Using Coatings Based on the ZrN System to Improve the Corrosion Resistance of Stainless Steel Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Deposition
- Rinsing in an alkaline solution at 80 °C under ultrasonication.
- Rinsing in specially purified water (filtration on a carbon filter).
- Blowing with a stream of purified air (filtration).
2.2. Coating Properties
3. Results
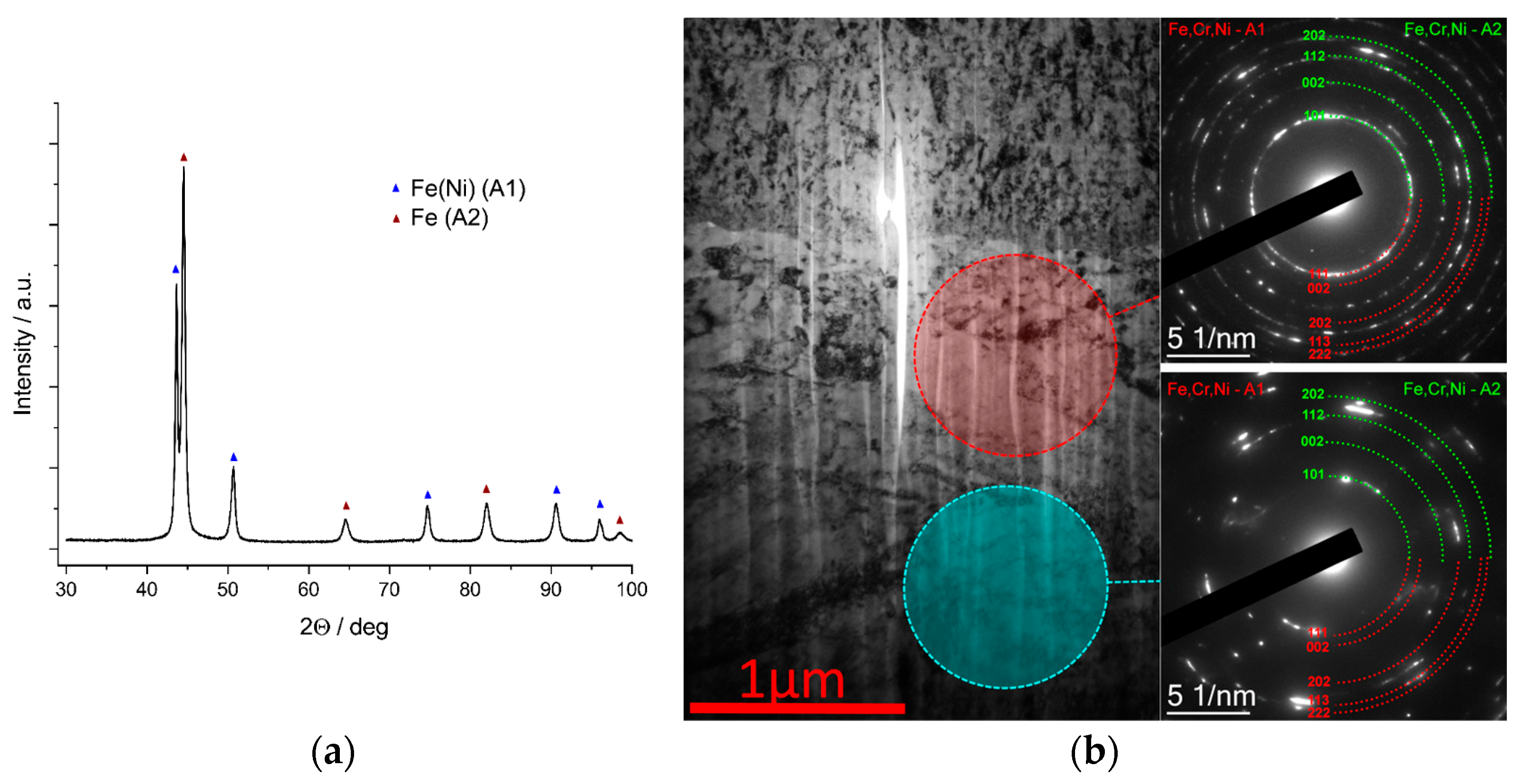
3.1. Substrate Properties
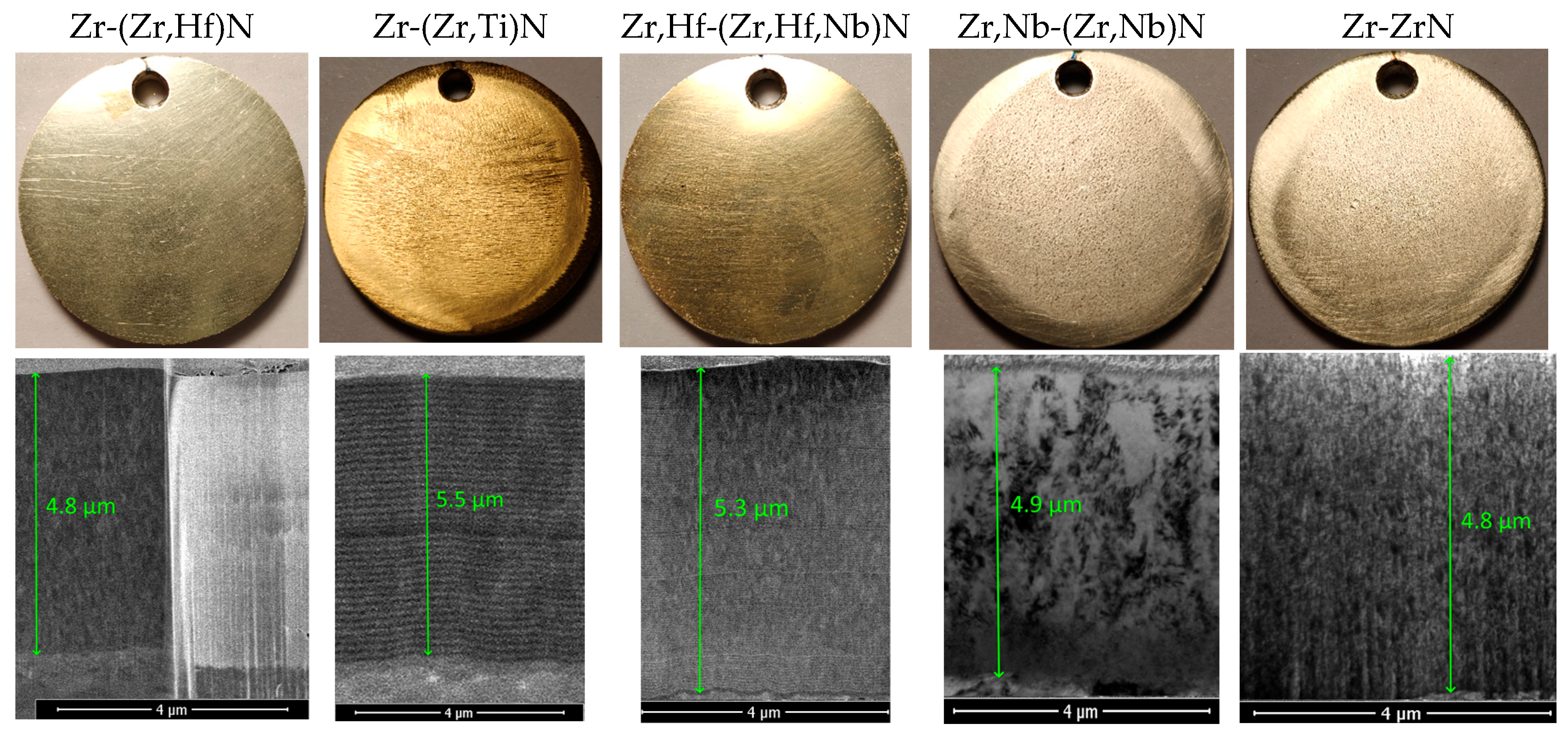
3.2. Mechanical Properties of the Coatings
