Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses
Abstract
1. Introduction
2. Microwave-Assisted Processes
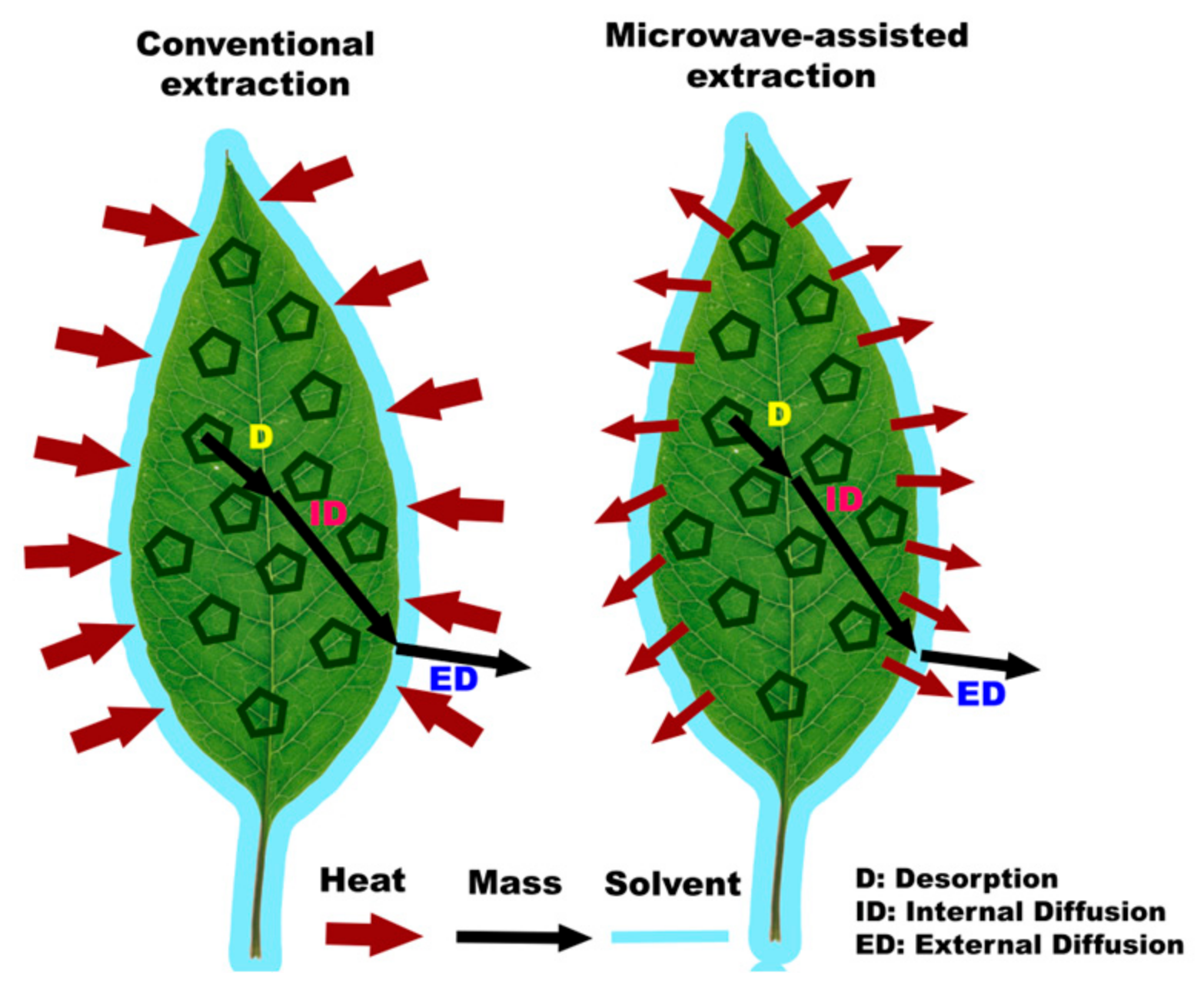
2.1. Microwave-Assisted Extraction (MAE)
2.2. Microwave-Assisted Pyrolysis (MAP)
2.3. Microwave-Assisted Hydrothermal Treatments (MAHT)
2.4. Microwave-Assisted Acid Hydrolysis (MAAH)
2.5. Microwave-Assisted Organosolv (MAO)
3. Previous Designs for Microwave-Assisted Processes
4. Added-Value Products from Biomass via Microwave-Assisted Processes
5. Strengths and Weaknesses of Microwave-Assisted Processes
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omer, A.M. Biomass energy resources utilisation and waste management. Agric. Sci. 2012, 3, 124–145. [Google Scholar] [CrossRef]
- Moustakas, K.; Loizidou, M. Waste and biomass management and valorization. Environ. Sci. Pollut. Res. 2021, 28, 24224–24229. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Chen, A.; Bai, M.; Peng, L.; Shao, J.; Yuan, J.; Shang, C.; Zhang, J.; Huang, H.; Peng, C. Valorization of heavy metal contaminated biomass: Recycling and expanding to functional materials. J. Clean. Prod. 2022, 366, 132771. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, R.; Kaushal, S.; Thakur, N.; Umar, A.; Akbar, S.; Ibrahim, A.A.; Baskoutas, S. Biomass waste-derived carbon materials for sustainable remediation of polluted environment: A comprehensive review. Chemosphere 2023, 345, 140419. [Google Scholar] [CrossRef]
- Tišma, M.; Bucić-Kojić, A.; Planinić, M. Bio-based Products from Lignocellulosic Waste Biomass. Chem. Biochem. Eng. Q. 2021, 35, 139–156. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Pereira, E.G.; da Silva, J.N.; de Oliveira, J.L.; Machado, C.S. Sustainable energy: A review of gasification technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.S.; Guerrero-Fajardo, C.A. Liquid Hot Water (LHW) and Hydrothermal Carbonization (HTC) of Coffee Berry Waste: Kinetics, Catalysis, and Optimization for the Synthesis of Platform Chemicals. Sustainability 2024, 16, 2854. [Google Scholar] [CrossRef]
- Galvis-Sandoval, D.E.; Lozano-Pérez, A.S.; Guerrero-Fajardo, C.A. Hydrothermal Valorization via Liquid Hot Water and Hydrothermal Carbonization of Pea Pod Waste: Characterization of the Biochar and Quantification of Platform Molecules. Appl. Sci. 2024, 14, 2329. [Google Scholar] [CrossRef]
- Palvasha, B.A.; Ahmad, S.; Abbasi, B.B.K.; Nazir, M.S.; Abdullah, M.A. Bioconversion of Straw Biomass into Bioproducts; Springer: Cham, Switzerland, 2021; pp. 369–383. [Google Scholar] [CrossRef]
- Lyu, Q.; Song, L.; Tong, Y.W.; Wang, W.; Zhou, J.; Yan, Z. Editorial: Highly efficient bioconversion of biomass waste: From theory to industry. Front. Bioeng. Biotechnol. 2023, 11, 1147993. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Sziple, F.; Vlazan, P.; Sfarloaga, P.; Grozesku, I.; Daniel, V. Biomass Extraction Methods. In Biomass Now—Sustainable Growth and Use; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T.; Ahmad, N.; Sher, F.; Khan, S.U.-D.; Ahmad, A.; Khan, R.; Wazeer, I. Valorisation and emerging perspective of biomass based waste-to-energy technologies and their socio-environmental impact: A review. J. Environ. Manag. 2021, 287, 112257. [Google Scholar] [CrossRef]
- Kapoore, R.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Nomanbhay, S.; Salman, B.; Hussain, R.; Ong, M.Y. Microwave pyrolysis of lignocellulosic biomass—A contribution to power Africa. Energy Sustain. Soc. 2017, 7, 23. [Google Scholar] [CrossRef]
- Allende, S.; Brodie, G.; Jacob, M.V. Breakdown of biomass for energy applications using microwave pyrolysis: A technological review. Environ. Res. 2023, 226, 115619. [Google Scholar] [CrossRef]
- Berko, E.; Adomako, D. FOSC 613 Industrial Food Processing Technologies. In Microwave Processing; 2016.
- Das, A.; Banik, B. Microwaves in Chemistry Applications: Fundamentals, Methods and Future Trends; Elsevier Science: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gaba, M.; Dhingra, N. Microwave Chemistry: General Features and Applications. Indian J. Pharm. Educ. Res. 2010, 45, 175–183. [Google Scholar]
- Wang, C.; Yao, W.; Zhu, H.; Yang, Y.; Yan, L. Uniform and highly efficient microwave heating based on dual-port phase-difference-shifting method. Int. J. RF Microw. Comput.-Aided Eng. 2021, 31, e22784. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, D. A new concept to improve microwave heating uniformity through data-driven process modelling. In Proceedings of the 17th International Conference on Microwave and High Frequency Heating, Valencia, Spain, 9–12 September 2019; Universitat Politècnica de València: Valencia, Spain, 2019. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L. Microwave dielectric heating in modern organic synthesis and drug discovery. In Microwave Heating; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, W. Microwave-assisted Inorganic Syntheses. In Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; pp. 173–195. [Google Scholar] [CrossRef]
