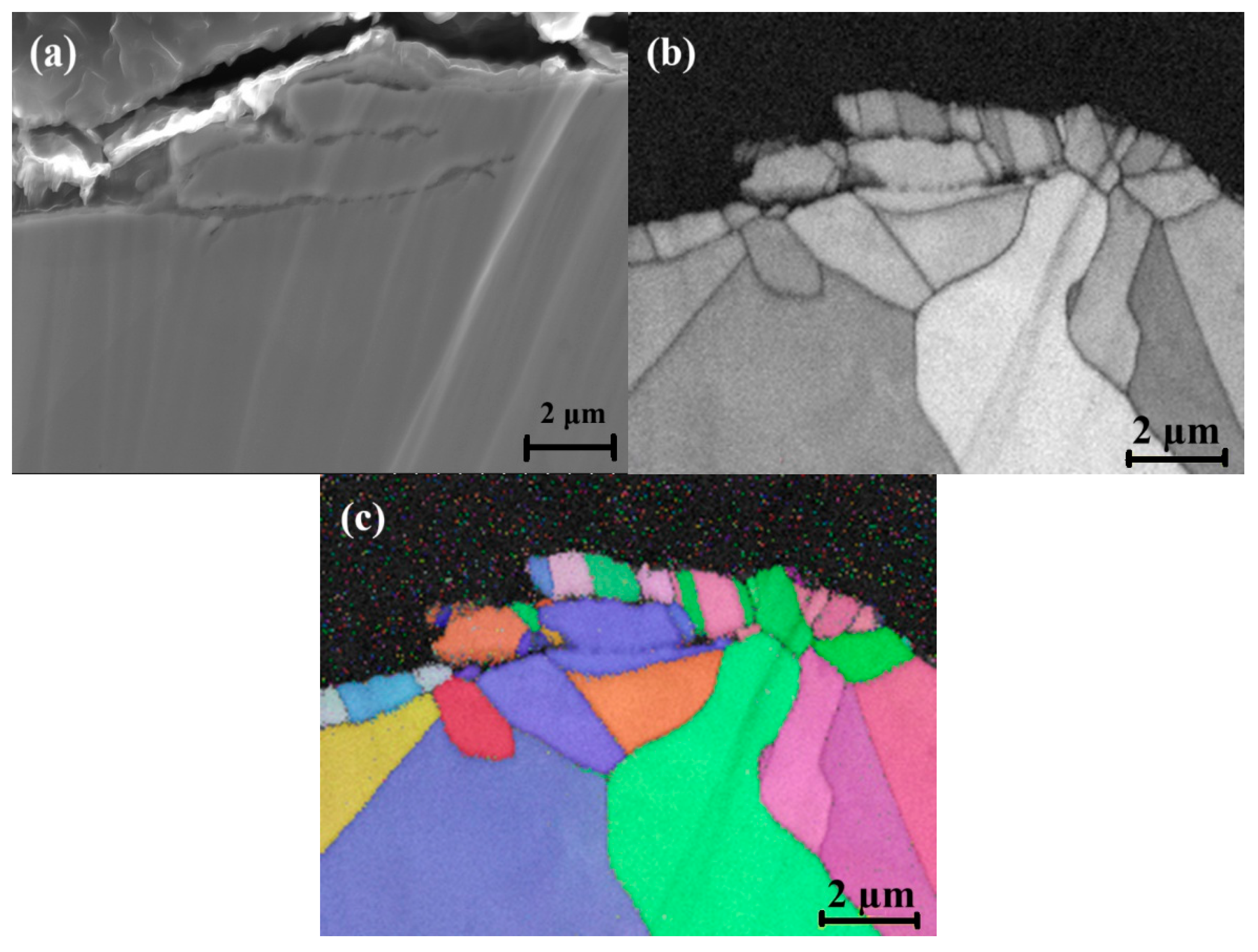
3.2. Surface Grain Size Before and After Welding
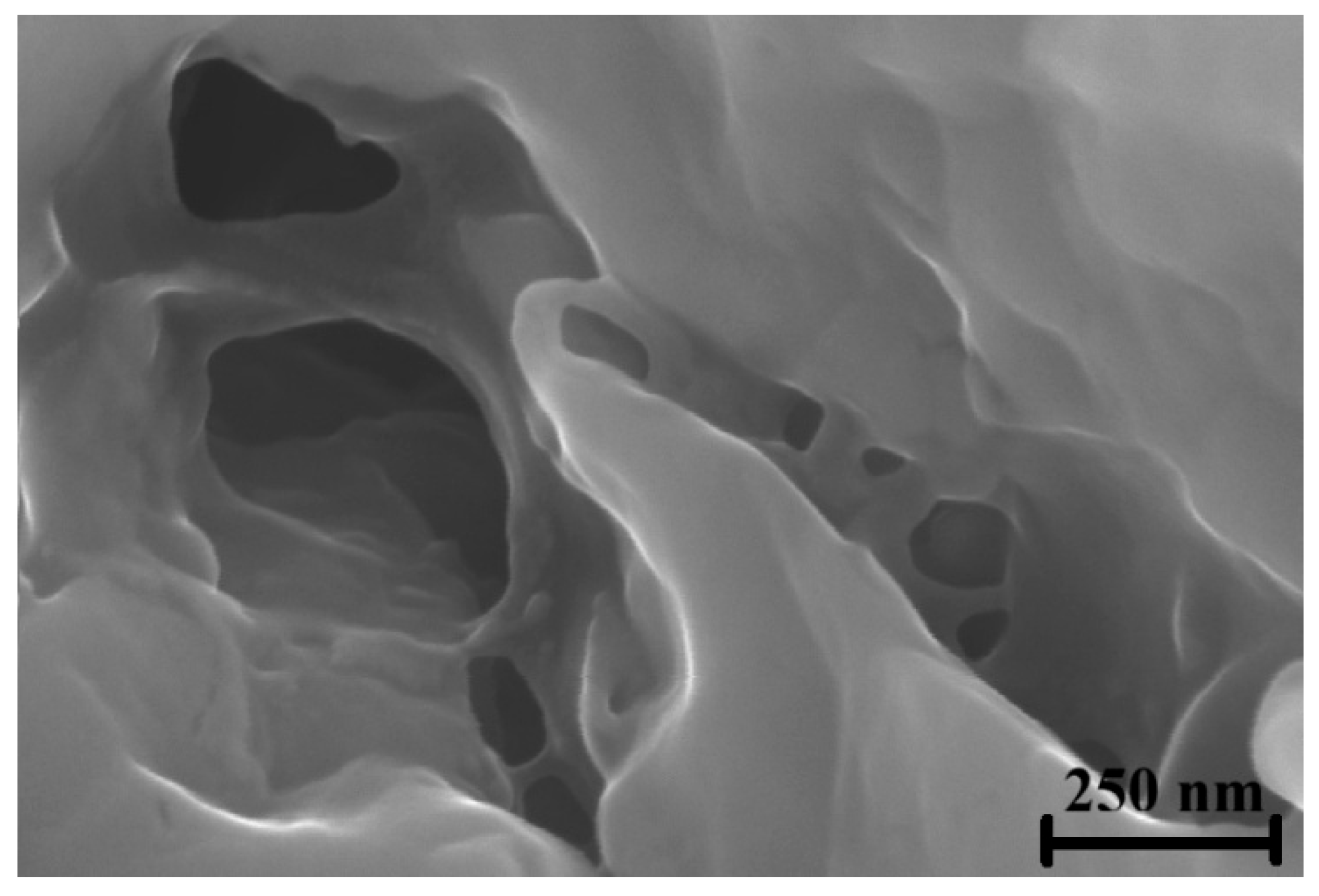
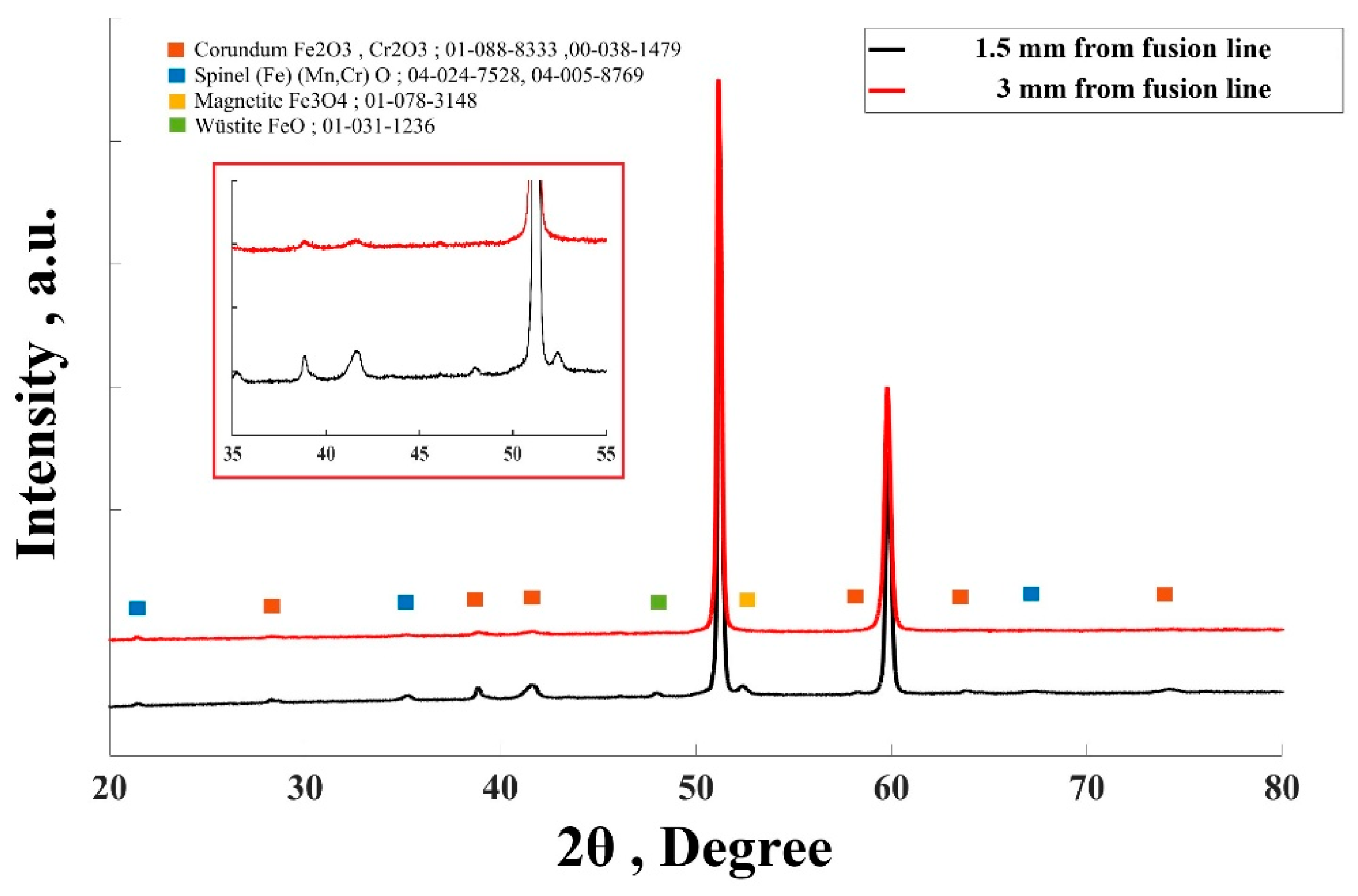
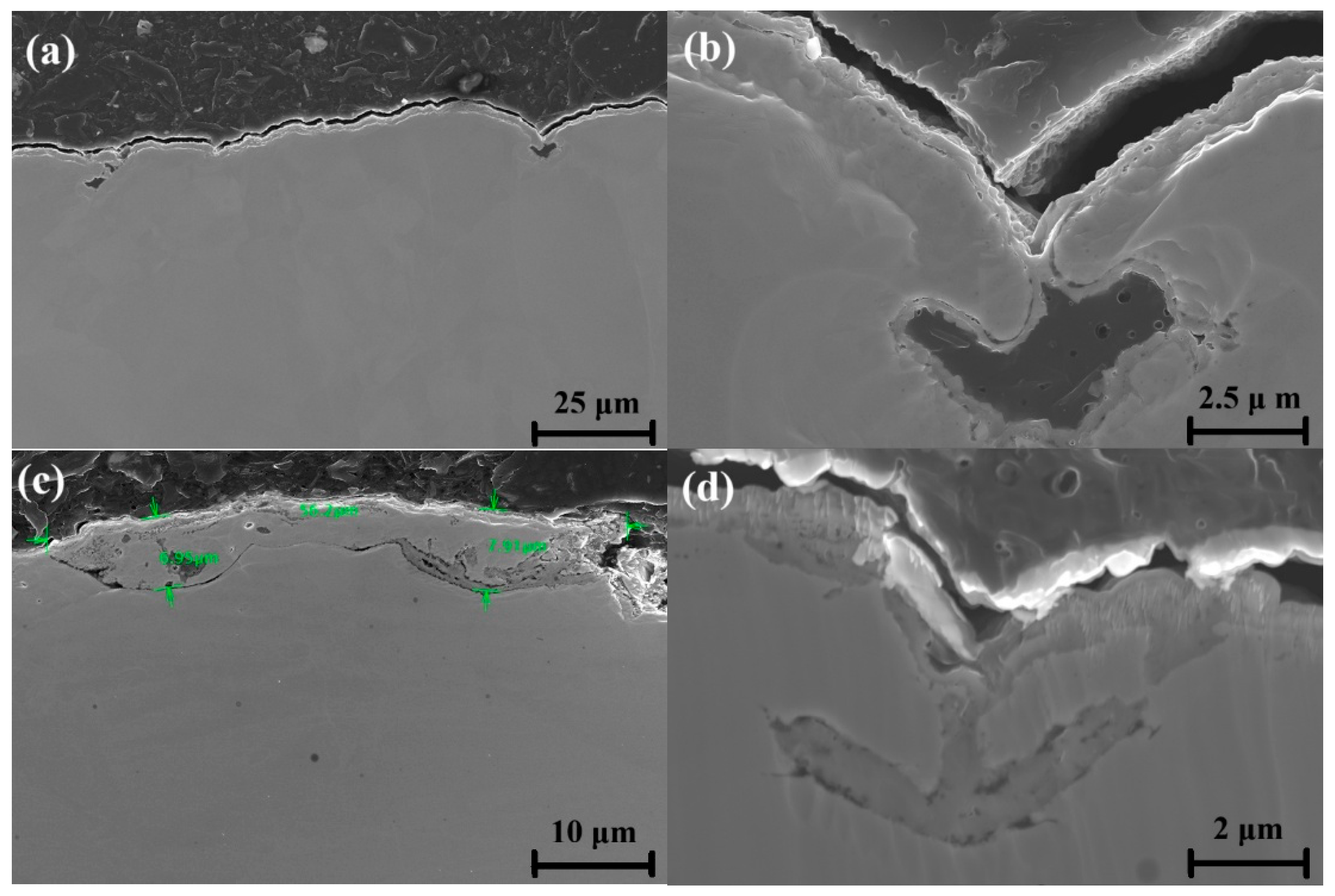
3.3. Oxidation Analysis
3.4. EIS Analysis
3.5. Analysis of the Pitting Corrosion
4. Conclusions
- Surface buffing significantly affected the misorientation of grains and recrystallization after welding. Higher misorientation levels were observed for the rougher surface, while the smoother surface exhibited a more uniform misorientation with lower values.
- Chromium (Cr) and manganese (Mn) depleted zones were revealed along the grain boundaries. The Cr-Mn oxide layer was thicker on the rougher surface. This thicker oxide layer offers initial resistance to corrosion.