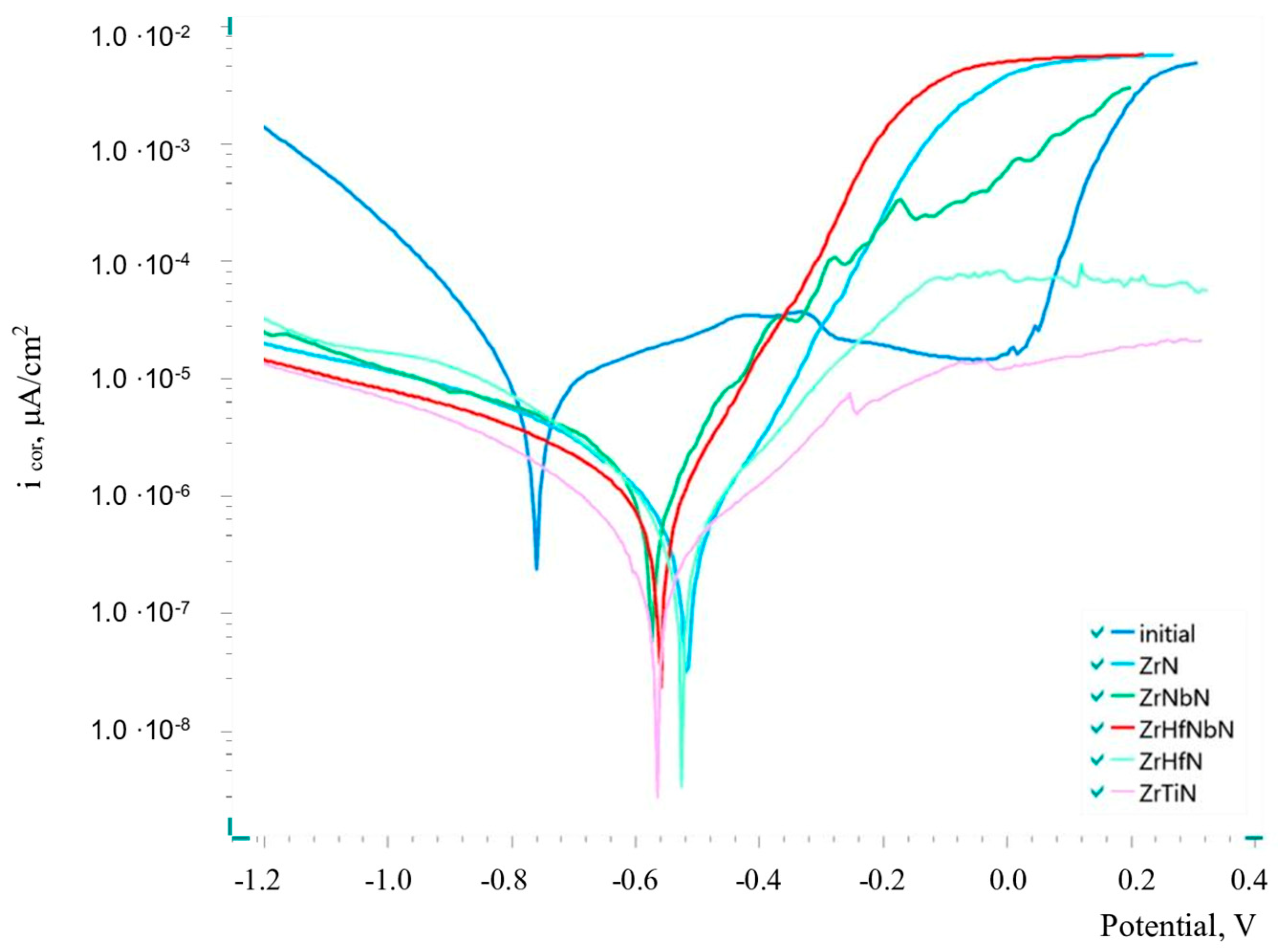
3.3. Corrosion Behaviour of the Samples with Nitride Coatings
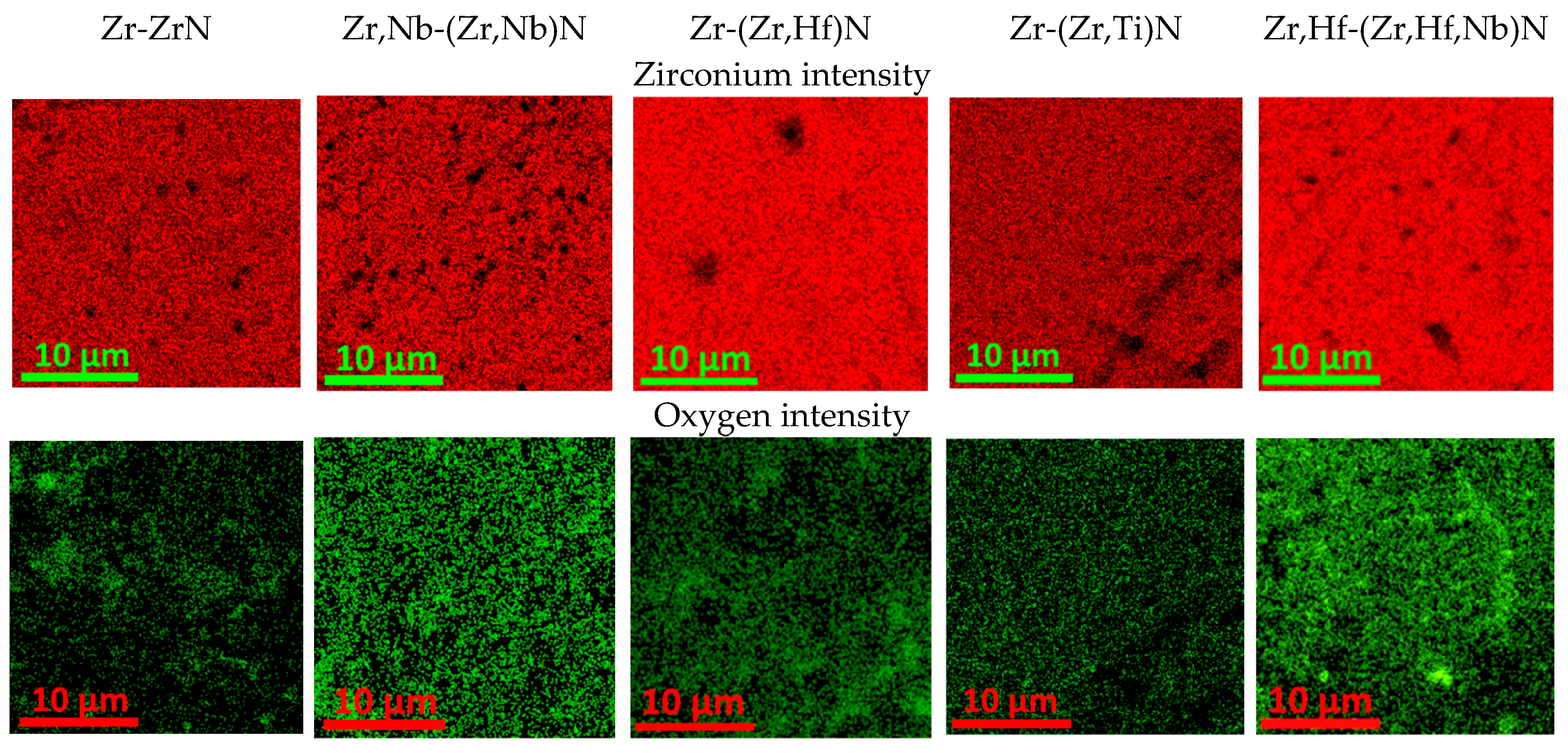
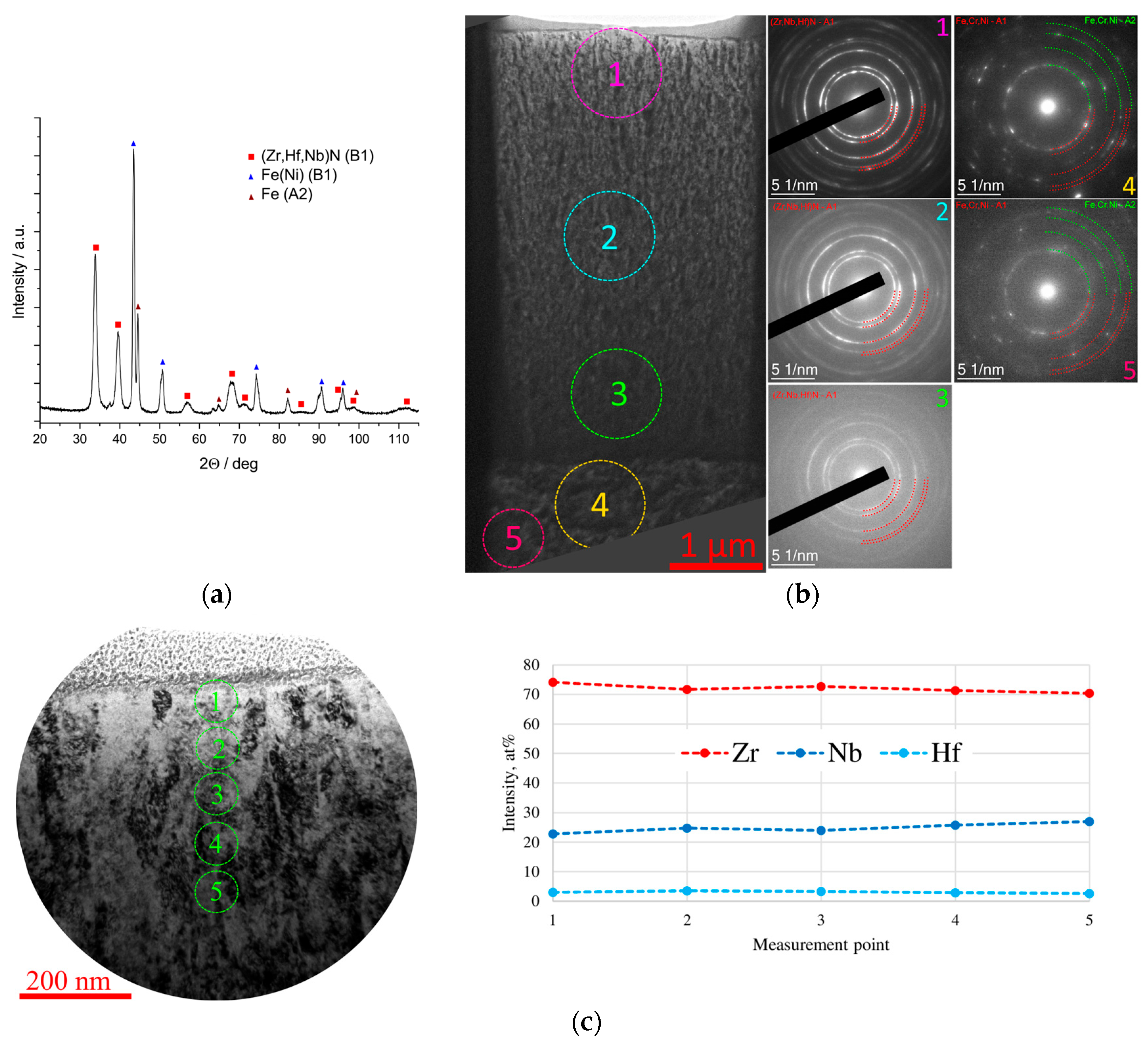
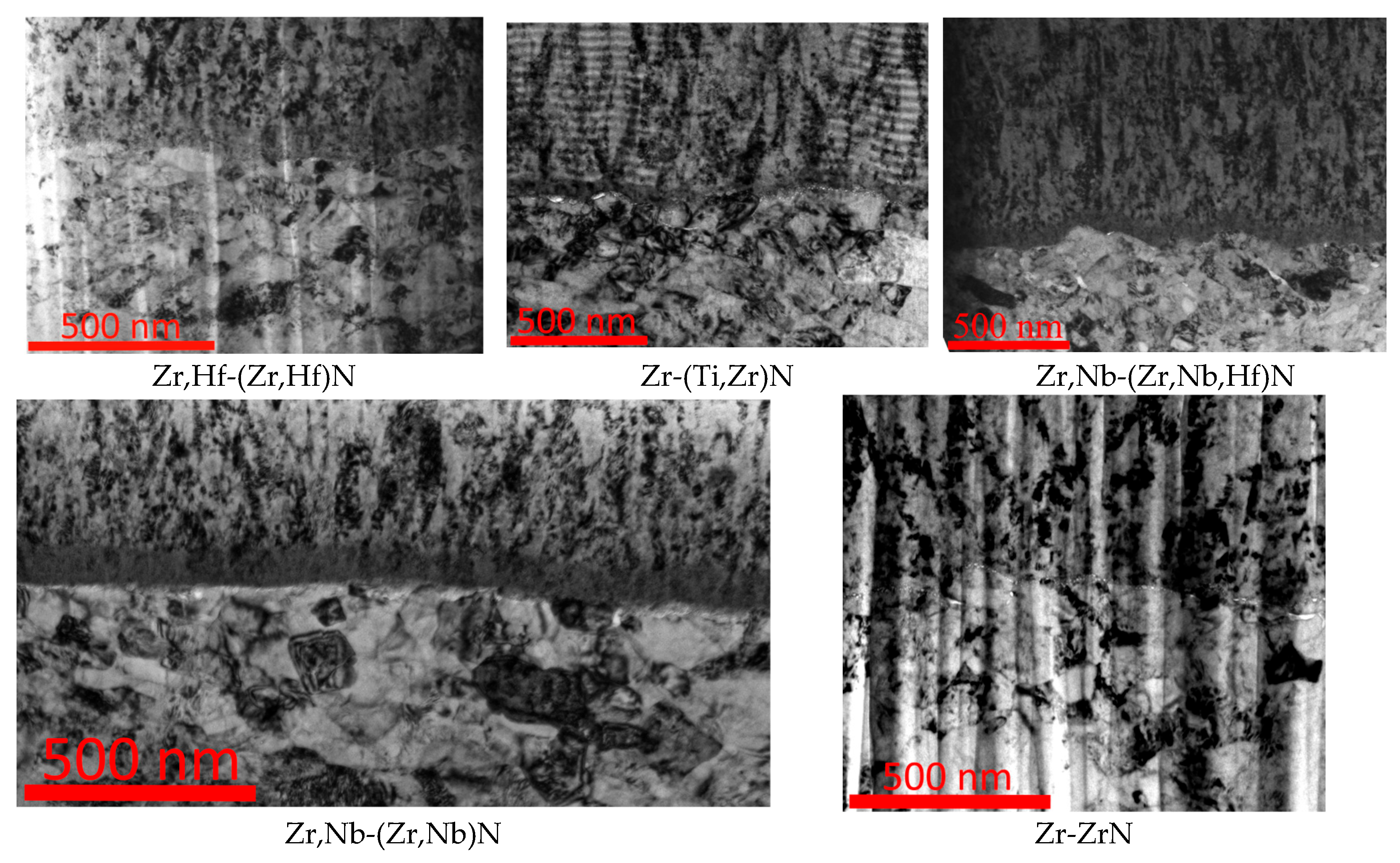
3.4. Elemental Distribution on the Surface of Coated Samples After Corrosion Exposure
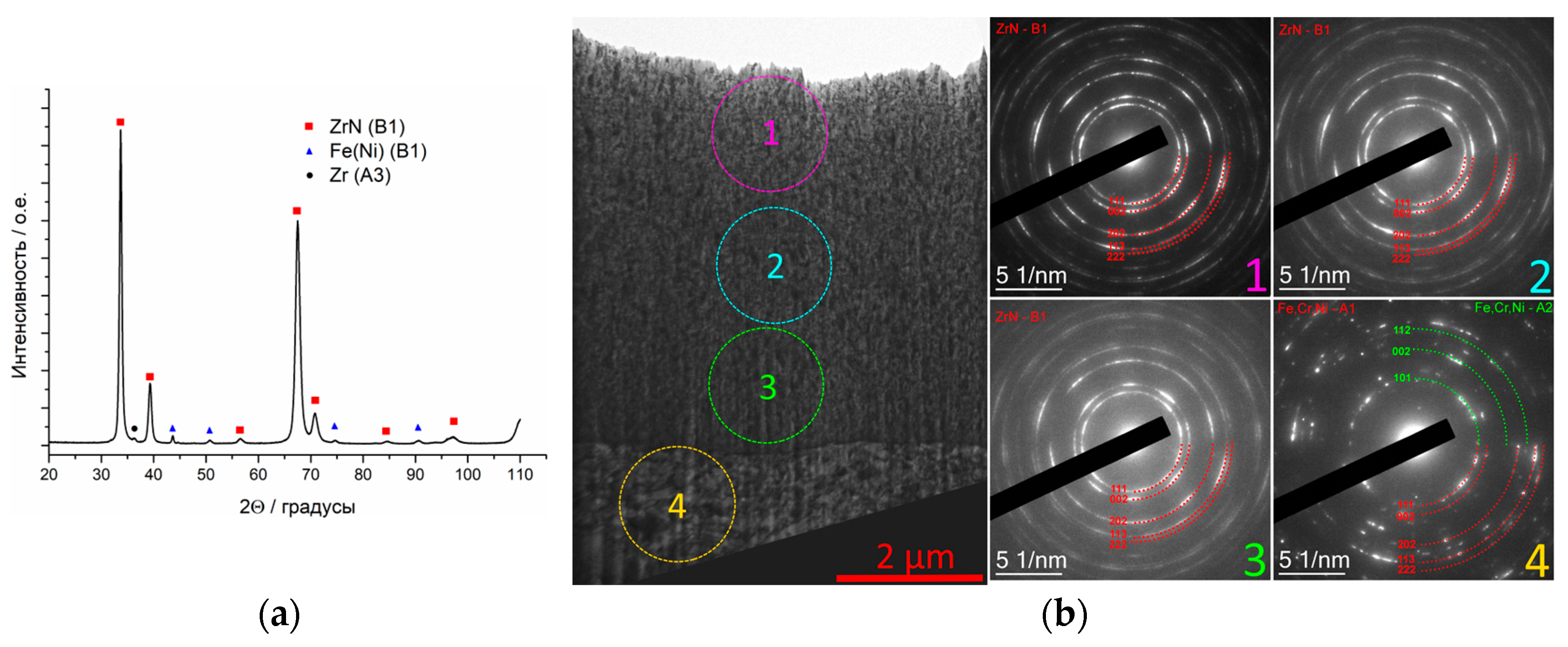
3.4.1. Coating ZrN
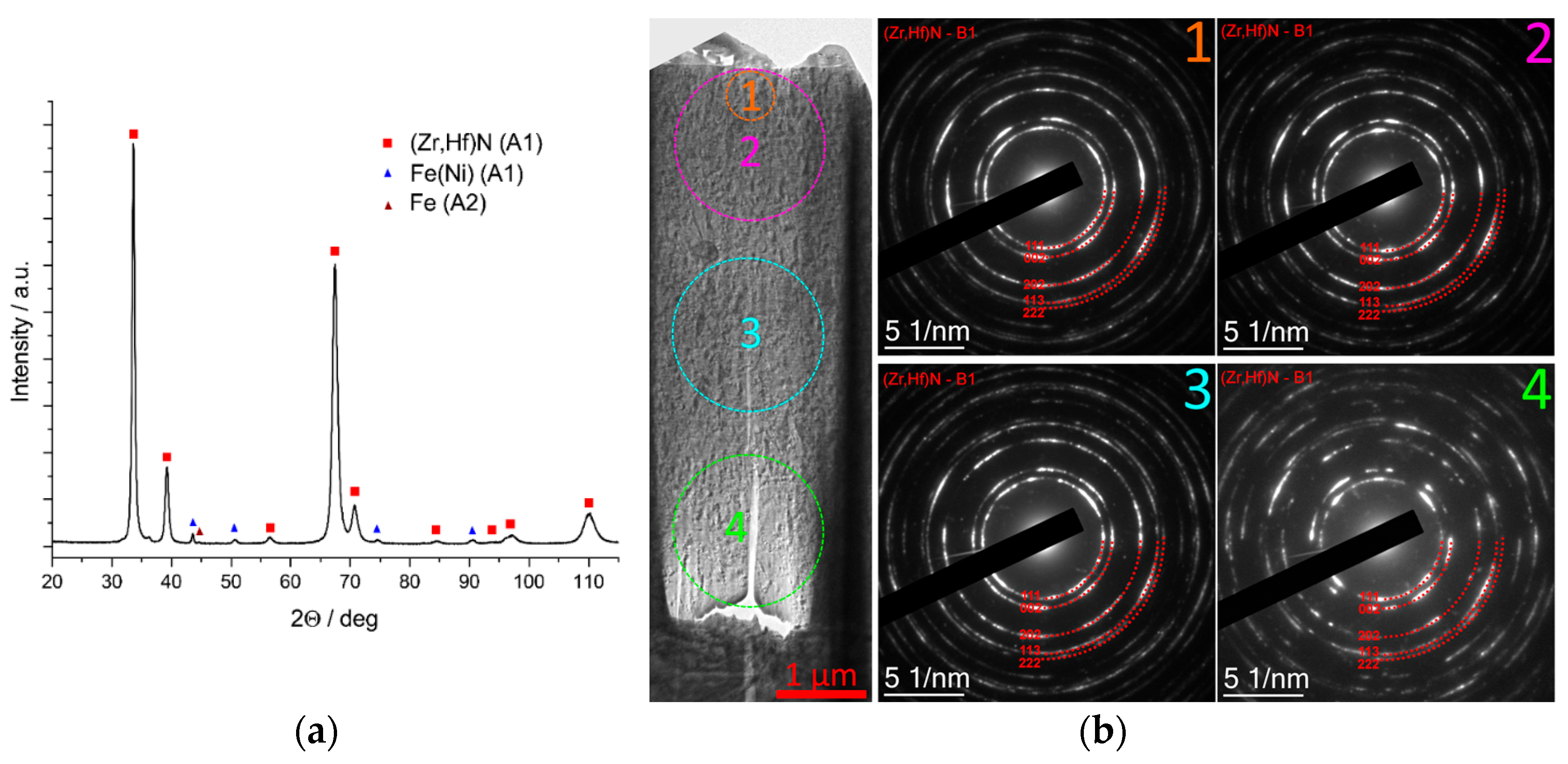
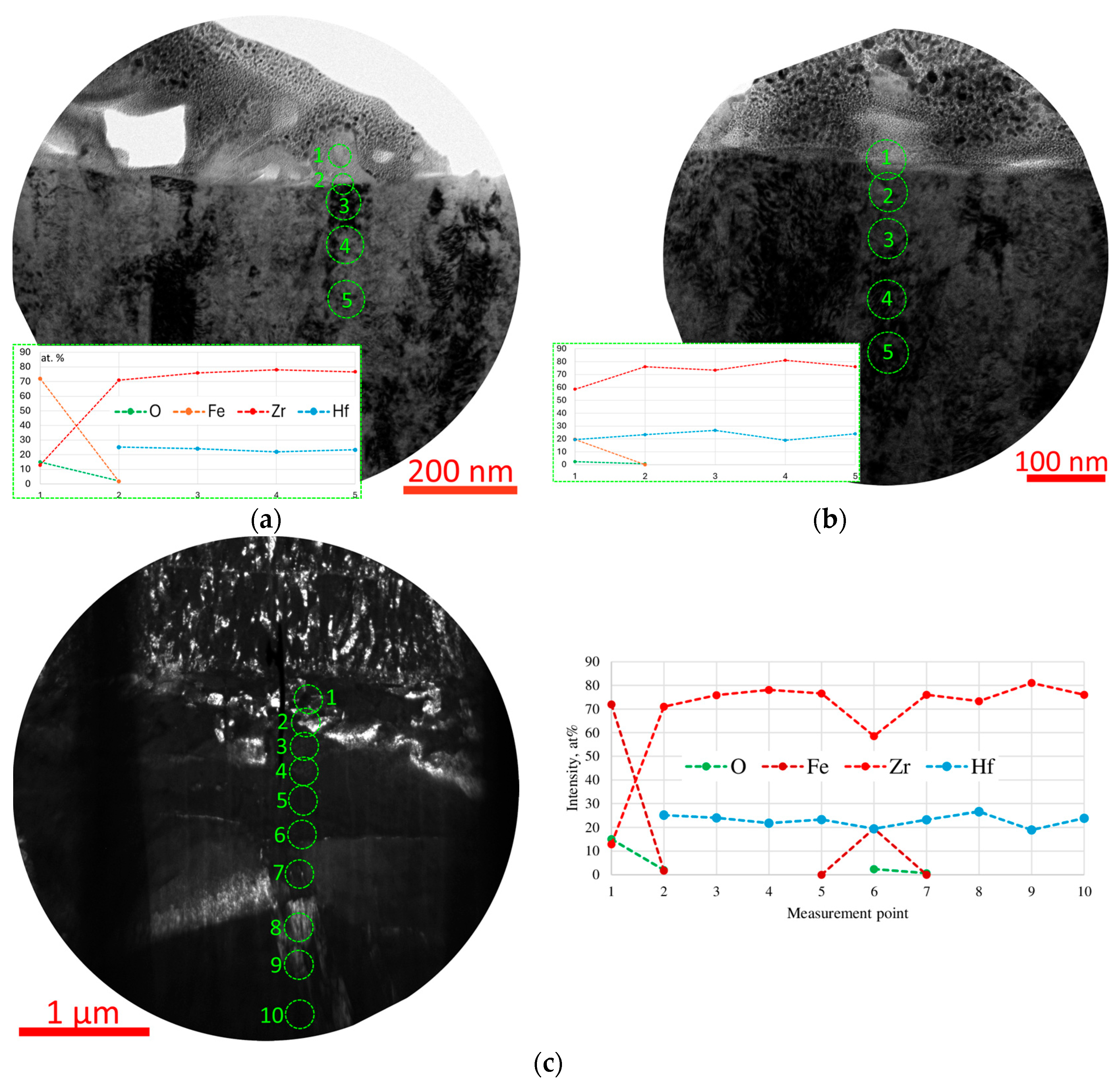
3.4.2. Coating Zr,Hf-(Zr,Hf)N
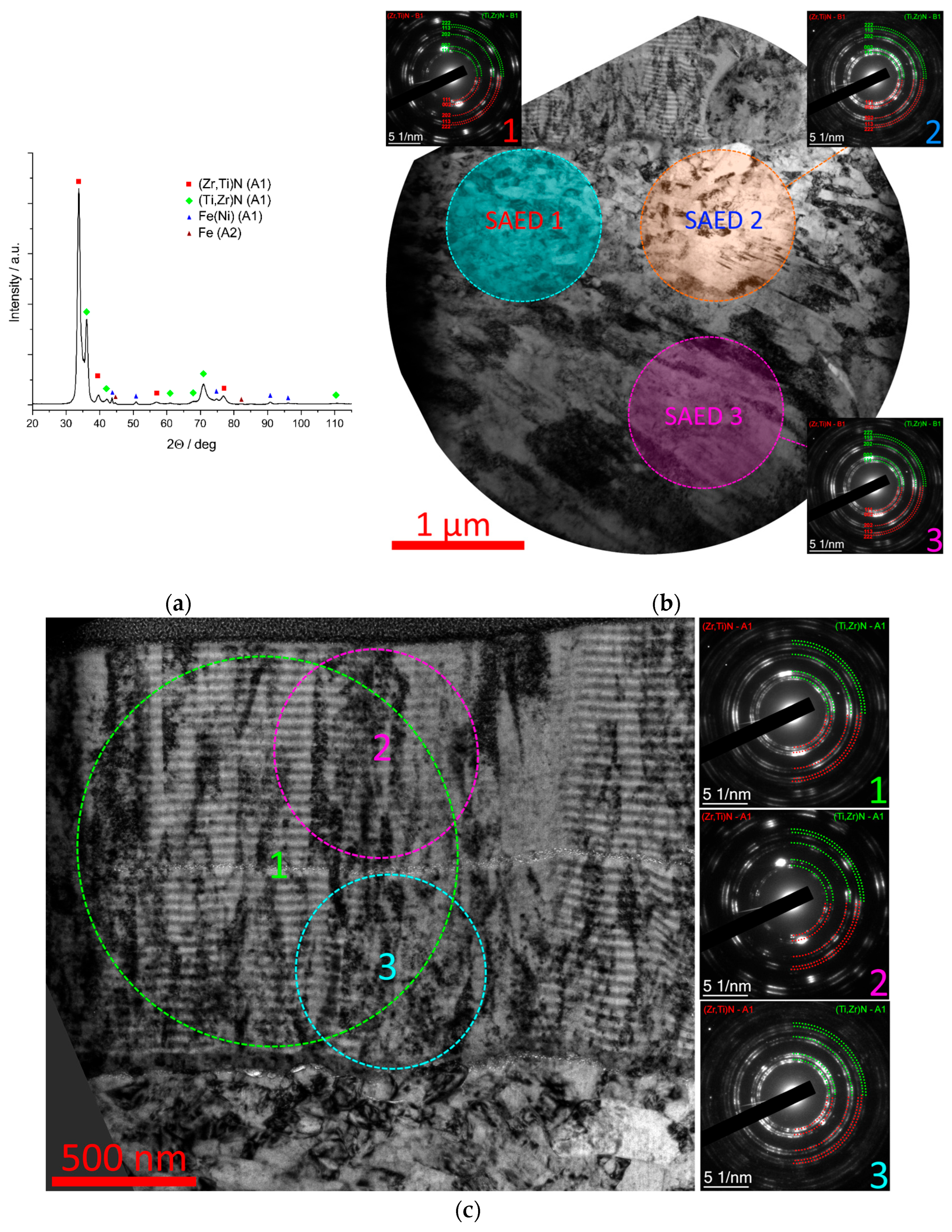