- Harrison, A.; Whittaker, A.G. Microwave Heating. In Comprehensive Coordination Chemistry II; Elsevier: Amsterdam, The Netherlands, 2003; pp. 741–745. [Google Scholar] [CrossRef]
- Církva, V. Microwaves in Photochemistry and Photocatalysis. In Microwaves in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2012; pp. 563–605. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Akanda, J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-Assisted Extraction in Natural Products Isolation; Humana Press: Totowa, NJ, USA, 2012; pp. 89–115. [Google Scholar] [CrossRef]
- Nour, A.H.; Oluwaseun, A.R.; Nour, A.H.; Omer, M.S.; Ahmed, N. Microwave-Assisted Extraction of Bioactive Compounds (Review). In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Rosa, R.; Ferrari, E.; Veronesi, P. From Field to Shelf: How Microwave-Assisted Extraction Techniques Foster an Integrated Green Approach. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Ridlo, M.; Kumalaningsih, S.; Pranowo, D. Process of microwave assisted extraction (MAE) for Rhodomyrtus tomentosa fruit and its bioactive compounds. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012038. [Google Scholar] [CrossRef]
- Prommuak, C.; Pavasant, P.; Quitain, A.T.; Goto, M.; Shotipruk, A. Microalgal Lipid Extraction and Evaluation of Single-Step Biodiesel Production. Eng. J. 2012, 16, 157–166. [Google Scholar] [CrossRef]
- Ma, G.; Hu, W.; Pei, H.; Jiang, L.; Song, M.; Mu, R. In situ heterogeneous transesterification of microalgae using combined ultrasound and microwave irradiation. Energy Convers. Manag. 2015, 90, 41–46. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of Extraction Methods for Recovery of Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Process. Process Intensif. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef]
- De Sousa e Silva, A.; de Magalhães, W.T.; Moreira, L.M.; Rocha, M.V.P.; Bastos, A.K.P. Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Carrera, C. Optimization of a Microwave Assisted Extraction Method for Maximum Flavonols and Antioxidant Activity of Onion Extracts. Antioxidants 2022, 11, 2393. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Zaini, M.A.A. Dielectric properties in microwave-assisted solvent extraction—Present trends and future outlook. Asia-Pac. J. Chem. Eng. 2018, 13, e2230. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Comparison of microwave-assisted extraction and conventional extraction techniques for the extraction of tanshinones from Salvia miltiorrhiza bunge. Biochem. Eng. J. 2002, 12, 71–77. [Google Scholar] [CrossRef]
- Abed, K.A. Effect of Microwave Power on the Yield of Essential Oil from Lavender. J. Alasmarya Univ. Basic Appl. Sci. 2020, 5, 35–53. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhang, M.; Zhou, H.-L. Microwave-Assisted Extraction, Purification, Partial Characterization, and Bioactivity of Polysaccharides from Panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Sen, K.; Chouhan, K.S.; Tandey, R.; Mehta, R.; Mandal, V. Impact of microwaves on the extraction yield of phenolics, flavonoids, and triterpenoids from centella leaves: An approach toward digitized robust botanical extraction. Pharmacogn. Mag. 2019, 15, 267. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Cui, B.; Xu, X.-R.; Song, Y.; Ai, X.-X.; Li, H.-B. Microwave-Assisted Extraction of Oxymatrine from Sophora flavescens. Molecules 2011, 16, 7391–7400. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.J.; Nillian, E. Microwave-assisted extraction of bioactive compounds from Sarawak Liberica sp. coffee pulp: Statistical optimization and comparison with conventional methods. Food Sci. Nutr. 2023, 11, 5364–5378. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Dewanjee, S.; Mandal, S.C. Role of Modifier in Microwave Assisted Extraction of Oleanolic Acid from Gymnema sylvestre: Application of Green Extraction Technology for Botanicals. Nat. Prod. Commun. 2009, 4, 1934578X0900400. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. CHAPTER 4. Microwave-Assisted Extraction; Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, B.; Chen, Y.; Xie, M.; Duan, H.; Li, Y.; Duan, G. Nitrogen-protected microwave-assisted extraction of ascorbic acid from fruit and vegetables. J. Sep. Sci. 2009, 32, 4227–4233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-H.; Wang, J.-X.; Wang, G.; Wang, J.-Y.; Li, G.-K. Evaluation of vacuum microwave-assisted extraction technique for the extraction of antioxidants from plant samples. J. Chromatogr. A 2009, 1216, 8867–8873. [Google Scholar] [CrossRef]
- Xiao, X.; Song, W.; Wang, J.; Li, G. Microwave-assisted extraction performed in low temperature and in vacuo for the extraction of labile compounds in food samples. Anal. Chim. Acta 2012, 712, 85–93. [Google Scholar] [CrossRef]
