Effect of Geometry on the Dissolution Behaviour of Complex Additively Manufactured Tablets
Abstract
1. Introduction
2. Materials
3. Methodology
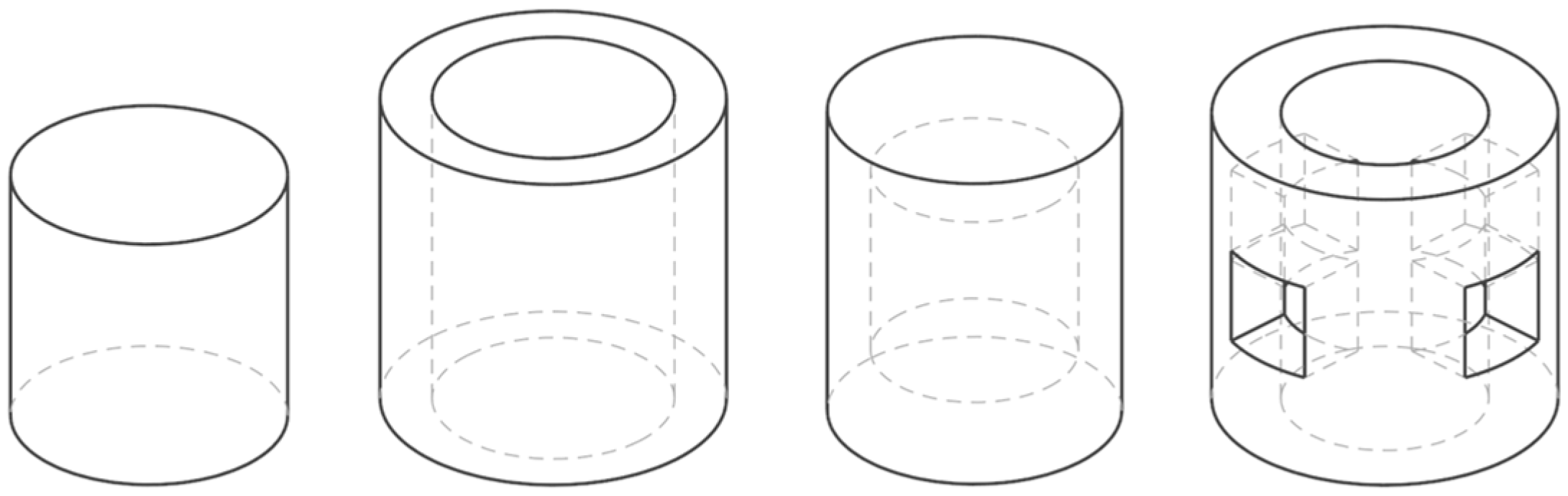
3.1. Design and Fabrication of the Specimens
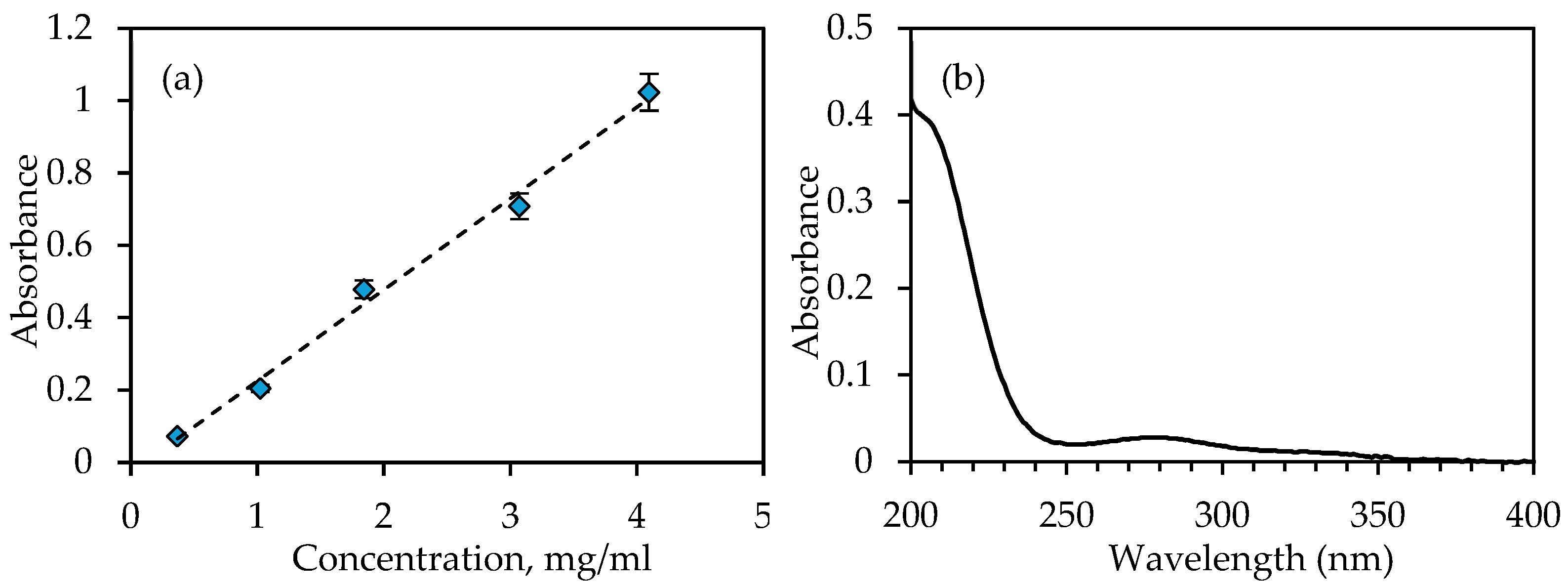
3.2. Dissolution Testing
4. Results and Discussion
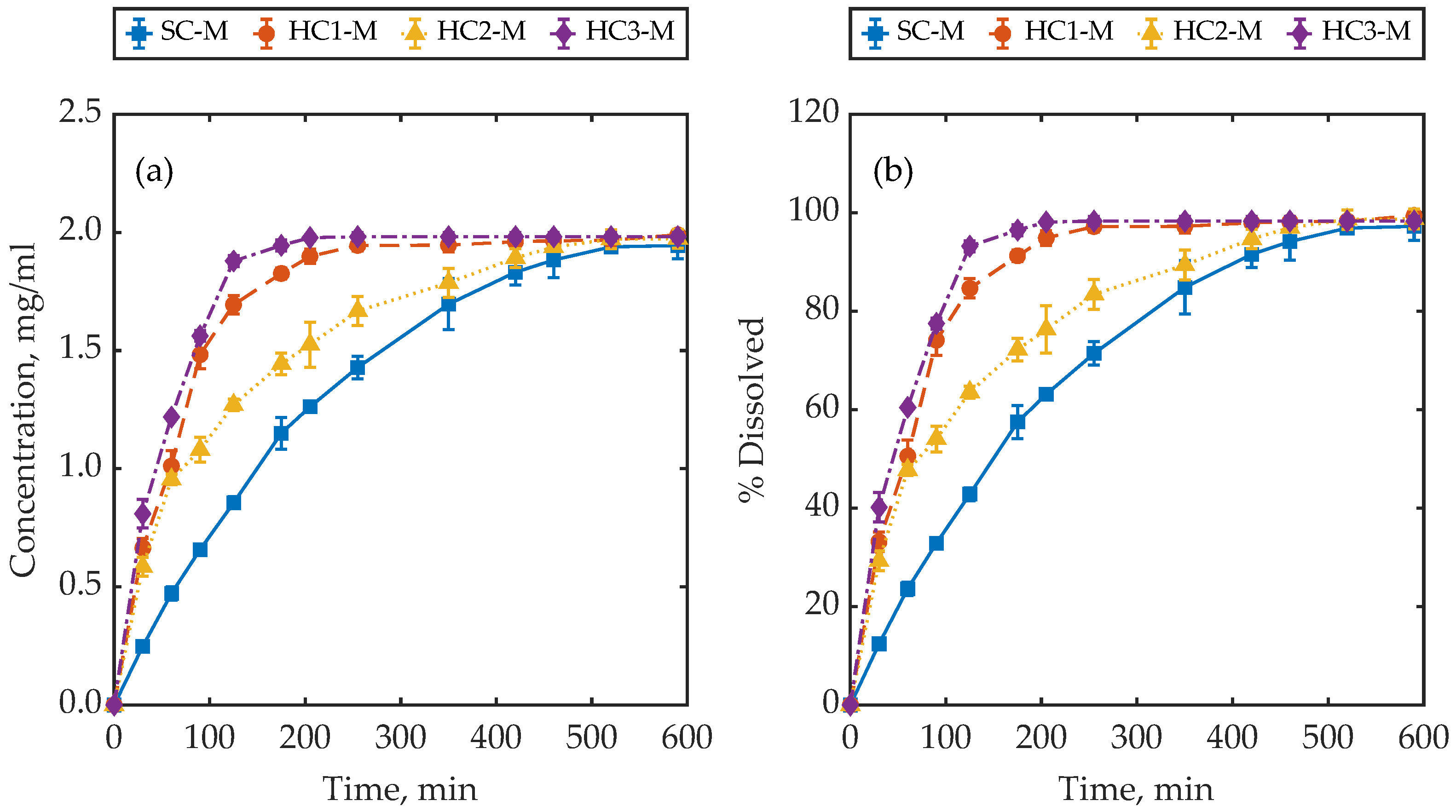
4.1. Dissolution Behaviour of Samples with a Similar Mass
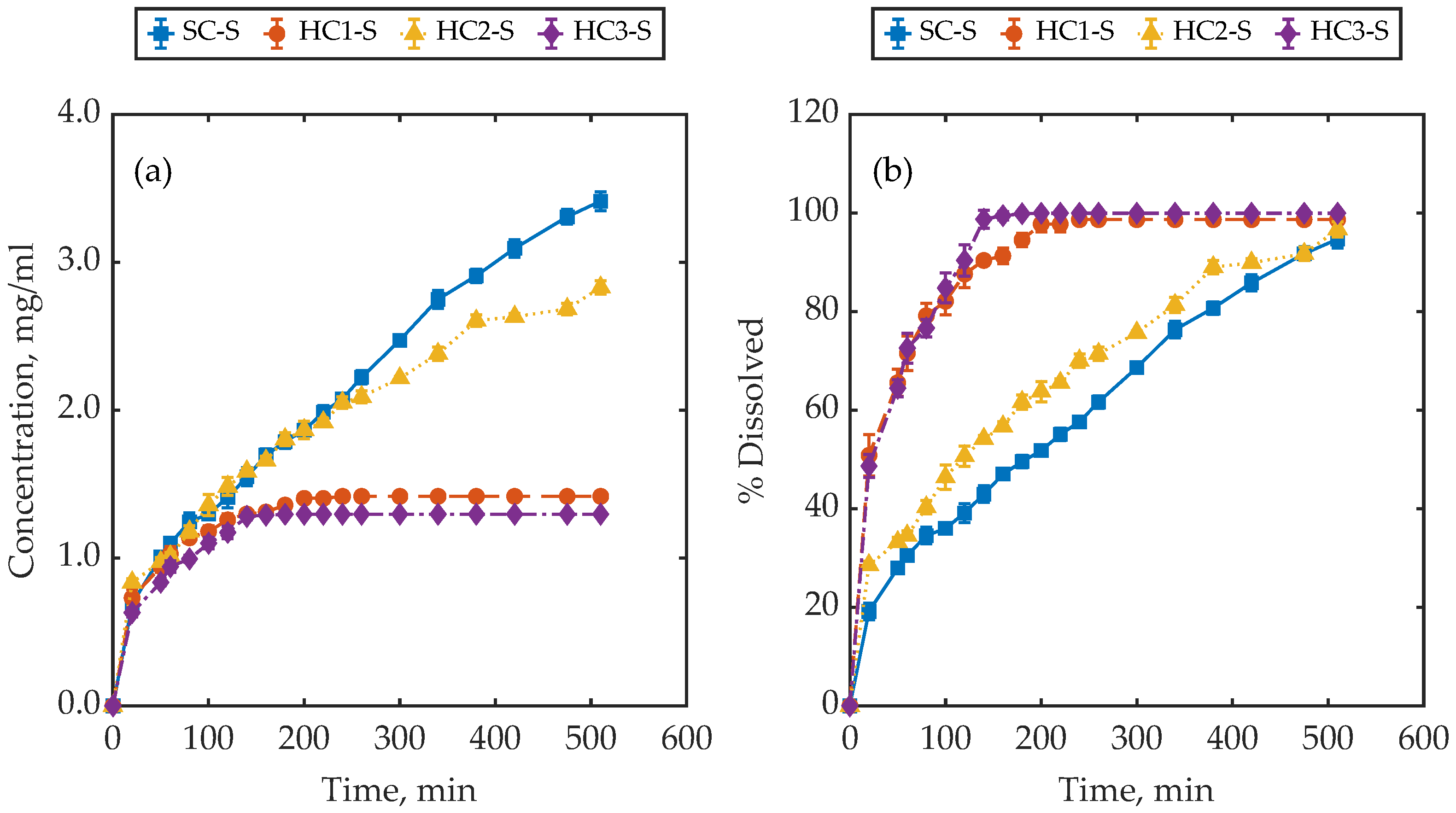
4.2. Dissolution Behaviour of Samples with a Similar Surface Area
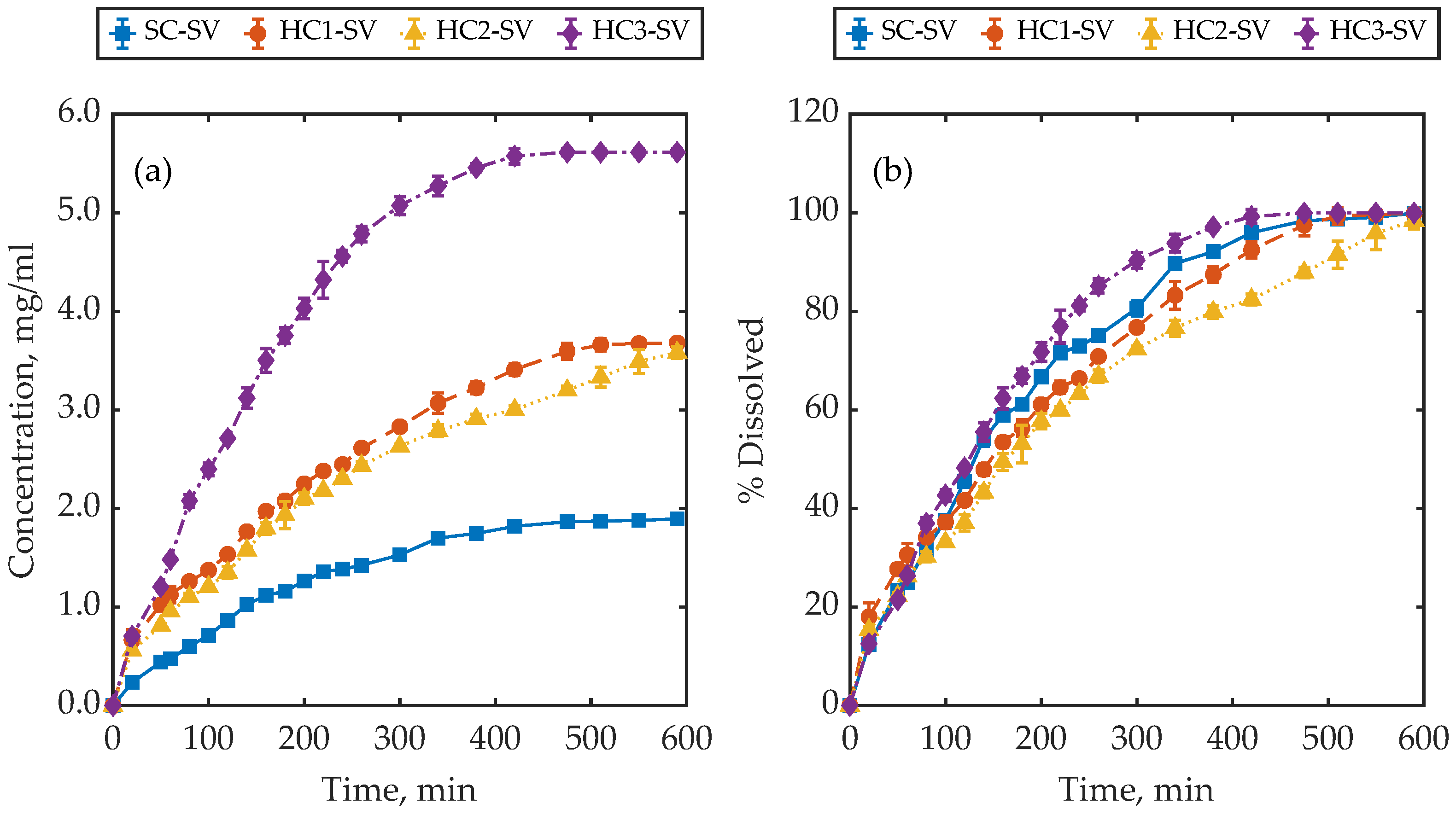
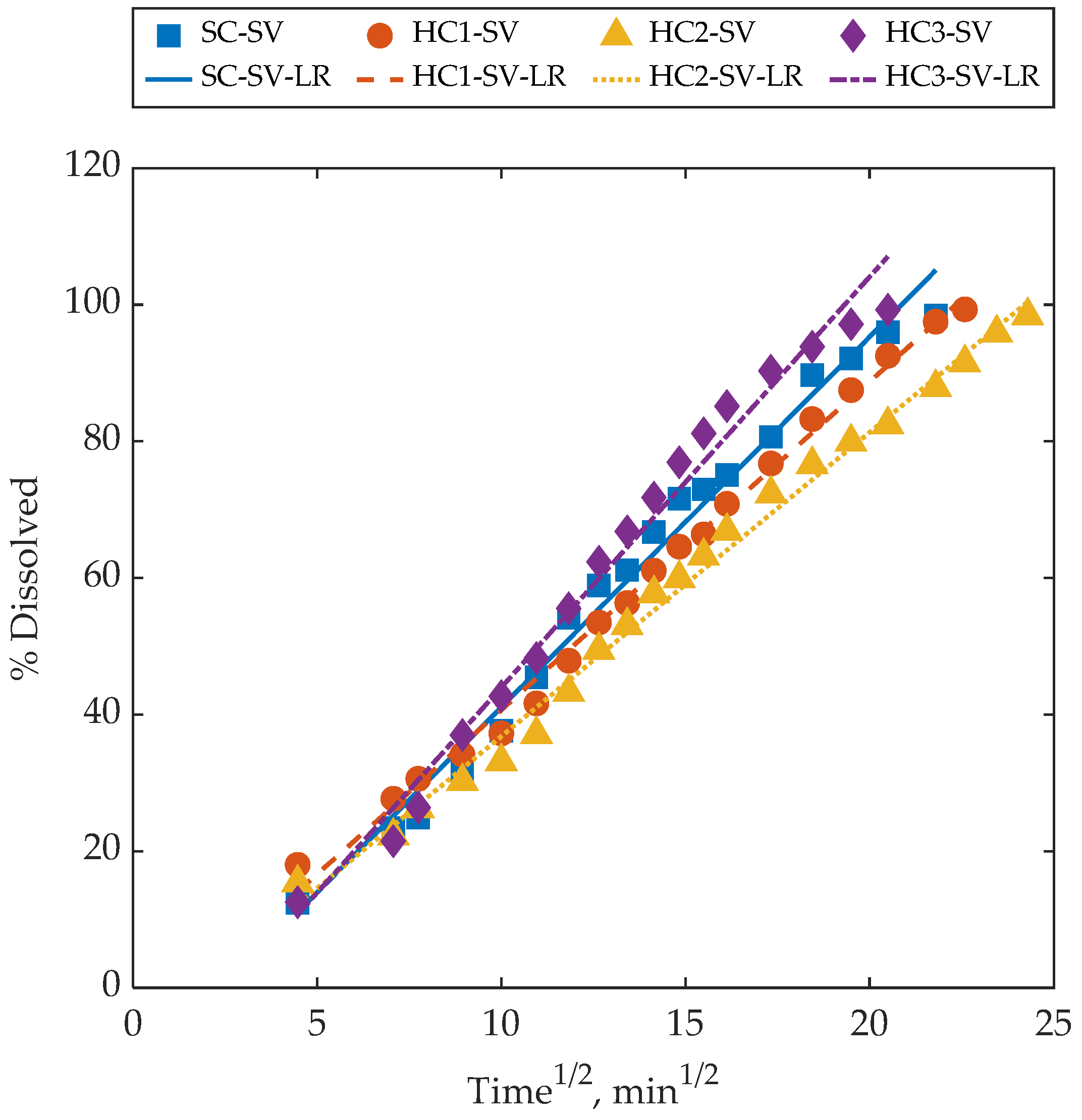
4.3. Dissolution Behaviour of Samples with a Similar Surface-Area-to-Volume Ratio
4.4. Further Remarks and Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The Role of Functional Excipients in Solid Oral Dosage Forms to Overcome Poor Drug Dissolution and Bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, E.; Ciepluch, N. Blend Segregation in Tablets Manufacturing and Its Effect on Drug Content Uniformity—A Review. Pharmaceutics 2021, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, I.; Friesen, I.; Puckhaber, D.; Schlegel, T.; Finke, J.H. Scaling Tableting Processes from Compaction Simulator to Rotary Presses-Mind the Sub-Processes. Pharmaceutics 2020, 12, 310. [Google Scholar] [CrossRef]