3.4.3. Coating Zr-(Ti,Zr)N
3.4.4. Coating Zr,Nb-(Zr,Nb,Hf)N
3.4.5. Coating Zr,Nb-(Zr,Nb)N
4. Discussion
5. Conclusions
- The (Zr,Hf)N coating had the highest hardness, and the (Zr,Nb)N coating had the lowest hardness. The (Zr,Nb)N coating demonstrated the highest adhesion strength to the substrate (based on the total coating failure rate, LC2), and the ZrN coating also demonstrated a high LC2 value, whereas the other coatings demonstrated relatively low values.
- The Zr-ZrN, Zr,Nb-(Zr,Nb)N, Zr-(Zr,Hf)N, Zr,Hf-(Zr,Hf,Nb)N, and Zr-(Zr,Ti)N coatings provided a 10-fold reduction in the corrosion current density in a 3% aqueous NaCl solution at 25 °C, decreasing from 6.96 for the uncoated AISI 321 steel to 0.17 μA/cm2 for the Zr-(Zr,Ti)N-coated sample.
- The addition of titanium to ZrN resulted in a significant decrease in corrosion current densities from 0.34 to 0.17 μA/cm2, which may be due to the formation of poorly soluble titanium oxide TiO2 on the surface. However, the addition of Nb increased the corrosion rate and reduced the polarisation resistance to corrosion. This may be due to the formation during corrosion of a non-stoichiometric composition of complex oxides NbOx with increased solubility in water due to the acid–base interaction. Hf did not affect the corrosion-protective properties of the nitride coatings.
- Although the presence of a surface oxide layer was directly observed only in the Zr,Hf-(Zr,Hf)N-coated sample, it was likely present in the other samples. Further research is necessary to achieve a more pronounced oxide layer, allowing for more detailed investigation.