- Luque-García, J.L.; Luque de Castro, M.D. Focused microwave-assisted Soxhlet extraction: Devices and applications. Talanta 2004, 64, 571–577. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Colnagui, G.; Visinoni, F.; Chemat, F. New microwave-integrated Soxhlet extraction. J. Chromatogr. A 2007, 1174, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, X.; Huang, S.; Li, J.; Wang, X.; Tang, J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol. 2010, 46, 429–435. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Jaramillo, B.E.; Martı, J.R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatography. J. Chromatogr. A 2004, 1025, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.-T.; Rezaei, K. Comparison of microwave-assisted hydrodistillation withthe traditional hydrodistillation method in the extractionof essential oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy. J. Chromatogr. A 2009, 1216, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, N.; Vian, M.A.; Bornard, I.; Boutekedjiret, C.; Chemat, F. Improved microwave steam distillation apparatus for isolation of essential oils. J. Chromatogr. A 2008, 1210, 229–233. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. An original solvent free microwave extraction of essential oils from spices. Flavour. Fragr. J. 2004, 19, 134–138. [Google Scholar] [CrossRef]
- López-Hortas, L.; Falqué, E.; Domínguez, H.; Torres, M.D. Microwave Hydrodiffusion and Gravity (MHG) Extraction from Different Raw Materials with Cosmetic Applications. Molecules 2019, 25, 92. [Google Scholar] [CrossRef]
- Camponogara, J.A.; Farias, C.A.A.; Moraes, D.P.; Bettio, L.; Citadin, I.; Mallman, C.A.; Schmiele, M.; Ballus, C.A.; Barin, J.S.; Barcia, M.T. Optimization of Microwave Hydrodiffusion and Gravity (MHG) for Pre-treatment of Dehydration and Obtaining a Jaboticaba Extract. Food Bioprocess Technol. 2023, 17, 1479–1491. [Google Scholar] [CrossRef]
- Chambaud, M.; Colas, C.; Destandau, E. Water-Based Microwave-Assisted Extraction of Pigments from Madder Optimized by a Box–Behnken Design. Separations 2023, 10, 433. [Google Scholar] [CrossRef]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Al Mamoori, F.; Al Janabi, R. Recent Advances in Microwave-Assisted Extraction (MAE) of Medicinal Plants: A Review. Int. Res. J. Pharm. 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Awang Biak, D.R.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; SosaMorales, M.E.; López-Malo, A. Microwave-assisted Extraction of Essential Oils from Herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kurian, V.; Gill, M.; Dhakal, B.; Kumar, A. Recent trends in the pyrolysis and gasification of lignocellulosic biomass. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 511–552. [Google Scholar] [CrossRef]
- Prakash, A.; Singh, R.; Balagurumurthy, B.; Bhaskar, T.; Arora, A.K.; Puri, S.K. Thermochemical Valorization of Lignin. In Recent Advances in Thermo-Chemical Conversion of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 455–478. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Mohammed Yunus, M.F.; Mohd Hakimi, N.I.N. Microwave-Assisted Pyrolysis of Oil Palm Biomass: Multi-Optimisation of Solid Char Yield and Its Calorific Value Using Response Surface Methodology. Front. Chem. Eng. 2022, 4, 864589. [Google Scholar] [CrossRef]
- Arshad, H.; Sulaiman, S.A.; Hussain, Z.; Naz, Y.; Basrawi, F. Microwave assisted pyrolysis of plastic waste for production of fuels: A review. MATEC Web Conf. 2017, 131, 02005. [Google Scholar] [CrossRef]
- Zulkornain, M.F.; Shamsuddin, A.H.; Normanbhay, S.; Saad, J.M.; Zhang, Y.S.; Samsuri, S.; Ghani, W.A.W.A.K. Microwave-assisted Hydrothermal Carbonization for Solid Biofuel Application: A Brief Review. Carbon Capture Sci. Technol. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2011, 102, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, K.N.; Sindhu, N. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef]
- Irfan, M.; Saleem, R.; Shoukat, B.; Hussain, H.; Shukrullah, S.; Naz, M.Y.; Rahman, S.; Ghanim, A.A.J.; Nawalany, G.; Jakubowski, T. Production of combustible fuels and carbon nanotubes from plastic wastes using an in-situ catalytic microwave pyrolysis process. Sci. Rep. 2023, 13, 9057. [Google Scholar] [CrossRef]
- Ahmed, A. Microwave Assisted Pyrolysis of Moringa Seed and Karanja for Bio-Oil Production. Int. J. Renew. Energy Resour. 2023, 13, 14–24. [Google Scholar] [CrossRef]
- Fodah, A.E.M.; Ghosal, M.K.; Behera, D. Studies on Microwave-Assisted Pyrolysis of Rice Straw Using Solar Photovoltaic Power. Bioenergy Res. 2021, 14, 190–208. [Google Scholar] [CrossRef]
- Quillope, J.C.C.; Carpio, R.B.; Gatdula, K.M.; Detras, M.C.M.; Doliente, S.S. Optimization of process parameters of self-purging microwave pyrolysis of corn cob for biochar production. Heliyon 2021, 7, e08417. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, Y.; Zhu, H.; Zhao, X.; Zhu, L.; Liu, W.; Sun, M.; Miao, G.; Li, S.; Kong, L.Z. Microwave-assisted low-temperature biomass pyrolysis: From mechanistic insights to pilot scale. Green Chem. 2021, 23, 821–827. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Min, M.; Cheng, Y.; Peng, P.; Anderson, E.; Wang, Y.; et al. Microwave-Assisted Pyrolysis of Biomass for Bio-Oil Production. In Pyrolysis; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Shao, Z. Microwave pyrolysis of sludge: A review. Sustain. Environ. Res. 2022, 32, 23. [Google Scholar] [CrossRef]