- Schomberg, A.K.; Kwade, A.; Finke, J.H. The Challenge of Die Filling in Rotary Presses—A Systematic Study of Material Properties and Process Parameters. Pharmaceutics 2020, 12, 248. [Google Scholar] [CrossRef]
- Schomberg, A.K.; Kwade, A.; Finke, J.H. Modeling gravity filling of dies on a rotary tablet press. Powder Technol. 2023, 413, 117998. [Google Scholar] [CrossRef]
- Sinka, I.; Motazedian, F.; Cocks, A.; Pitt, K. The effect of processing parameters on pharmaceutical tablet properties. Powder Technol. 2009, 189, 276–284. [Google Scholar] [CrossRef]
- Manley, L.; Hilden, J.; Valero, P.; Kramer, T. Tablet Compression Force as a Process Analytical Technology (PAT): 100% Inspection and Control of Tablet Weight Uniformity. J. Pharm. Sci. 2019, 108, 485–493. [Google Scholar] [CrossRef]
- Molavi, F.; Hamishehkar, H.; Nokhodchi, A. Impact of Tablet Shape on Drug Dissolution Rate Through Immediate Released Tablets. Adv. Pharm. Bull. 2020, 10, 656–661. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Prashar, G.; Vasudev, H.; Bhuddhi, D. Additive manufacturing: Expanding 3D printing horizon in industry 4.0. Int. J. Interact. Des. Manuf. IJIDeM 2023, 17, 2221–2235. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and limitations in 3D printing materials and technologies: A critical review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef] [PubMed]
- Milliken, R.L.; Quinten, T.; Andersen, S.K.; Lamprou, D.A. Application of 3D printing in early phase development of pharmaceutical solid dosage forms. Int. J. Pharm. 2024, 653, 123902. [Google Scholar] [CrossRef]
- Li, C.; Pisignano, D.; Zhao, Y.; Xue, J. Advances in Medical Applications of Additive Manufacturing. Engineering 2020, 6, 1222–1231. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS Pharmscitech 2018, 19, 3403–3413. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, H.; Jia, D.; Guan, X.; Pan, H.; Yang, Y.; Pan, W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int. J. Pharm. 2017, 525, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef]
- Crișan, A.G.; Porfire, A.; Ambrus, R.; Katona, G.; Rus, L.M.; Porav, A.S.; Ilyés, K.; Tomuță, I. Polyvinyl Alcohol-Based 3D Printed Tablets: Novel Insight into the Influence of Polymer Particle Size on Filament Preparation and Drug Release Performance. Pharmaceuticals 2021, 14, 418. [Google Scholar] [CrossRef]