- Surface roughness significantly affects corrosion resistance, with smoother surfaces exhibiting lower pitting corrosion resistance compared to rougher surfaces. However, smoother surfaces demonstrated better pitting propagation resistance. The findings are explained in terms of the smoother surface’s lower grain boundary density, which helps prevent initiation of localized corrosion, though it does not prevent the development of deeper pits once they have formed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Z.; Wang, R.; Li, J.; Xu, L.; Qiao, L. Effect of cold rolling on the pitting corrosion of 316L and 304 austenitic stainless steels. J. Mater. Sci. 2022, 57, 20503–20520. [Google Scholar] [CrossRef]
- Bedoya-Zapata, Á.D.; Franco-Rendón, C.M.; León-Henao, H.; Santa, J.F.; Barrada, J.E.G. Failure analysis of a welded stainless-steel piping system with premature pitting. Eng. Fail. Anal. 2021, 119, 104986. [Google Scholar] [CrossRef]
- Maroufkhani, M.; Khodabandeh, A.; Radu, I.; Moosavi-Khoonsari, E.; Jahazi, M. Thermodynamic and kinetic analyses of high temperature oxidation of 316L stainless steel. Results Mater. 2025, 28, 100774. [Google Scholar] [CrossRef]
- Maroufkhani, M.; Hakimian, S.; Khodabandeh, A.; Radu, I.; Hof, L.A.; Jahazi, M. Influence of oxygen content in the protective gas on pitting corrosion resistance of a 316l stainless steel weld joint. Materials 2023, 16, 5968. [Google Scholar] [CrossRef] [PubMed]
- Aumpiem, A.; Prateepasen, A. The relation of oxygen value, heat tint and pitting corrosion in super duplex pipe. In Proceedings of the 3rd International Conference on Mechanical and Production Engineering, Pattaya, Thailand, 14–15 January 2017; ISBN 9788193137390. [Google Scholar]
- Garcia, J.H.N.; Santos, N.F.d.; Esteves, L.; Campos, W.R.d.C.; Rabelo, E.G. Corrosion behavior of 316L and alloy 182 dissimilar weld joint with post-weld heat treatment. Matéria 2019, 24, e12471. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, P.; Wang, D.; Wu, L.; Ni, D.; Xiao, B.; Ma, Z. Effect of heat-input on pitting corrosion behavior of friction stir welded high nitrogen stainless steel. J. Mater. Sci. Technol. 2019, 35, 1278–1283. [Google Scholar] [CrossRef]
- Li, M.; Zou, D.; Li, Y.; Tong, L. Effect of cooling rate on pitting corrosion behavior of 904L austenitic stainless steel in a simulated flue gas desulfurization solution. Met. Mater. Int. 2023, 29, 730–747. [Google Scholar] [CrossRef]
- Ezuber, H.; Alshater, A.; Nisar, S.; Gonsalvez, A.; Aslam, S. Effect of surface finish on the pitting corrosion behavior of sensitized AISI 304 austenitic stainless steel alloys in 3.5% NaCl solutions. Surf. Eng. Appl. Electrochem. 2018, 54, 73–80. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Tong, H.; Sui, Y.; Li, X.; Hou, J. Effect of Surface Roughness on the Corrosion Behavior of 304 Stainless Steel in Seawater. J. Mater. Eng. Perform. 2024, 34, 18287–18297. [Google Scholar] [CrossRef]
- Kim, B.; Lee, C.; Kim, J.; Lee, J. Corrosion Assessment and Quantification of Discoloration in the Weld Root Bead and Heat-Affected Zone of Stainless Steel Pipe Weldments. In Proceedings of the ISOPE International Ocean and Polar Engineering Conference, Rhodes, Greece, 16–21 June 2024; ISOPE: Mountain View, CA, USA, 2024. [Google Scholar]
- Kimbrel, K. Determining Acceptable Levels of Weld Discoloration on Mechanically Polished and Electropolished Stainless Steel Surface. Pharm. Eng. 2011, 31, 1–7. [Google Scholar]
- Ling, L.; Liu, T.; Lu, Y.; Guo, P. Investigation of the oxides film on 304L base metal produced during welding process without inert gas shielding. Appl. Surf. Sci. 2019, 465, 780–786. [Google Scholar] [CrossRef]
- Bergquist, E.-L.; Huhtala, T.; Karlsson, L. The effect of purging gas on 308L TIG root pass ferrite content. Weld. World 2011, 55, 57–64. [Google Scholar] [CrossRef]