- Although the Zr,Hf-(Zr,Hf,Nb)N coating provided the best wear resistance and the lowest friction coefficient in the ball-on-disk test, consistent with previous work [56], this coating is not preferred for corrosion protection. In terms of corrosion resistance, the highest performance was provided by the Zr-(Zr,Ti)N, Zr-ZrN, and Zr-(Zr,Hf)N coatings. Thus, in terms of both high corrosion resistance and high wear resistance, the Zr-(Zr,Hf)N coating is recommended. In the absence of noticeable mechanical wear, the Zr-(Zr,Ti)N coating is the most appropriate for corrosion protection in NaCl solutions (e.g., seawater).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiiala, U.K.; Penttinen, I.M.; Korhonen, A.S.; Aromaa, J.; Ristolainen, E. Improved corrosion resistance of physical vapour deposition coated TiN and Zr-ZrN. Surf. Coat. Technol. 1990, 41, 191–204. [Google Scholar] [CrossRef]
- Brown, R.; Alias, M.N.; Fontana, R. Effect of composition and thickness on corrosion behavior of TiN and Zr-ZrN thin films. Surf. Coat. Technol. 1993, 62, 467–473. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, Q.; Zhu, X.; Ma, D.; Ma, F.; Song, Z.; Fu, Y.Q. Corrosion performance of Zr-ZrN/ZrO2 multilayer coatings deposited on 304 stainless steel using multi-arc ion plating. Appl. Surf. Sci. 2018, 431, 170–176. [Google Scholar] [CrossRef]
- Chou, W.-J.; Yu, G.P.; Huang, J.-H. Corrosion resistance of Zr-ZrN films on AISI 304 stainless steel substrate. Surf. Coat. Technol. 2003, 167, 59–67. [Google Scholar] [CrossRef]
- Yi, P.; Zhu, L.; Dong, C.; Xiao, K. Corrosion and interfacial contact resistance of 316L stainless steel coated with magnetron sputtered Zr-ZrN and TiN in the simulated cathodic environment of a proton-exchange membrane fuel cell. Surf. Coat. Technol. 2019, 363, 198–202. [Google Scholar] [CrossRef]
- Dur, E.; Cora, Ö.N.; Koç, M. Effect of manufacturing process sequence on the corrosion resistance characteristics of coated metallic bipolar plates. J. Power Sources 2014, 246, 788–799. [Google Scholar] [CrossRef]
- Lin, M.-T.; Wan, C.-H.; Wu, W. Enhanced corrosion resistance of SS304 stainless steel and titanium coated with alternate layers of TiN and Zr-ZrN in a simulated O2-rich environment of a unitized regenerative fuel cell. Int. J. Electrochem. Sci. 2014, 9, 7832–7845. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Huang, J.-H.; Yu, G.-P. Microstructure and corrosion resistance of nanocrystalline TiZr-ZrN films on AISI 304 stainless steel substrate. J. Vac. Sci. Technol. A Vac. Surf. Film. 2010, 28, 774–778. [Google Scholar] [CrossRef]
- Xi, Y.; Wan, L.; Hou, J.; Wang, Z.; Wang, L.; Yang, D.; Liu, D.; Lei, S. Improvement of erosion-corrosion behavior of AISI 420 stainless steel by ion-assisted deposition Zr-ZrN coatings. Metals 2021, 11, 1811. [Google Scholar] [CrossRef]
- Das Mercês Reis De Castro, M.; Ferreira, C.P.; Da Silva, B.P.; Tentardini, E.K.; De Freitas Cunha Lins, V. Corrosion performance evaluation of Zr-ZrN and ZrSiN coatings deposited on steel surface by magnetron sputtering. Eur. Corros. Congr. Eurocorr. 2016, 2, 1417–1424. [Google Scholar]
- Ferreira, C.P.; Castro, M.D.M.R.D.; Tentardini, E.K.; Lins, V.D.F.C.; Saliba, P.A. Silicon influence on corrosion resistance of magnetron sputtered Zr-ZrN and ZrSiN thin films. Surf. Eng. 2020, 36, 33–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, M.; Gyawali, G.; Zhang, T.F.; Zhang, S. Effect of nitrogen pressure on the microstructure, conductivity, and corrosion resistance of Zr-ZrN-coated stainless steel as bipolar plate for proton exchange membrane fuel cell. Ceram. Int. 2024, 50, 44678–44688. [Google Scholar] [CrossRef]
- Huang, J.-H.; Hsu, C.-Y.; Chen, S.-S.; Yu, G.-P. Effect of substrate bias on the structure and properties of ion-plated Zr-ZrN on Si and stainless steel substrates. Mater. Chem. Phys. 2003, 77, 14–21. [Google Scholar] [CrossRef]
- Huang, J.-H.; Kuo, K.-L.; Yu, G.-P. Oxidation behavior and corrosion resistance of vacuum annealed Zr-ZrN-coated stainless steel. Surf. Coat. Technol. 2019, 358, 308–319. [Google Scholar] [CrossRef]
- Samim, P.M.; Fattah-Alhosseini, A.; Elmkhah, H.; Imantalab, O. A study on the corrosion resistance of Zr-ZrN/CrN multilayer nanostructured coating applied on AISI 304 stainless steel using Arc-PVD method in 3.5 wt% NaCl solution. Mater. Res. Express 2019, 6, 126426. [Google Scholar] [CrossRef]
- Mohamadian Samim, P.; Fattah-Alhosseini, A.; Elmkhah, H.; Imantalab, O. Structure and corrosion behavior of Zr-ZrN/CrN nano-multilayer coating deposited on AISI 304 stainless steel by CAE-PVD technique. J. Asian Ceram. Soc. 2020, 8, 460–469. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, M.; Shi, H.; Wang, Y.; Wang, Z.; Cheng, Y.; Liu, M. CrZr-ZrN/Zr-ZrN multilayer coatings on 316L stainless steel towards anticorrosion application. Thin Solid Film. 2022, 755, 139330. [Google Scholar] [CrossRef]
- Rajabi, T.; Atapour, M.; Elmkhah, H.; Nahvi, S.M. Nanometric CrN/CrAlN and CrN/Zr-ZrN multilayer physical vapor deposited coatings on 316L stainless steel as bipolar plate for proton exchange membrane fuel cells. Thin Solid Film. 2022, 753, 139288. [Google Scholar] [CrossRef]
- De Sánchez, N.A.; Jaramillo, H.E.; Vivas, Z.; Aperador, W.; Amaya, C.; Caicedo, J.C. Fracture resistant and wear corrosion performance of CrN/Zr-ZrN bilayers deposited onto AISI 420 stainless steel. Adv. Mater. Res. 2008, 38, 63–75. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Prakash, M.S.; Poojari, A.; Rajam, K.S. Corrosion behavior of nanolayered TiN/NbN multilayer coatings prepared by reactive direct current magnetron sputtering process. Thin Solid Film. 2004, 460, 133–142. [Google Scholar] [CrossRef]
- Subramanian, B. Enhancement of biocompatibility of metal implants by nanoscale TiN/NbN multilayer coatings. J. Nanosci. Nanotechnol. 2013, 13, 4565–4572. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Rajam, K.S. Structure and properties of reactive DC magnetron sputtered TiN/NbN hard superlattices. Surf. Coat. Technol. 2004, 183, 174–183. [Google Scholar] [CrossRef]
- Purandare, Y.P.; Stack, M.M.; Hovsepian, P.E. Velocity effects on erosion-corrosion of CrN/NbN superlattice PVD coatings. Surf. Coat. Technol. 2006, 201, 361–370. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Li, J.; Chen, Z.; Sun, D. Effects of phase transformation on the corrosion resistance and conductivity of NbN coatings for metal bipolar plates. Surf. Coat. Technol. 2025, 497, 131800. [Google Scholar] [CrossRef]
- Monticelli, C.; Balbo, A.; Zucchi, F. Corrosion and tribocorrosion behaviour of cermet and cermet/nanoscale multilayer CrN/NbN coatings. Surf. Coat. Technol. 2010, 204, 1452–1460. [Google Scholar] [CrossRef]
- Purandare, Y.; Stack, M.M.; Hovsepian, P. A study of the erosion-corrosion of PVD CrN/NbN superlattice coatings in aqueous slurries. Wear 2005, 259, 256–262. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Lewis, D.B.; Luo, Q.; Farinotti, A. Corrosion resistance of CrN/NbN superlattice coatings grown by various physical vapour deposition techniques. Thin Solid Film. 2005, 488, 1–8. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Lewis, D.B.; Münz, W.D.; Lyon, S.B.; Tomlinson, M. Combined cathodic arc/unbalanced magnetron grown CrN/NbN superlattice coatings for corrosion resistant applications. Surf. Coat. Technol. 1999, 120–121, 535–541. [Google Scholar] [CrossRef]