- McDonagh, T.; Belton, P.; Qi, S. An investigation into the effects of geometric scaling and pore structure on drug dose and release of 3D printed solid dosage forms. Eur. J. Pharm. Biopharm. 2022, 177, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M. Challenges, current status and emerging strategies in the development of rapidly dissolving FDM 3D-printed tablets: An overview and commentary. Admet Dmpk 2023, 11, 33–55. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.H.; Choi, J.S.; Choi, Y.C.; Kim, J.D.; Cho, Y.W. Fabrication of drug-loaded polymer microparticles with arbitrary geometries using a piezoelectric inkjet printing system. Int. J. Pharm. 2012, 427, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gleadall, A.; Belton, P.; Mcdonagh, T.; Bibb, R.; Qi, S. New insights into the effects of porosity, pore length, pore shape and pore alignment on drug release from extrusionbased additive manufactured pharmaceuticals. Addit. Manuf. 2021, 46, 102196. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS Pharmscitech 2018, 19, 3355–3361. [Google Scholar] [CrossRef]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Bettini, R.; Santi, P.; De Ascentiis, A.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behavior, mechanisms and optimal performance. Pharm. Sci. Technol. Today 1996, 1, 202–208. [Google Scholar] [CrossRef]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA fibers for drug delivery: A review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Otarbayeva, S.; Berillo, D. Poly (Vinyl Alcohol) Drug and PVA–Drug–Surfactant Complex Organogel with Dimethyl Sulfoxide as a Drug Delivery System. Gels 2024, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Basa, B.; Jakab, G.; Kállai-Szabó, N.; Borbás, B.; Fülöp, V.; Balogh, E.; Antal, I. Evaluation of biodegradable PVA-based 3D printed carriers during dissolution. Materials 2021, 14, 1350. [Google Scholar] [CrossRef]
- Al-Taie, A.; Pan, J.; Polak, P.; Barer, M.R.; Han, X.; Abbott, A.P. Mechanical properties of 3-D printed polyvinyl alcohol matrix for detection of respiratory pathogens. J. Mech. Behav. Biomed. Mater. 2020, 112, 104066. [Google Scholar] [CrossRef] [PubMed]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Predicting drug release from 3D printed oral medicines based on the surface area to volume ratio of tablet geometry. Pharmaceutics 2021, 13, 1453. [Google Scholar] [CrossRef]