- Panmongkol, P.; Phung-on, I. Effect of backing gas mixtures on corrosion properties of stainless steel grade 304 weld metal by autogenous GTAW. J. Mater. Res. Technol. 2021, 11, 1559–1570. [Google Scholar] [CrossRef]
- Trigwell, S.; Selvaduray, G. Effects of welding on the passive oxide film of electropolished 316L stainless steel. J. Mater. Process. Technol. 2005, 166, 30–43. [Google Scholar] [CrossRef]
- Macleod, H.A.; Macleod, H.A. Thin-Film Optical Filters; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Pedrotti, L.S. Basic physical optics. Fundam. Photonics 2008, 1, 152–154. [Google Scholar]
- Khafaji, N.Y.; Demir, A.G.; Vitali, L.; Fustinoni, D.; Niro, A.; Previtali, B.; Taha, Z.A. Optical characterization of laser coloured titanium under different processing atmospheres. Surf. Coat. Technol. 2017, 321, 156–163. [Google Scholar] [CrossRef]
- Avery, R.E. Sanitary Welding Standards. In Proceedings of the ASME Citrus Engineering Symposium, Lake Alfred, FL, USA, 23 March 2000; American Society of Mechanical Engineers: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Hong, T.; Nagumo, M. Effect of surface roughness on early stages of pitting corrosion of type 301 stainless steel. Corros. Sci. 1997, 39, 1665–1672. [Google Scholar] [CrossRef]
- Nowak, W.J. Effect of surface roughness on oxidation resistance of stainless steel AISI 316Ti during exposure at high temperature. J. Mater. Eng. Perform. 2020, 29, 8060–8069. [Google Scholar] [CrossRef]
- Wang, J.; Xue, H.; Zhao, Y.; Zhang, T.; Wang, F. Effect of Surface Roughness on the Corrosion of HP-13Cr Stainless Steel in the Dynamic Aggressive Oilfield Environment. Metals 2024, 14, 280. [Google Scholar] [CrossRef]
- Jaffré, K.; Ter-Ovanessian, B.; Abe, H.; Mary, N.; Normand, B.; Watanabe, Y. Effect of mechanical surface treatments on the surface state and passive behavior of 304L stainless steel. Metals 2021, 11, 135. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, M.; Wang, W.; Hou, J.; Kuang, W. The effects of heat treatment and surface state on the corrosion resistance of laser powder bed fusion 304L stainless steel in 3.5 wt% NaCl solution. J. Mater. Res. Technol. 2024, 29, 5620–5632. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, P.; Zhang, J.; Yue, F.; Nie, X.; Chi, R.; Fu, M. Influence of Surface Roughness on the Corrosion Behavior of ENiCrFe-7 Weld Overlay Cladding Materials. J. Mater. Eng. Perform. 2025, 1–9. [Google Scholar] [CrossRef]
- Messinese, E.; Casanova, L.; Paterlini, L.; Capelli, F.; Bolzoni, F.; Ormellese, M.; Brenna, A. A Comprehensive Investigation on the Effects of Surface Finishing on the Resistance of Stainless Steel to Localized Corrosion. Metals 2022, 12, 1751. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, N.; Wu, J.; Jiang, Y.; Li, J. Effect of surface roughness on pitting corrosion of 2205 duplex stainless steel investigated by electrochemical noise measurements. Materials 2019, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Salah-Rousset, N.B.; Chaouachi, M.; Chellouf, A. Role of surface finishing on pitting corrosion of a duplex stainless steel in seawater. J. Mater. Eng. Perform. 1996, 5, 225–231. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, N.; Wu, J.; Jiang, Y.; Li, J. Investigation of influence of surface roughness on pitting corrosion of duplex stainless steel 2205 using various electrochemical techniques. Int. J. Electrochem. Sci. 2019, 14, 6790–6813. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, H.; Lu, J.; Sun, G.; Du, L. Effect of grain size on mechanical property and corrosion behavior of a metastable austenitic stainless steel. Mater. Charact. 2022, 194, 112360. [Google Scholar] [CrossRef]