- Alam, S.S.; Khan, A.H. Microwave-assisted pyrolysis for waste plastic recycling: A review on critical parameters, benefits, challenges, and scalability perspectives. Int. J. Environ. Sci. Technol. 2024, 21, 5311–5330. [Google Scholar] [CrossRef]
- Yang, C.; Shang, H.; Li, J.; Fan, X.; Sun, J.; Duan, A. A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes 2023, 11, 1487. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Deng, C.; Kang, X.; Lin, R.; Murphy, J.D. Microwave assisted low-temperature hydrothermal treatment of solid anaerobic digestate for optimising hydrochar and energy recovery. Chem. Eng. J. 2020, 395, 124999. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, K.; Yuan, Q. Comparative study of microwave and conventional hydrothermal treatment of chicken carcasses: Bio-oil yields and properties. Energy 2020, 200, 117539. [Google Scholar] [CrossRef]
- Sininart, C.; Chakrit, T. Optimization of soluble sugar production from pineapple peel by microwave-assisted water pretreatment. Songklanakarin J. Sci. Technol. 2019, 41, 237–245. [Google Scholar]
- Li, Y.; Li, B.; Du, F.; Wang, Y.; Pan, L.; Chen, D. Microwave-assisted hydrothermal liquefaction of lignin for the preparation of phenolic formaldehyde adhesive. J. Appl. Polym. Sci. 2017, 134, 44510. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Li, C.; Shupe, T.F.; Hu, T.; Qi, J.; De Hoop, C.F. Characterization of Microwave Liquefied Bamboo Residue and Its Potential Use in the Generation of Nanofibrillated Cellulosic Fiber. ACS Sustain. Chem. Eng. 2016, 4, 3477–3485. [Google Scholar] [CrossRef]
- Torrado, I.; Neves, B.G.; da Conceição Fernandes, M.; Carvalheiro, F.; Pereira, H.; Duarte, L.C. Microwave-assisted hydrothermal processing of pine nut shells for oligosaccharide production. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Nassar, A.E.; El-Aswar, E.I.; Rizk, S.A.; Gaber, S.E.-S.; Jahin, H.S. Microwave-assisted hydrothermal preparation of magnetic hydrochar for the removal of organophosphorus insecticides from aqueous solutions. Sep. Purif. Technol. 2023, 306, 122569. [Google Scholar] [CrossRef]
- Knappe, V.; Paczkowski, S.; Tejada, J.; Diaz Robles, L.A.; Gonzales, A.; Pelz, S. Low temperature microwave assisted hydrothermal carbonization (MAHC) reduces combustion emission precursors in short rotation coppice willow wood. J. Anal. Appl. Pyrolysis 2018, 134, 162–166. [Google Scholar] [CrossRef]
- Ifrah, S.; Kaddouri, A.; Gelin, P.; Leonard, D. Conventional hydrothermal process versus microwave-assisted hydrothermal synthesis of La1−xAgxMnO3+δ (x = 0, 0.2) perovskites used in methane combustion. Comptes Rendus Chim. 2007, 10, 1216–1226. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, L.; Mo, H.; Chen, Y.; Hu, X. Microwave-assisted catalytic hydrothermal carbonization of Laminaria japonica for hydrochars catalyzed and activated by potassium compounds. Bioresour. Technol. 2021, 341, 125835. [Google Scholar] [CrossRef] [PubMed]
- Khushk, S.; Zhang, L.; Li, A.; Irfan, M.; Zhang, X. Microwave-Assisted Hydrothermal Carbonization of Furfural Residue for Adsorption of Cr(VI): Adsorption and Kinetic Study. Pol. J. Environ. Stud. 2020, 29, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Wang, N.; Chen, Y.; Li, G. Process characteristics for microwave assisted hydrothermal carbonization of cellulose. Bioresour. Technol. 2018, 259, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lorente, A.; Remon, J.; Salgado, M.; Huertas-Alonso, A.J.; Sanchez-Verdu, P.; Moreno, A.; Clark, J.H. Sustainable Production of Solid Biofuels and Biomaterials by Microwave-Assisted, Hydrothermal Carbonization (MA-HTC) of Brewers’ Spent Grain (BSG). ACS Sustain. Chem. Eng. 2020, 8, 18982–18991. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, H.; Zou, J.; Mai, Z.; Cao, Z.; Li, M.; Fan, B.; Shao, G.; Wang, H.; Xu, H.; et al. Effect of process factors of microwave hydrothermal method on the preparation of micron-sized spherical α-Al2O3 particles. Inorg. Chem. Commun. 2021, 133, 108938. [Google Scholar] [CrossRef]
- Álvarez-Chávez, B.J.; Godbout, S.; Raghavan, V. Optimization of microwave-assisted hydrothermal pretreatment and its effect on pyrolytic oil quality obtained by an auger reactor. Biofuel Res. J. 2021, 8, 1316–1329. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Z.; Yin, D.; Xu, Q.; Liu, F.; Lu, C.; Mao, L. Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem. 2010, 12, 696. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Cantero-Bahillo, E.; Fernández-Felipe, M.T.; Martin, D. Microwave-Assisted Acid Hydrolysis vs. Conventional Hydrolysis to Produce Sapogenin-Rich Products from Fenugreek Extracts. Foods 2022, 11, 1934. [Google Scholar] [CrossRef]