- Tsunematsu, H.; Hifumi, H.; Kitamura, R.; Hirai, D.; Takeuchi, M.; Ohara, M.; Itai, S.; Iwao, Y. Analysis of available surface area can predict the long-term dissolution profile of tablets using short-term stability studies. Int. J. Pharm. 2020, 586, 119504. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Ikeda, S.; Kobayashi, M.; Aoki, S.; Terukina, T.; Kanazawa, T.; Kojima, H.; Kondo, H. 3D-printed fast-dissolving oral dosage forms via fused deposition modeling based on sugar alcohol and poly (vinyl alcohol)—Preparation, drug release studies and in vivo oral absorption. Pharmaceutics 2023, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, M.P.; Clarelli, F.; Våbenø, J.; Lesley, C.; Rahman, S.D.; Cauzzo, J.; Stein, P.C. Experimental determination of drug diffusion coefficients in unstirred aqueous environments by temporally resolved concentration measurements. Mol. Pharm. 2018, 15, 1488–1494. [Google Scholar] [CrossRef] [PubMed]


| PVA Filament Specifications | |
|---|---|
| Diameter | 2.85 ± 0.10 mm |
| Colour | PVA Natural |
| Melting temperature | 163 °C |
| 3D-printing temperature | 190–210 °C |
| Specific gravity | 1.23 gr/cm3 |
| Geometry | Designed Mass (mg) | Designed Surface Area (mm2) | Surface-Area-to-Volume (mm−1) | Average Measured Mass (mg) | Mass Error (%) | |
|---|---|---|---|---|---|---|
| Similar Mass (M) | Solid Cylinder (SC-M) | 502 | 302 | 0.75 | 487 ± 2.9 | 2.9 |
| Hollow Cylinder 1 (HC1-M) | 501 | 614 | 1.53 | 500 ± 11.9 | 0.1 | |
| Hollow Cylinder 2 (HC2-M) | 497 | 380 | 0.95 | 500 ± 6.5 | 0.5 | |
| Hollow Cylinder 3 (HC3-M) | 504 | 605 | 1.50 | 503 ± 5.8 | 0.1 | |
| Similar Surface Area (S) | Solid Cylinder (SC-S) | 950 | 466 | 0.61 | 901 ± 10.5 | 5.1 |
| Hollow Cylinder 1 (HC1-S) | 371 | 466 | 1.57 | 359 ± 2.6 | 3.2 | |
| Hollow Cylinder 2 (HC2-S) | 758 | 466 | 0.77 | 732 ± 8.5 | 3.4 | |
| Hollow Cylinder 3 (HC3-S) | 346 | 468 | 1.69 | 324 ± 10.9 | 6.2 | |
| Similar Surface-Area-to-Volume Ratio (S/V) | Solid Cylinder (SC-SV) | 497 | 300 | 0.75 | 470 ± 4 | 5.4 |
| Hollow Cylinder 1 (HC1-SV) | 958 | 528 | 0.75 | 922 ± 12.3 | 3.8 | |
| Hollow Cylinder 2 (HC2-SV) | 981 | 604 | 0.77 | 910 ± 19.6 | 7.2 | |
| Hollow Cylinder 3 (HC3-SV) | 1489 | 923 | 0.77 | 1404 ± 35.1 | 5.6 | |