- Balusamy, T.; Kumar, S.; Narayanan, T.S. Effect of surface nanocrystallization on the corrosion behaviour of AISI 409 stainless steel. Corros. Sci. 2010, 52, 3826–3834. [Google Scholar] [CrossRef]
- Wang, S.; Sun, M.; Xu, Y.; Long, K.; Zhang, Z. Enhanced localized and uniform corrosion resistances of bulk nanocrystalline 304 stainless steel in high-concentration hydrochloric acid solutions at room temperature. J. Mater. Sci. Technol. 2018, 34, 2498–2506. [Google Scholar] [CrossRef]
- Hao, Y.-W.; Deng, B.; Zhong, C.; Jiang, Y.-M.; Li, J. Effect of surface mechanical attrition treatment on corrosion behavior of 316 stainless steel. J. Iron Steel Res. Int. 2009, 16, 68–72. [Google Scholar] [CrossRef]
- Wang, P.-j.; Ma, L.-w.; Cheng, X.-q.; Li, X.-g. Influence of grain refinement on the corrosion behavior of metallic materials: A review. Int. J. Miner. Metall. Mater. 2021, 28, 1112–1126. [Google Scholar] [CrossRef]
- Aghuy, A.A.; Zakeri, M.; Moayed, M.H.; Mazinani, M. Effect of grain size on pitting corrosion of 304L austenitic stainless steel. Corros. Sci. 2015, 94, 368–376. [Google Scholar] [CrossRef]
- ASTM G5-13; Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2013.
- Loto, R.T. Study of the corrosion behaviour of S32101 duplex and 410 martensitic stainless steel for application in oil refinery distillation systems. J. Mater. Res. Technol. 2017, 6, 203–212. [Google Scholar] [CrossRef]
- Azuma, S.; Kudo, T.; Miyuki, H.; Yamashita, M.; Uchida, H. Effect of nickel alloying on crevice corrosion resistance of stainless steels. Corros. Sci. 2004, 46, 2265–2280. [Google Scholar] [CrossRef]
- Sanni, O.; Popoola, A. Corrosion inhibition effect of a non-toxic waste compound on stainless steel in 0.5 molar concentration of sulfuric acid. J. Bio-Tribo-Corros. 2021, 7, 88. [Google Scholar] [CrossRef]
- Bellezze, T.; Giuliani, G.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 1: Cyclic potentiodynamic polarization curves examined by means of an analytical method. Corros. Sci. 2018, 130, 113–125. [Google Scholar] [CrossRef]
- Miranda-Pérez, A.F.; Rodríguez-Vargas, B.R.; Calliari, I.; Pezzato, L. Corrosion resistance of GMAW duplex stainless steels welds. Materials 2023, 16, 1847. [Google Scholar] [CrossRef]
- Lippold, J.C. Welding Metallurgy and Weldability; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Huang, X.; Xiao, K.; Fang, X.; Xiong, Z.; Wei, L.; Zhu, P.; Li, X. Oxidation behavior of 316L austenitic stainless steel in high temperature air with long-term exposure. Mater. Res. Express 2020, 7, 066517. [Google Scholar] [CrossRef]
- Xie, B.; Sun, M.; Xu, B.; Wang, C.; Jiang, H.; Li, D.; Li, Y. Oxidation of stainless steel in vacuum and evolution of surface oxide scales during hot-compression bonding. Corros. Sci. 2019, 147, 41–52. [Google Scholar] [CrossRef]
- Habib, K.; Damra, M.; Saura, J.; Cervera, I.; Bellés, J. Breakdown and Evolution of the Protective Oxide Scales of AISI 304 and AISI 316 Stainless Steels under High-Temperature Oxidation. Int. J. Corros. 2011, 2011, 824676. [Google Scholar] [CrossRef]
- Balaško, T.; Batič, B.Š.; Burja, J. Influence of the Cooling Rate on the Wüstite Content in Oxide Layers Formed During High-Temperature Oxidation of Hot-Worked Tool Steel with High Thermal Conductivity. High Temp. Corros. Mater. 2025, 102, 2. [Google Scholar] [CrossRef]
- Zhao, G.; Tian, Y.; Li, H.; Ma, L.; Li, Y.; Li, J. Microstructure evolution and dynamic recrystallization mechanisms of 316L stainless steel during hot deformation. Arch. Civ. Mech. Eng. 2024, 24, 35. [Google Scholar] [CrossRef]
- Celik, S.; Ersozlu, I. Dynamic recrystallization of friction welded AISI 316 stainless steel joints. Mater. Test. 2020, 62, 1126–1130. [Google Scholar] [CrossRef]