- Jill, J. Microwave-Assisted Acid Hydrolysis. In Microwave-Assisted Proteomics; The Royal Society of Chemistry: London, UK, 2009; pp. 56–70. [Google Scholar] [CrossRef]
- Seo, M.-Y.; Kim, J.-H.; Park, S.-H.; Kim, T.-H.; Lee, J.-H.; Kim, J.-K. Microwave-assisted Weak Acid Hydrolysis of Proteins. Mass Spectrom. Lett. 2012, 3, 47–49. [Google Scholar] [CrossRef]
- Colleary, C.; Little, N.C.; Cleland, T.P. Microwave-assisted acid hydrolysis for whole-bone proteomics and paleoproteomics. Rapid Commun. Mass Spectrom. 2020, 34, e8568. [Google Scholar] [CrossRef]
- Tanaka, Y.; Okamoto, K.; Matsushima, A.; Ota, T.; Matsumoto, Y.; Akasaki, T. Microwave-assisted acid hydrolysis of konjac products for determining the konjac powder content. Anal. Sci. 2013, 29, 1049–1053. [Google Scholar] [CrossRef]
- Sunarti, T.C.; Yanti, S.D.; Ruriani, E. Two-steps microwave-assisted treatment on acid hydrolysis of sago pith for bioethanol production. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012052. [Google Scholar] [CrossRef]
- Mayer, N.; Dirauf, M.P.; Kaltschmitt, M. Microwave-assisted acidic hydrolysis of phytate from rye bran—Experimental procedure and model prediction. LWT 2023, 189, 115499. [Google Scholar] [CrossRef]
- Noguerra, J. Parametric and Kinetic Studies of Microwave-Assisted Dilute Sulfuric Acid Hydrolysis of Peanut (Arachis hypogaea L.) Shells. Bachelor’s Thesis, University of the Philippines, Quezon City, Philippines, 2018. [Google Scholar]
- Tong, K.T.X.; Tan, I.S.; Foo, H.C.Y.; Tiong, A.C.Y.; Lam, M.K.; Lee, K.T. Third-generation L-Lactic acid production by the microwave-assisted hydrolysis of red macroalgae Eucheuma denticulatum extract. Bioresour. Technol. 2021, 342, 125880. [Google Scholar] [CrossRef]
- Alio, M.A.; Tugui, O.-C.; Vial, C.; Pons, A. Microwave-assisted Organosolv pretreatment of a sawmill mixed feedstock for bioethanol production in a wood biorefinery. Bioresour. Technol. 2019, 276, 170–176. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Rodríguez, A.; Espinosa, E.; Domínguez-Robles, J.; Sánchez, R.; Bascón, I.; Rosal, A. Different Solvents for Organosolv Pulping. In Pulp and Paper Processing; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Pauly, M.; Dama, M. Microwave Assisted Pretreatment of Szarvasi (Agropyron elongatum) Biomass to Enhance Enzymatic Saccharification and Direct Glucose Production. Front. Plant Sci. 2022, 12, 767254. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Mateo, S.; Spaccini, F.; Buratti, C.; Moya, A.J. Optimization of Microwave Assisted Organosolv Pretreatment for Enzymatic Hydrolysis of Cherry Tree Pruning and Pistachio Shells. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Merino-Pérez, O.; Martínez-Palou, R.; Labidi, J.; Luque, R. Microwave-Assisted Pretreatment of Lignocellulosic Biomass to Produce Biofuels and Value-Added Products. In Production of Biofuels and Chemicals with Microwave. Biofuels and Biorefineries; Springer: Berlin/Heidelberg, Germany, 2015; pp. 197–224. [Google Scholar] [CrossRef]
- Yang, X.; Cui, C.; Zheng, A.; Zhao, Z.; Wang, C.; Xia, S.; Huang, Z.; Wei, G.; Li, H. Ultrasonic and microwave assisted organosolv pretreatment of pine wood for producing pyrolytic sugars and phenols. Ind. Crops Prod. 2020, 157, 112921. [Google Scholar] [CrossRef]
- Yao, J.; Karlsson, M.; Lawoko, M.; Odelius, K.; Hakkarainen, M. Microwave-assisted organosolv extraction for more native-like lignin and its application as a property-enhancing filler in a light processable biobased resin. RSC Sustain. 2023, 1, 1211–1222. [Google Scholar] [CrossRef]
- Avelino, F.; da Silva, K.T.; de Souza Filho, M.d.S.M.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F.; da Silva, K.T.M.; de Souza Filho, M.d.S.M.; Mazzetto, S.E. Microwave-assisted organosolv extraction of coconut shell lignin by Brønsted and Lewis acids catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Corsi, L.; Mateo, S.; Spaccini, F.; Buratti, C.; Moya, A.J. Optimization of Microwave-Assisted Organosolv Pretreatment for Enzymatic Hydrolysis of Cherry Tree Pruning and Pistachio Shells: A Step to Bioethanol Production. Bioenergy Res. 2023, 17, 294–308. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Huang, G.H.; Paquet, L.; Deschamps, S.; Beaulieu, C.; Hawari, J. Microwave-assisted extraction of lignin from triticale straw: Optimization and microwave effects. Bioresour. Technol. 2012, 104, 775–782. [Google Scholar] [CrossRef]
- Yimtrakarn, T.; Kaveevivitchai, W.; Lee, W.-C.; Lerkkasemsan, N. Study of Lignin Extracted from Rubberwood Using Microwave Assisted Technology for Fuel Additive. Polymers 2022, 14, 814. [Google Scholar] [CrossRef]
- Avelino, F.; Marques, F.; Soares, A.K.L.; Silva, K.T.; Leitão, R.C.; Mazzetto, S.E.; Lomonaco, D. Microwave-Assisted Organosolv Delignification: A Potential Eco-Designed Process for Scalable Valorization of Agroindustrial Wastes. Ind. Eng. Chem. Res. 2019, 58, 10698–10706. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; Van Vuong, Q. Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts. Processes 2021, 9, 2212. [Google Scholar] [CrossRef]