- Lewis, D.B.; Creasey, S.J.; Wüstefeld, C.; Ehiasarian, A.P.; Hovsepian, P.E. The role of the growth defects on the corrosion resistance of CrN/NbN superlattice coatings deposited at low temperatures. Thin Solid Film. 2006, 503, 143–148. [Google Scholar] [CrossRef]
- Chen, M.; Ding, J.C.; Kwon, S.-H.; Wang, Q.; Zhang, S. Corrosion resistance and conductivity of NbN-coated 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2022, 196, 110042. [Google Scholar] [CrossRef]
- Carvalho, R.G.; Fonseca, R.M.; Lins, V.D.F.C.; Castro, M.D.M.R.D.; Tentardini, E.K. Corrosion resistance of NbN and NbxAlyN coatings on stainless steel. Surf. Eng. 2021, 37, 1579–1585. [Google Scholar] [CrossRef]
- Huang, W.; Zalnezhad, E.; Musharavati, F.; Jahanshahi, P. Investigation of the tribological and biomechanical properties of CrAlTiN and CrN/NbN coatings on SST 304. Ceram. Int. 2017, 43, 7992–8003. [Google Scholar] [CrossRef]
- Fonseca, R.M.; Soares, R.B.; Carvalho, R.G.; Tentardini, E.K.; Lins, V.F.C.; Castro, M.M.R. Corrosion behavior of magnetron sputtered NbN and Nb1-xAlxN coatings on AISI 316L stainless steel. Surf. Coat. Technol. 2019, 378, 124987. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, Z.; Wei, B.; Shen, H.; Wang, Z. Microstructure and corrosion behaviors of conductive Hf/HfN multilayer coatings on magnesium alloys. Ceram. Int. 2018, 44, 9958–9966. [Google Scholar] [CrossRef]
- Escobar, C.; Villarreal, M.; Caicedo, J.C.; Aperador, W.; Prieto, P. Novel performance in physical and corrosion resistance HfN/VN coating system. Surf. Coat. Technol. 2013, 221, 182–190. [Google Scholar] [CrossRef]
- Bian, S.; Chen, C.; Yu, L.; Lu, G.; Xu, J. Improvement of structures and properties of hafnium nitride coatings by yttrium introduction. Surf. Coat. Technol. 2024, 478, 130358. [Google Scholar] [CrossRef]
- He, T.; Valery, Z.; Vereschaka, A.; Keshin, A.; Huo, Y.; Milovich, F.; Sotova, C.; Seleznev, A. Influence of niobium and hafnium doping on the wear and corrosion resistance of coatings based on ZrN. J. Mater. Res. Technol. 2023, 27, 6386–6399. [Google Scholar] [CrossRef]
- Tao, H.; Zhylinski, V.; Vereschaka, A.; Chayeuski, V.; Yuanming, H.; Milovich, F.; Sotova, C.; Seleznev, A.; Salychits, O. Comparison of the Mechanical Properties and Corrosion Resistance of the Cr-CrN, Ti-TiN, Zr-ZrN, and Mo-MoN Coatings. Coatings 2023, 13, 750. [Google Scholar] [CrossRef]
- Takahashi, M.; Kikuchi, M.; Takada, Y.; Okuno, O. Corrosion resistance of dental Ti-Ag alloys in NaCl solution. Mater. Trans. 2010, 51, 762–766. [Google Scholar] [CrossRef]
- Nichul, U.; Tambe, P.; Hiwarkar, V. Electrochemical Behaviour of Textured Beta C Titanium Alloy in 3.5% NaCl Solution. Arch. Metall. Mater. 2024, 69, 571–577. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Peng, X.; Shan, C. Corrosion behavior of three titanium alloys in 3.5%NaCl solution. J. Chin. Soc. Corros. Prot. 2015, 35, 75–80. [Google Scholar]
- Grigoriev, S.; Volosova, M.; Zhylinski, V.; Sotova, C.; Milovich, F.; Seleznev, A.; Pianka, H.; Makarevich, K.; Potapov, P.; Vereschaka, A. Increasing the Corrosion Resistance of Austenitic Stainless Steel Products by Depositing Vanadium Nitride-Based Coatings on Their Surface. J. Compos. Sci. 2025, 9, 498. [Google Scholar] [CrossRef]
- Olsson, C.O.A. Passivation of Stainless Steels and Other Chromium Bearing Alloys. In Encyclopedia of Interfacial Chemistry Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 357–364. [Google Scholar]
- Mansfeld, F. The Polarization Resistance Technique for Measuring Corrosion Currents. In Advances in Corrosion Science and Technology; Fontana, G., Staehle, R.W., Eds.; Plenum Press: New York, NY, USA, 1976; Volume 6, p. 163. [Google Scholar]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Andreev, N.; Bublikov, J.; Kutina, N. Investigation of the properties of the Cr,Mo-(Cr,Mo,Zr,Nb)N-(Cr,Mo,Zr,Nb,Al)N multilayer composite multicomponent coating with nanostructured wear-resistant layer. Wear 2021, 468–469, 203597. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Bublikov, J.I.; Sitnikov, N.N.; Oganyan, G.V.; Sotova, C.S. Influence of nanolayer thickness on the perfor-mance properties of multilayer composite nano-structured modified coatings for metal-cutting tools. Int. J. Adv. Manuf. Technol. 2018, 95, 2625–2640. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Migranov, M.; Andreev, N.; Bublikov, J.; Sitnikov, N.; Oganyan, G. Investigation of the tribological properties of Ti-TiN-(Ti,Al,Nb,Zr)N composite coating and its efficiency in increasing wear resistance of metal cutting tools. Tribol. Int. 2021, 164, 107236. [Google Scholar] [CrossRef]
- Vereschaka, A.S.; Grigoriev, S.N.; Sotova, E.S.; Vereschaka, A.A. Improving the efficiency of the cutting tools made of mixed ceramics by applying modifying nano-scale multilayered coatings. Adv. Mat. Res. 2013, 712–715, 391–394. [Google Scholar]
- Volosova, M.; Grigoriev, S.; Metel, A.; Shein, A. The Role of Thin-Film Vacuum-Plasma Coatings and Their Influence on the Efficiency of Ceramic Cutting Inserts. Coatings 2018, 8, 287. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Vereschaka, A.A.; Sitnikov, N.N.; Milovich, F. Properties of (Cr,Al,Si)N-(DLC-Si) composite coatings deposited on a cutting ceramic substrate. Ceram. Int. 2020, 46, 18241–18255. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Seleznev, A.; Sotova, C.; Bublikov, J. Influence of the yttrium cathode arc current on the yttrium content in the (Ti,Y,Al) N coating and the coating properties. Vacuum 2024, 222, 113028. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Zelenkov, V.; Sitnikov, N.; Bublikov, J.; Milovich, F.; Andreev, N.; Sotova, C. Investigation of the influence of the features of the deposition process on the structural features of microparticles in PVD coatings. Vacuum 2022, 202, 111144. [Google Scholar] [CrossRef]
- ASTM C1624-05; Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Poorqasemi, E.; Abootalebi, O.; Peikari, M.; Haqdar, F. Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 2009, 51, 1043–1054. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 265–291. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Vereschaka, A.S.; Bublikov, J.I.; Aksenenko, A.Y.; Sitnikov, N.N. Study of properties of nanostructured multilayer composite coatings of Ti-TiN-(TiCrAl)N and Zr-ZrN-(ZrNbCrAl)N. J. Nano Res. 2016, 40, 90–98. [Google Scholar] [CrossRef]
- Volosova, M.; Zhylinski, V.; Sotova, C.; Milovich, F.; Seleznev, A.; Pyanka, H.; Makarevich, K.; Vereschaka, A. Features of the Application of Coatings Based on the ZrN System to Increase Resistance to Mechanical Wear and Corrosion of Titanium Alloy Products. Coatings 2024, 14, 1304. [Google Scholar] [CrossRef]
- Roman, D.; Bernardi, J.; de Amorim, C.L.; de Souza, F.S.; Spinelli, A.; Giacomelli, C.; Figueroa, C.A.; Baumvol, I.J.; Basso, R.L. Effect of deposition temperature on microstructure and corrosion resistance of ZrN thin films deposited by DC reactive magnetron sputtering. Mater. Chem. Phys. 2011, 130, 147–153. [Google Scholar] [CrossRef]