- Pinto, F.C.; Aota, L.S.; Souza Filho, I.R.d.; Raabe, D.; Sandim, H.R.Z. Recrystallization in non-conventional microstructures of 316L stainless steel produced via laser powder-bed fusion: Effect of particle coarsening kinetics. J. Mater. Sci. 2022, 57, 9576–9598. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, C.; Zhang, Y.; Liu, X.; Qin, C.; Wang, Z.; Lin, X.; Wang, J.; Wang, L.; He, F. Recovery-Assisted Abnormal Grain Evolution of Selective Laser-Melted 316L Stainless Steel at Intermediate Temperatures. Metall. Mater. Trans. A 2024, 55, 4613–4622. [Google Scholar] [CrossRef]
- Gao, S.; Hu, Z.; Duchamp, M.; Krishnan, P.S.R.; Tekumalla, S.; Song, X.; Seita, M. Recrystallization-based grain boundary engineering of 316L stainless steel produced via selective laser melting. Acta Mater. 2020, 200, 366–377. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wei, L.; Wang, Y.; Misra, R.; Chen, J. Understanding the high-temperature oxidation resistance of heat-resistant austenitic stainless steel with gradient nanostructure. Corros. Sci. 2024, 231, 111966. [Google Scholar] [CrossRef]
- Hart, E. On the role of dislocations in bulk diffusion. Acta Metall. 1957, 5, 597. [Google Scholar] [CrossRef]
- Sabioni, A.C.S.; Ramos, R.P.B.; Ji, V.; Jomard, F.; Macedo, W.A.d.A.; Gastelois, P.L.; Trindade, V.B. About the role of chromium and oxygen ion diffusion on the growth mechanism of oxidation films of the AISI 304 austenitic stainless steel. Oxid. Met. 2012, 78, 211–220. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wang, J.; Wang, W.; Xue, Z.; Lu, Y.; Huang, H. Influence of surface plastic deformation on oxidation behavior for low Cr content nickel superalloy: Focus on the Cr diffusion. Surf. Coat. Technol. 2024, 494, 131347. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, B.K.; Kim, D.-I.; Choi, P.-P.; Raabe, D.; Yi, K.-W. The role of grain boundaries in the initial oxidation behavior of austenitic stainless steel containing alloyed Cu at 700 C for advanced thermal power plant applications. Corros. Sci. 2015, 96, 52–66. [Google Scholar] [CrossRef]
- Lobb, R.; Evans, H. Formation of protective oxide film on chromium-depleted stainless steel. Met. Sci. 1981, 15, 267–274. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Wen, W.; Deng, Y.; Peng, K.; Liu, Y. Effect of Mn content on the high-temperature oxidation behaviors of Mn-substituted-for-Ni alumina-forming austenitic stainless steel. J. Mater. Res. Technol. 2023, 26, 7816–7828. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takayama, T.; Minamino, Y.; Koizumi, Y.; Tokunaga, T.; Hagihara, K. Modification of grain boundary microstructure by controlling dissolution behavior of θ particles in Cr-containing hypereutectoid steel. Mater. Charact. 2023, 205, 113241. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, S.; Liu, P.; Kuang, W. The structure dependence of grain boundary passivation of Alloy 690 in high temperature water. Acta Mater. 2023, 261, 119368. [Google Scholar] [CrossRef]
- Chen, J.; Xie, X.; Zou, T.; Zhang, Y.; Wang, H.; Liang, Z. Improvement of the high-temperature oxidation resistance of 254SMo using ultrasonic strengthening grinding. J. Mater. Res. Technol. 2023, 27, 2052–2065. [Google Scholar] [CrossRef]
- Ji, Y.; Hao, L.; Wang, J.; Ke, W. EIS investigation on surface roughness induced oxide film evolution on 304 SS in simulated secondary circuit water of PWR system. J. Mater. Sci. 2025, 60, 5511–5532. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Vafaeian, S. Influence of grain refinement on the electrochemical behavior of AISI 430 ferritic stainless steel in an alkaline solution. Appl. Surf. Sci. 2016, 360, 921–928. [Google Scholar] [CrossRef]
- Li, Z.; Mei, K.; Dong, J.; Yang, Y.; Sun, J.; Luo, Z. An investigation on the wear and corrosion resistance of AlCoCrFeNi high-entropy alloy coatings enhanced by Ti and Si. Surf. Coat. Technol. 2024, 487, 130949. [Google Scholar] [CrossRef]