| Sample | Slope | Intercept | R2 |
|---|---|---|---|
| SC-SV | 5.42 | −13.16 | 0.99 |
| HC1-SV | 4.81 | −7.41 | 0.99 |
| HC2-SV | 4.45 | −7.70 | 0.99 |
| HC3-SV | 6.01 | −16.15 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afkhami, S.; Abdi, M.; Baserinia, R. Effect of Geometry on the Dissolution Behaviour of Complex Additively Manufactured Tablets. J. Manuf. Mater. Process. 2025, 9, 11. https://doi.org/10.3390/jmmp9010011
Afkhami S, Abdi M, Baserinia R. Effect of Geometry on the Dissolution Behaviour of Complex Additively Manufactured Tablets. Journal of Manufacturing and Materials Processing. 2025; 9(1):11. https://doi.org/10.3390/jmmp9010011
Chicago/Turabian StyleAfkhami, Seyedebrahim, Meisam Abdi, and Reza Baserinia. 2025. "Effect of Geometry on the Dissolution Behaviour of Complex Additively Manufactured Tablets" Journal of Manufacturing and Materials Processing 9, no. 1: 11. https://doi.org/10.3390/jmmp9010011
APA StyleAfkhami, S., Abdi, M., & Baserinia, R. (2025). Effect of Geometry on the Dissolution Behaviour of Complex Additively Manufactured Tablets. Journal of Manufacturing and Materials Processing, 9(1), 11. https://doi.org/10.3390/jmmp9010011