- Shi, K.; Yan, J.; Menéndez, J.A.; Luo, X.; Yang, G.; Chen, Y.; Lester, E.; Wu, T. Production of H2-Rich Syngas From Lignocellulosic Biomass Using Microwave-Assisted Pyrolysis Coupled with Activated Carbon Enabled Reforming. Front. Chem. 2020, 8, 3. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, S.-W.; Lai, F.-Y.; Liu, S.-Y.; Xiong, J.-B.; Zhou, C.-F.; Yi-Yu, Y.; Huang, H.-J. Microwave-assisted hydrothermal carbonization of pig feces for the production of hydrochar. J. Supercrit. Fluids 2020, 162, 104858. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.; Sun, D.; Li, L. Microwave-assisted acid hydrolysis of proteins combined with peptide fractionation and mass spectrometry analysis for characterizing protein terminal sequences. J. Proteom. 2014, 100, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Microwave-assisted biodiesel production using –SO3H functionalized heterogeneous catalyst derived from a lignin-rich biomass. Sci. Rep. 2023, 13, 9074. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, Y.; Arenillas, A.; Angel, J. Microwave Heating Applied to Pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Fia, A.Z.; Amorim, J. Microwave pretreatment of biomass for conversion of lignocellulosic materials into renewable biofuels. J. Energy Inst. 2023, 106, 101146. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.; Jeong, C.; Kim, H.; Kim, C. Determination of Relationship between Higher Heating Value and Atomic Ratio of Hydrogen to Carbon in Spent Coffee Grounds by Hydrothermal Carbonization. Energies 2021, 14, 6551. [Google Scholar] [CrossRef]
- Coelho, J.; Robalo, M.; Boyadzhieva, S.; Stateva, R. Microwave-Assisted Extraction of Phenolic Compounds from Spent Coffee Grounds. Process Optimization Applying Design of Experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Menéndez, J.; Fernández, Y.; Pis, J.; Nabais, J.V.; Carrott, P.; Carrott, M.R. Conventional and microwave induced pyrolysis of coffee hulls for the production of a hydrogen rich fuel gas. J. Anal. Appl. Pyrolysis 2007, 79, 128–135. [Google Scholar] [CrossRef]
- Divyashri, G.; Murthy, T.P.K.; Ragavan, K.V.; Sumukh, G.M.; Sudha, L.S.; Nishka, S.; Himanshi, G.; Misriya, N.; Sharada, B.; Venkataramanaiah, R.A. Valorization of coffee bean processing waste for the sustainable extraction of biologically active pectin. Heliyon 2023, 9, e20212. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A biorefinery approach for the valorization of spent coffee grounds to produce antioxidant compounds and biobutanol. Biomass Bioenergy 2021, 147, 106026. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Corscadden, K.; He, Q.; Zhou, N. MW-assisted hydrothermal liquefaction of spent coffee grounds. Can. J. Chem. Eng. 2022, 100, 1729–1738. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020, 129, 108864. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S.; Nunes, A.A.; Alves, C.C.O. Microwave assisted thermal treatment of defective coffee beans press cake for the production of adsorbents. Bioresour. Technol. 2010, 101, 1068–1074. [Google Scholar] [CrossRef]
- Ondruschka, B.; Bonrath, W.; Stuerga, D. Development and Design of Laboratory and Pilot Scale Reactors for Microwave-assisted Chemistry. In Microwaves in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2006; pp. 62–107. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 461–483. [Google Scholar] [CrossRef]
- Ambrozic, G.; Crnjak, Z.; Zigon, M. Microwave-Assisted Non-Aqueous Synthesisof ZnO Nanoparticles. Mater. Technol. 2011, 45, 173–177. [Google Scholar]
- Danlami, J.M.; Arsad, A.; Ahmad Zaini, M.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Gotama, B.; Rahman, A.K.; Ahmad, A.; Hariyadi, A. Extraction of rice bran oil using microwave-assisted extraction and green solvents. IOP Conf. Ser. Earth Environ. Sci. 2022, 1105, 012052. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rivas, S.; Torres, M.D.; Domínguez, H. Microwave-Assisted Extraction of Carrageenan from Sarcopeltis skottsbergii. Mar. Drugs 2023, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Dertli, H.; Saloglu, D. A valorization approach of food industry wastewater using microwave-assisted extraction. In Advanced Technologies in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–178. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-assisted extraction of functional compounds from plants: A Review. BioResources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Robinson, J.; Binner, E.; Vallejo, D.B.; Perez, N.D.; Al Mughairi, K.; Ryan, J.; Shepherd, B.; Adam, M.; Budarin, V.; Fan, J.; et al. Unravelling the mechanisms of microwave pyrolysis of biomass. Chem. Eng. J. 2022, 430, 132975. [Google Scholar] [CrossRef]
- Bing, W.; Hongbin, Z.; Zeng, D.; Yuefeng, F.; Yu, Q.; Rui, X. Microwave fast pyrolysis of waste tires: Effect of microwave power on product composition and quality. J. Anal. Appl. Pyrolysis 2021, 155, 104979. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, J.; Dai, L.; Guo, F.; Wang, Y.; Li, H.; Deng, W.; Lei, H.; Chen, P.; Liu, Y.; et al. Syngas production from biomass pyrolysis in a continuous microwave assisted pyrolysis system. Bioresour. Technol. 2020, 314, 123756. [Google Scholar] [CrossRef]