- Hernández, H.H.; Reynoso, A.R.; González, J.T.; Morán, C.G.; Hernández, J.M.; Ruiz, A.M.; Hernández, J.M.; Cruz, R.O. Electrochemical impedance spectroscopy (EIS): A review study of basic aspects of the corrosion mechanism applied to steels. In Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2020; pp. 137–144. [Google Scholar] [CrossRef]







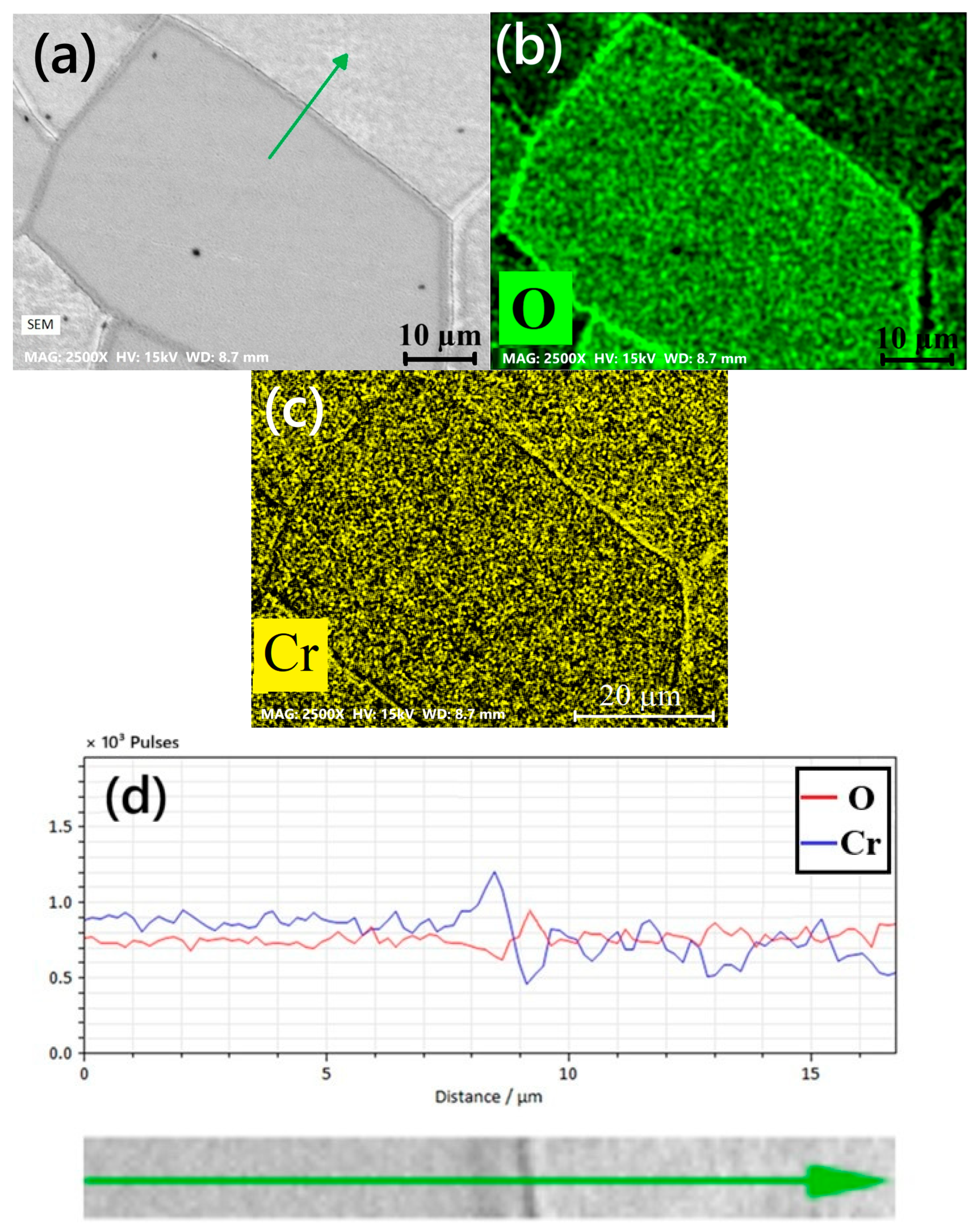
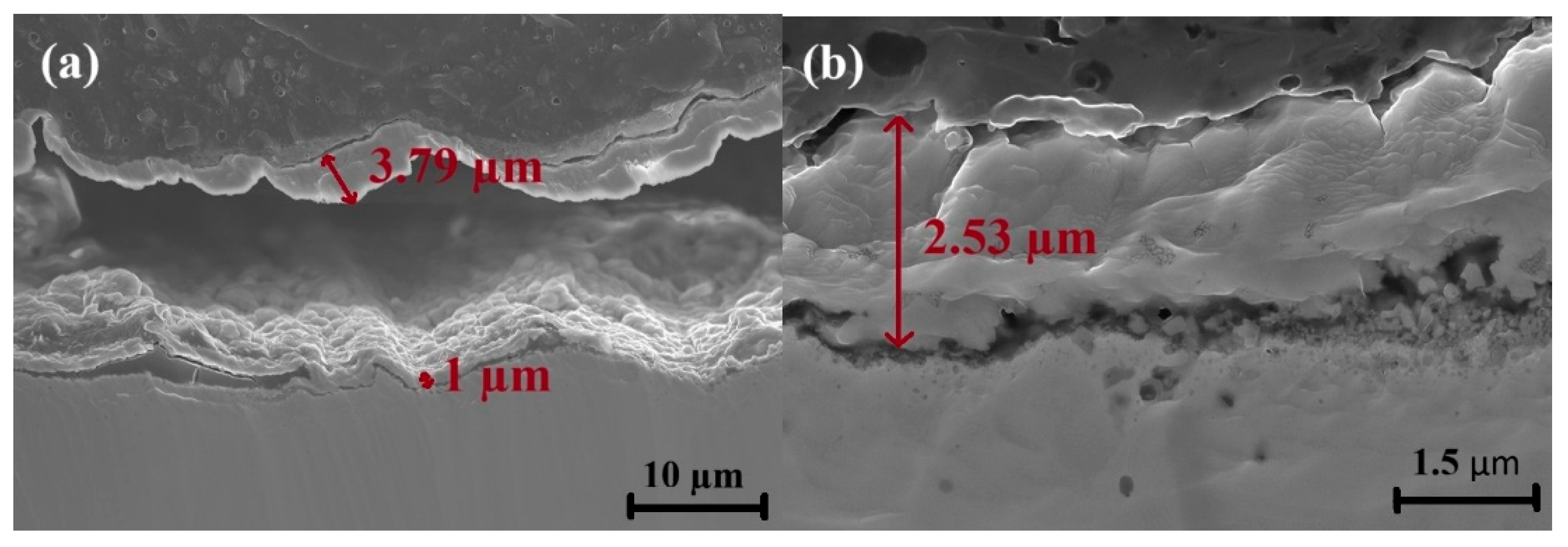



| Element | C | Mn | Si | P | S | Mo | Cr |
|---|---|---|---|---|---|---|---|
| wt.% | 0.0212 ± 0.0004 | 1.70 ± 0.0070 | 0.439 ± 0.0020 | 0.0333 ± 0.0004 | 0.0290 ± 0.0023 | 2.03 ± 0.0054 | 16.70 ± 0.0240 |
| Element | Ni | Al | Co | Cu | W | Others | Fe |
| wt.% | 10.13 ± 0.0327 | 0.0061 ± 0 | 0.209 ± 0.00055 | 0.55 ± 0.0016 | 0.0601 ± 0.0003 | 0.17 | Balance |
| Process | GTAW | FCAW | FCAW |
|---|---|---|---|
| Pass | 1 | 2 | 3 |
| Shielding gas | Ar | Ar | Ar |
| Purge gas flow rate (CFH) | 30 | 40 | 40 |
| Current (A) | 170 ± 20 | 236 ± 20 | 262 ± 20 |
| Voltage (V) | 12.6 ± 1 | 29.5 ± 1 | 29.5 ± 0.2 |
| Tube-to-work distance (mm) | 9.52 | 9.52 | 9.52 |
| Heat input (Kj/mm) | 1.24 | 0.87 | 1.18 |
| Oxygen Content (ppm) | 500 | 5000 |
|---|---|---|
| Component | Ar +0.05% O | Ar +0.5% O |
| Parameter | CPE1 (μs°/Ω) | α | CPE2 (μs°/Ω) | α | Rs (Ω) | Rct1 (Ω) | Rct2(Ω) | X2 |
|---|---|---|---|---|---|---|---|---|
| 500B1 | 236.35 ± 38.84 | 0.86 ± 0.01 | 2962.53 ± 479.05 | 0.5 ± 0.01 | 3.30 ± 0.01 | 1195.47± 318.18 | 6.57 ± 3.2 | 0.049 ± 0.042 |
| 5000B1 | 310.67 ± 0 | 0.84 ± 0.06 | 7427.93 ± 56.7 | 0.5 ± 0.03 | 3.60 ± 1.12 | 999.49 ± 98.55 | 4.55 ± 2.06 | 0.0039 ± 0.003 |
| 500B2 | 563.2 ± 151.25 | 0.85 ± 0.03 | 3443.84 ± 56.66 | 0.62 ± 0.01 | 3.83 ± 0.43 | 534.51 ± 150.97 | 1.34 ± 0.2 | 0.0077 ± 0.003 |
| 5000B2 | 692.58 ± 136.83 | 0.83 ± 0.06 | 7039.1 ± 44.66 | 0.5 ± 0.01 | 3.47± 0.95 | 344.81± 34.25 | 3.31 ± 10.40 | 0.0054 ± 0.002 |
| Parameter | I Pitt (µA) | E Pitt (V) | I Passive (µA) | E passive (V) | I Repassive (µA) | E Repassive (V) | Hysteresis Loop Area (W) |
|---|---|---|---|---|---|---|---|
| 500B1 | 234 ± 16 | 0.48 ± 0.02 | 271 ± 24 | 0.07 ± 0.01 | 379 ± 1 | 0.07 ± 0.01 | 1.26 ± 0.00 |
| 5000B1 | 251 ± 14 | 0.50 ± 0.01 | 251 ± 19 | 0.08 ± 0.01 | 483 ± 3 | 0.05 ± 0.01 | 1.33 ± 0.05 |
| 500B2 | 253 ± 55 | 0.48± 0.00 | 257 ± 27 | 0.03 ± 0.01 | 429 ±11 | 0.06 ± 0.02 | 1.15 ± 0.11 |
| 5000B2 | 409 ± 37 | 0.53 ± 0.02 | 461 ± 89 | 0.13 ± 0.02 | 748 ± 71 | 0.1 ± 0.01 | 1.12 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroufkhani, M.; Khodabandeh, A.; Radu, I.; Jahazi, M. Hybrid GTAW–FCAW of 316L Stainless Steel Pipes: Influence of Oxygen Content in Baking Gas and Surface Preparation on Oxide Characteristics and Corrosion Behavior. J. Manuf. Mater. Process. 2025, 9, 377. https://doi.org/10.3390/jmmp9110377
Maroufkhani M, Khodabandeh A, Radu I, Jahazi M. Hybrid GTAW–FCAW of 316L Stainless Steel Pipes: Influence of Oxygen Content in Baking Gas and Surface Preparation on Oxide Characteristics and Corrosion Behavior. Journal of Manufacturing and Materials Processing. 2025; 9(11):377. https://doi.org/10.3390/jmmp9110377
Chicago/Turabian StyleMaroufkhani, Mohammad, Alireza Khodabandeh, Iulian Radu, and Mohammad Jahazi. 2025. "Hybrid GTAW–FCAW of 316L Stainless Steel Pipes: Influence of Oxygen Content in Baking Gas and Surface Preparation on Oxide Characteristics and Corrosion Behavior" Journal of Manufacturing and Materials Processing 9, no. 11: 377. https://doi.org/10.3390/jmmp9110377
APA StyleMaroufkhani, M., Khodabandeh, A., Radu, I., & Jahazi, M. (2025). Hybrid GTAW–FCAW of 316L Stainless Steel Pipes: Influence of Oxygen Content in Baking Gas and Surface Preparation on Oxide Characteristics and Corrosion Behavior. Journal of Manufacturing and Materials Processing, 9(11), 377. https://doi.org/10.3390/jmmp9110377