- Ma, Z.-K.; Gao, Y.; Cai, H.-W.; Wang, C.; Yuan, L.; Zhang, Y.; Wu, W.-Q. Corrosion resistance of TiN and CrN coatings with arc ion plating on 201 stainless steel surface. Corros. Prot. 2013, 34, 670–672. [Google Scholar]
- Bekermann, D.; Barreca, D.; Gasparotto, A.; Becker, H.W.; Fischer, R.A.; Devi, A. Investigation of niobium nitride and oxy-nitride films grown by MOCVD. Surf. Coat. Technol. 2009, 204, 404–409. [Google Scholar] [CrossRef]
- Lukaszkowicz, K.; Dobrzański, L.A. Structure and mechanical properties of gradient coatings deposited by PVD technology onto the X40CrMoV5-1 steel substrate. J. Mater. Sci. 2008, 43, 3400–3407. [Google Scholar] [CrossRef]
- Lukaszkowicz, K.; Sondor, J.; Kriz, A.; Pancielejko, M. Structure, mechanical properties and corrosion resistance of nanocomposite coatings deposited by PVD technology onto the X6CrNiMoTi17-12-2 and X40CrMoV5-1 steel substrates. J. Mater. Sci. 2010, 45, 1629–1637. [Google Scholar] [CrossRef]
- Grigoriev, S.; Volosova, M.; Sotova, C.; Milovich, F.; Seleznev, A.; Makarevich, K.; Potapov, P.; Vereschaka, A. Increasing the Wear Resistance of Stainless Steel Products by Depositing Modifying Coatings Based on Zirconium Nitride with the Addition of Niobium, Hafnium, and Titanium. J. Manuf. Mater. Process. 2025, 9, 316. [Google Scholar] [CrossRef]
- Lukaszkowicz, K.; Konieczny, J. Microstructure and mechanical properties of PVD nanoncrystalline layer. Solid State Phenom. 2012, 186, 230–233. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Liu, Q.; Su, Y.; Wu, J.; Xie, Z.; Guo, J.; Zhu, W. Effect of interface roughness on the CMAS + molten salt (Na2SO4 + NaCl + NaVO3) corrosion resistance of EB-PVD thermal barrier coatings. Surf. Coat. Technol. 2025, 503, 13202. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, W.; Liu, S.; Guo, J.; Ma, Z. Real-time acoustic emission detection of damage mode and failure of EB-PVD thermal barrier coating under CMAS and sea salt corrosion. J. Eur. Ceram. Soc. 2025, 45, 117404. [Google Scholar] [CrossRef]
- Wang, X.; Pei, Y.; Ma, Y. The effect of microstructure at interface between coating and substrate on damping capacity of coating systems. Appl. Surf. Sci. 2013, 282, 60–66. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhu, J.-F.; Zhong, Z.-Q.; Gu, J.-H. Cohesive failure and film adhesion of PVD coating: Cemented carbide substrate phase effect and its micro-mechanism. Int. J. Refract. Met. Hard Mater. 2023, 111, 106066. [Google Scholar] [CrossRef]
- Tillmann, W.; Hagen, L.; Stangier, D.; Dias, N.F.L.; Görtz, J.; Kensy, M.D. Lapping and polishing of additively manufactured 316L substrates and their effects on the microstructural evolution and adhesion of PVD CrAlN coatings. Surf. Coat. Technol. 2021, 428, 127905. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, Q.; Jian, Q.; Qiu, X.; Li, C. Effect of laser interface pretreatment on the microstructure, adhesion strength, and corrosion resistance of PVD-AlTiN coatings on Mg alloy substrates. Surf. Coat. Technol. 2025, 516, 13267. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Guo, X.; Wang, C.; Sun, L. Preparation of micro-textures on cemented carbide substrate surface by plasma-assisted laser machining to enhance the PVD tool coatings adhesion. J. Mater. Process. Technol. 2021, 288, 116870. [Google Scholar] [CrossRef]
- Teles, V.C.; de Mello, J.D.B.; da Silva, W.M. Abrasive wear of multilayered/gradient CrAlSiN PVD coatings: Effect of interface roughness and of superficial flaws. Wear 2017, 376–377, 1691–1701. [Google Scholar] [CrossRef]
- Herrera-Jimenez, E.J.; Bousser, E.; Schmitt, T.; Klemberg-Sapieha, J.E.; Martinu, L. Effect of plasma interface treatment on the microstructure, residual stress profile, and mechanical properties of PVD TiN coatings on Ti-6Al-4V substrates. Surf. Coat. Technol. 2021, 413, 127058. [Google Scholar] [CrossRef]
- Sveen, S.; Andersson, J.M.; M’Saoubi, R.; Olsson, M. Scratch adhesion characteristics of PVD TiAlN deposited on high speed steel, cemented carbide and PCBN substrates. Wear 2013, 308, 133–141. [Google Scholar] [CrossRef]


| Element | Mass Fraction (%) |
|---|---|
| Si | Max 1.0 |
| C | Max 0.08 |
| P | Max 0.045 |
| Mn | Max 2.0 |
| Cr | 17–19 |
| S | Max 0.03 |
| Ni | 9–11 |
| Ti | 0.5 |
| Fe | Rest |
| Coating | Elemental Composition [at.%] | Hardness | Adhesion Strength to the Substrate, LC2 (N) | |||
|---|---|---|---|---|---|---|
| Zr | Nb | Hf | Ti | |||
| (Zr,Nb)N | 50.5 | 49.5 | − | − | HV 2336 ± 115 | 15 ± 2 |
| ZrN | 100 | − | − | − | HV 2993 ± 145 | 12 ± 2 |
| (Zr,Hf)N | 76.12 | − | 23.88 | − | HV 3350 ± 120 | 7 ± 1 |
| (Zr,Ti)N | 46.58 | − | − | 53.42 | HV 2727 ± 86 | 6 ± 2 |
| (Zr,Nb,Hf)N | 72.07 | 24.87 | 3.05 | − | HV 2860 ± 95 | 6 ± 1 |
| № | Coating | Eoc, V | Ecorr, V | Tafel Plot Extrapolation Method | |||||
|---|---|---|---|---|---|---|---|---|---|
| icorr, μA/cm2 | P, μm/year | βc, V/dec | βa, V/dec | Ecorr calc, V | R0, kΩ | ||||
| 1 | uncoated | −0.492 | −0.759 | 6.96 | 80.8 | 0.15 | 0.45 | −0.767 | 6.9 |
| 3 | Zr-ZrN | −0.529 | −0.516 | 0.25 | 2.9 | 0.11 | 0.11 | −0.519 | 98.0 |
| 4 | Zr,Nb-(Zr,Nb)N | −0.599 | −0.571 | 0.78 | 9.1 | 0.19 | 0.11 | −0.565 | 39.6 |
| 8 | Zr-(Zr,Hf)N | −0.474 | −0.523 | 0.31 | 3.6 | 0.15 | 0.12 | −0.517 | 94.6 |
| 9 | Zr,Hf-(Zr,Hf,Nb)N | −0.578 | −0.559 | 0.45 | 5.2 | 0.16 | 0.10 | −0.562 | 61.9 |
| 10 | Zr-(Zr,Ti)N | −0.484 | −0.563 | 0.16 | 1.9 | 0.15 | 0.16 | −0.563 | 207.0 |
| № | Coating | Ecorr, V | Polarisation Resistance Method | ||
|---|---|---|---|---|---|
| icorr, μA/cm2 | P, μm/year | R0, kΩ cm2 | |||
| 1 | uncoated | −0.759 | 4.77 | 55.4 | 5.5 |
| 3 | Zr-ZrN | −0.516 | 0.34 | 3.9 | 77.8 |
| 4 | Zr,Nb-(Zr,Nb)N | −0.571 | 0.84 | 9.7 | 31.1 |
| 8 | Zr-(Zr,Hf)N | −0.523 | 0.34 | 3.9 | 76.9 |
| 9 | Zr,Hf-(Zr,Hf,Nb)N | −0.559 | 0.57 | 6.6 | 45.8 |
| 10 | Zr-(Zr,Ti)N | −0.563 | 0.17 | 2.0 | 154.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.; Volosova, M.; Zhylinski, V.; Sotova, C.; Milovich, F.; Kalinichenko, A.; Taleb, A.; Eganova, E.; Borovik, T.; Vereschaka, A. Using Coatings Based on the ZrN System to Improve the Corrosion Resistance of Stainless Steel Products. J. Manuf. Mater. Process. 2025, 9, 369. https://doi.org/10.3390/jmmp9110369
Grigoriev S, Volosova M, Zhylinski V, Sotova C, Milovich F, Kalinichenko A, Taleb A, Eganova E, Borovik T, Vereschaka A. Using Coatings Based on the ZrN System to Improve the Corrosion Resistance of Stainless Steel Products. Journal of Manufacturing and Materials Processing. 2025; 9(11):369. https://doi.org/10.3390/jmmp9110369
Chicago/Turabian StyleGrigoriev, Sergey, Marina Volosova, Valery Zhylinski, Catherine Sotova, Filipp Milovich, Alexander Kalinichenko, Abdelhafed Taleb, Elena Eganova, Tatyana Borovik, and Alexey Vereschaka. 2025. "Using Coatings Based on the ZrN System to Improve the Corrosion Resistance of Stainless Steel Products" Journal of Manufacturing and Materials Processing 9, no. 11: 369. https://doi.org/10.3390/jmmp9110369
APA StyleGrigoriev, S., Volosova, M., Zhylinski, V., Sotova, C., Milovich, F., Kalinichenko, A., Taleb, A., Eganova, E., Borovik, T., & Vereschaka, A. (2025). Using Coatings Based on the ZrN System to Improve the Corrosion Resistance of Stainless Steel Products. Journal of Manufacturing and Materials Processing, 9(11), 369. https://doi.org/10.3390/jmmp9110369