- Ahmad, F.; Idrees, F.; Fazal-e-Aleem. Recent Advancements in Microwave-Assisted Synthesis of NiO Nanostructures and their Supercapacitor Properties: A Comprehensive Review. Curr. Nanomater. 2018, 3, 5–17. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Rajkumar, T.; Pandi, D.K.; Ahmed, A.S.; Ani, F.N. Microwave assisted acid hydrolysis for bioethanol fuel production from sago pith waste. Waste Manag. 2019, 86, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefinery 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Goyal, H.; Chen, T.-Y.; Chen, W.; Vlachos, D.G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2022, 430, 133183. [Google Scholar] [CrossRef]
- Radoiu, M.; Mello, A. Technical advances, barriers, and solutions in microwave—Assisted technology for industrial processing. Chem. Eng. Res. Des. 2022, 181, 331–342. [Google Scholar] [CrossRef]
- Sun, J.; Chen, W.; Jia, K.; Li, S.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Chen, S. Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes 2023, 11, 1451. [Google Scholar] [CrossRef]

| Vegetal Matter (VM) | MAE Conditions | Solvent Used and Volumes Added | Valorized Product | Yield | Ref. |
|---|---|---|---|---|---|
| Rhodomyrtus tomentosa | 204.84 s, 400 W, ΔT = 26.8 °C–115 °C | Ethanol 10 g fruit, 150 mL ethanol | Flavonoids Anthocyanin | 1.68% (v/w) | [32] |
| Chlorella vulgaris | 300 W, 50 °C, 30 min | 1 g algae, 100 mL chloroform:methanol (1:1) | DW lipid | 31.9% | [33] |
| Mixed microalgal culture | 2.45 GHz, 10–80 W, 60 °C, 45 min | 5 g algae, KFCaO catalyst, 40 mL methanol | Biodiesel | 58.12% | [34] |
| Stigeoclonium sp.; Monoraphidium sp.; Nitzchia sp.; and Navicula sp. | 2.45 GHz, 900 W, 3 min | Wet biomass, no solvent added. 150 mL algal biomass | Carbohydrates, proteins | 915 mg/L soluble carbohydrates; 127.7 mL/g volatile solids and 193 mg/L protein | [35] |
| Haematococcus pluvialis | 2.45 GHz, 60% of 1200 W output, 75 °C, 5 min | 0.1 g algae, 10 mL acetone | Pigments | 74% astaxanthin recovery | [36] |
| Cucurbita pepo var. styriaca | 1200 W, 10 min, 102.2 °C | 1 g pumpkin, 50 mL water | Pectin | 7.3% | [37] |
| Rhizopus oryzae NRRL1526 | 300 W, 22 min | 1 g fungus, 50 mL NaOH 1 N | Chitosan | 13.43% | [38] |
| Spirulina (Arthrospira) platensis | 434 W, 20 min | 2.6 g of biomass to 80 mL of ethanol | Polysacarides | g 127 ± 5 mg of carbohydrate/g of biomass | [39] |
| Feedstock | MAP Conditions | Valorized Product | Yield | Ref. |
|---|---|---|---|---|
| Oil palm biomass | 300 W, 2.45 GHz, quartz reactor, 17 min, N2 system | Solid char Liquid Gas | 27.6% | [73] |
| Soapstock | 800 W at a frequency of 2450 MHz, N2 system, 550 °C and 6 g/min | Alkenes, cycloalkenes, alkadienes, alkynes, aromatics | 65% | [74] |
| Rice straw | 2.45 GHz, 300 W, N2 system, 600–700 °C, 30 min | Gas fraction | H2 (50.67 vol%), CO2 (22.56 vol%), CO (16.09 vol%), and CH4 (7.42 vol%) | [76] |
| Sewage sludge | 400 W, 6 min, 500 °C, 2450 MHz | Bio-oil | 49.8 wt.% | [77] |
| Mixture of plastics | 5 kW, quartz vessel | Bio-oil and biochar | - | [78] |
| Plastic Waste | 1 kW, m 400 to 450 °C, 2450 MHz | Combustible fuels and carbon nanotubes | 80% | [79] |
| Moringa seed | 800 W, 13 min, 450 °C | Karanja bio-oil | 10.6% | [80] |
| Feedstock | Microwave Conditions | Valorized Product | Yield | Ref. |
|---|---|---|---|---|
| Grass silage | 2.45 GHz, 1600 W, 0.7 g of dried feedstock/14 mL water, 30 min, 180 °C | Hydrochar, biogas | Hydrochar: f 0.79 g/g Biogas: 68.7 mL/g | [91] |
| Chicken carcasses | Temperature 240 °C, time (2 h), power (400 W), biomass loading (20/45 g/mL) | Biocrude | 59.41% | [92,93] |
| Pineapple peal | Time (9 m), power (900 W), biomass loading (100 g/L) | Sugars | 80.2% | [94] |
| Bagasse | Temperature (105 °C), time (30 min), biomass loading (1:15), catalysts H2SO4, CH3COOH | Lignin | 78.69% | [95] |
| Bamboo | Temperature (180 °C), time (3 min), power (550 W), biomass loading (1:4) | Nanofibrillated cellulosic fiber | 56.41% | [96] |
| Pine nut shells | Temperature (190 °C), time (60 min), biomass loading (1:3), 2450 MHz | Oligosaccharides | 1.59% | [97] |
| Feedstock | Microwave Conditions | Product | Yield | Ref. |
|---|---|---|---|---|
| Myoglobin | 800 W, 60 Hz, 1 h, 30–100 °C, 2% formic acid | Small peptide fragments | - | [110] |
| Whole moosebone | 140 °C, formic acid and acetic acid, 30 min | Peptides | 70.16% | [111] |
| Brown seaweed | H2SO4 (0.01–0.4 M), temperature (120–180 °C), biomass loading (solid/liquid ratio: 0.6–6%, w/v), time (0–30 min) | Monosaccharides, ethanol | 127 mg/g monosaccharides, 20.8 mg/g ethanol | [89] |
| Konjac flour | Diluted sulfuric acid, 135 °C, 600 W, 45 min, 0.25 M H2SO4 | Glucomannan | 80% | [112] |
| Sago pith | 0.5 M H2SO4 | Ethanol | 0.361% | [113] |
| Feedstock | Microwave conditions | Product | Yield | Ref. |
|---|---|---|---|---|
| Coconut shell | 2.45 GHz, 500 W, 100 mL of acetic acid/water solution (9/1, v/v), 110 °C, 20 min | Lignin | 3.82% | [124] |
| Sawmill | 60:40 ethanol-water, 175 °C, 0.25% H2SO4 | Cellulose | 82% | [116] |
| Triticale straw | 800 W, 83–167 °C, 30 min | Lignin | 91% | [126] |
| Rubberwood | 2450 MHz, 200 W, 30 min, ethanol or isopropanol | Ethanol/Lignin | 6.26% | [127] |
| Pine wood | 480 W, 150 °C, 10 min | Levoglucosan | 55.87% | [122] |
| Coffee Residue | Hemicellulose (%) | Cellulose (%) | Lignin (%) |
|---|---|---|---|
| Coffee Cherry | 12.5 | 27.6 | 13.7 |
| Pulp | 3.6 | 25.88 | 20.07 |
| Husk | 7 | 43 | 9 |
| SCG | 12.1 | 23.6 | 17.8 |
| Feedstock | Microwave-Assisted Process | Conditions | Product and Yield | Source |
|---|---|---|---|---|
| SCG | MAE | 60–120 W, 75 °C, 1 g SGC/15 mL Ethanol (60%), 3–6 min | Total polyphenols content: 175.08 mg/g | [137] |
| Parchment | MAP | Single-mode microwave oven at 500, 800, and 1000 °C, 15 min, quartz reactor, N2 60 mL/min, 130, 270, and 420 W | Hydrogen rich fuel gas: 68.72%, Oil: 8.58%, Char: 22.70% | [138] |
| Husk | MAAH | 2.5 g in 50 mL Citric acid solution, 100–800 W, 5–26 min, 50–100 °C, quartz reactor | Pectin: 40.2% | [139] |
| SCG | MAO | 50 Hz, choline chloride: glycerol (ChCl:Glyc), 60–120 °C, 5–15 min/(ethanol-water 25:75, v/v), 60 °C, 15 min | Antioxidants: 0.48 mg GAE/SCG (TPC) 81 kg butanol t−1 SCG | [140] |
| SCG | MA-HTL | Quartz vessels, N2 atmosphere, 270 °C, 200 rpm, 3 g SCG, Multi-wave PRO MW, 20 min | Biocrude: 30.1 wt.% Solid: 28.6 wt.% Aqueous Phase: 28.0 wt.%, Gas: 13.3 wt.% | [141] |
| Toasted coffee | MAE | 1–10 min, 120–180 °C, 2–6 g/mL, 1 kW, 2.45 GHz, MicroSYNTH Labstation | Carbohydrate (18–43% w/w) Caffeine (4–7% w/w) 5-caffeoylquinic acid (1–2% w/w) | [142] |
| Defective coffee beans | MAHT | 800 W (Panasonic NN6460A), 3 min, 15 g, quartz reactor | Absorbent: 54% | [143] |
| Microwave-Assisted Process | Advantages | Disadvantages | Sources |
|---|---|---|---|
| MAE | Higher yield, selectivity, extraction efficiency, reduced time and solvent consumption, less environmental pollution, and reduced degradation of thermolabile constituents. | Not robust to outliers, bigger error terms are not punished, not feasible for reaction monitoring, expensive equipment, non-differentiable nature of graphs, penalizes underestimates more than overestimates, and limited choice of solvents. | [148,149,150,151] |
| MAP | Uniform heating of large particulate sizes of feed, no requirement of fluidization, fast switching on and off controls, time and energy savings, and fewer ashes in the liquid product. | Complexity of the microwave heating phenomena, need for proper heating control devices, limited feedstock options, limited scale-up potential, and high capital cost. | [151,152,153,154] |
| MHT | High efficiency and productivity in the hydrothermal process, uniform distribution of heat during the pretreatment process, rapid heat generation leading to shorter treatment times, energy efficiency and rapid heat generation in the pretreatment process, and potential for higher yields of products with lower operating costs. | Low penetration of radiation in bulk products during microwave heating, complexity of the microwave heating phenomena leading to challenges in control, agitation problems with high loading substrate in the microwave reactor, and not suitable for all types of feedstock, limiting its versatility. | [155,156] |
| MAAH | High uniformity and selectivity, short process time, and lower energy requirements compared to traditional heating methods. | Degradation of glucose into toxic compounds, need of neutralization of hydrolysates, and no notable enhancement in protein hydrolysis. | [110,155,157] |
| MAO | High efficiency and productivity, uniform distribution, easy recovery and reuse of organic solvents, fast heating rates, and high temperatures. | High capital investment equipment, unsuitable for scaling up, and not feasible for monitoring. | [123,128,158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano Pérez, A.S.; Lozada Castro, J.J.; Guerrero Fajardo, C.A. Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses. J. Manuf. Mater. Process. 2024, 8, 121. https://doi.org/10.3390/jmmp8030121
Lozano Pérez AS, Lozada Castro JJ, Guerrero Fajardo CA. Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses. Journal of Manufacturing and Materials Processing. 2024; 8(3):121. https://doi.org/10.3390/jmmp8030121
Chicago/Turabian StyleLozano Pérez, Alejandra Sophia, Juan José Lozada Castro, and Carlos Alberto Guerrero Fajardo. 2024. "Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses" Journal of Manufacturing and Materials Processing 8, no. 3: 121. https://doi.org/10.3390/jmmp8030121
APA StyleLozano Pérez, A. S., Lozada Castro, J. J., & Guerrero Fajardo, C. A. (2024). Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses. Journal of Manufacturing and Materials Processing, 8(3), 121. https://doi.org/10.3390/jmmp8030121






