Effect of Liquid Miscibility Gap on Defects in Inconel 625–GRCop42 Joints through Analysis of Gradient Composition Microstructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Arc Melting Process
2.2. Microstructural Characterization
2.3. Computation
3. Results
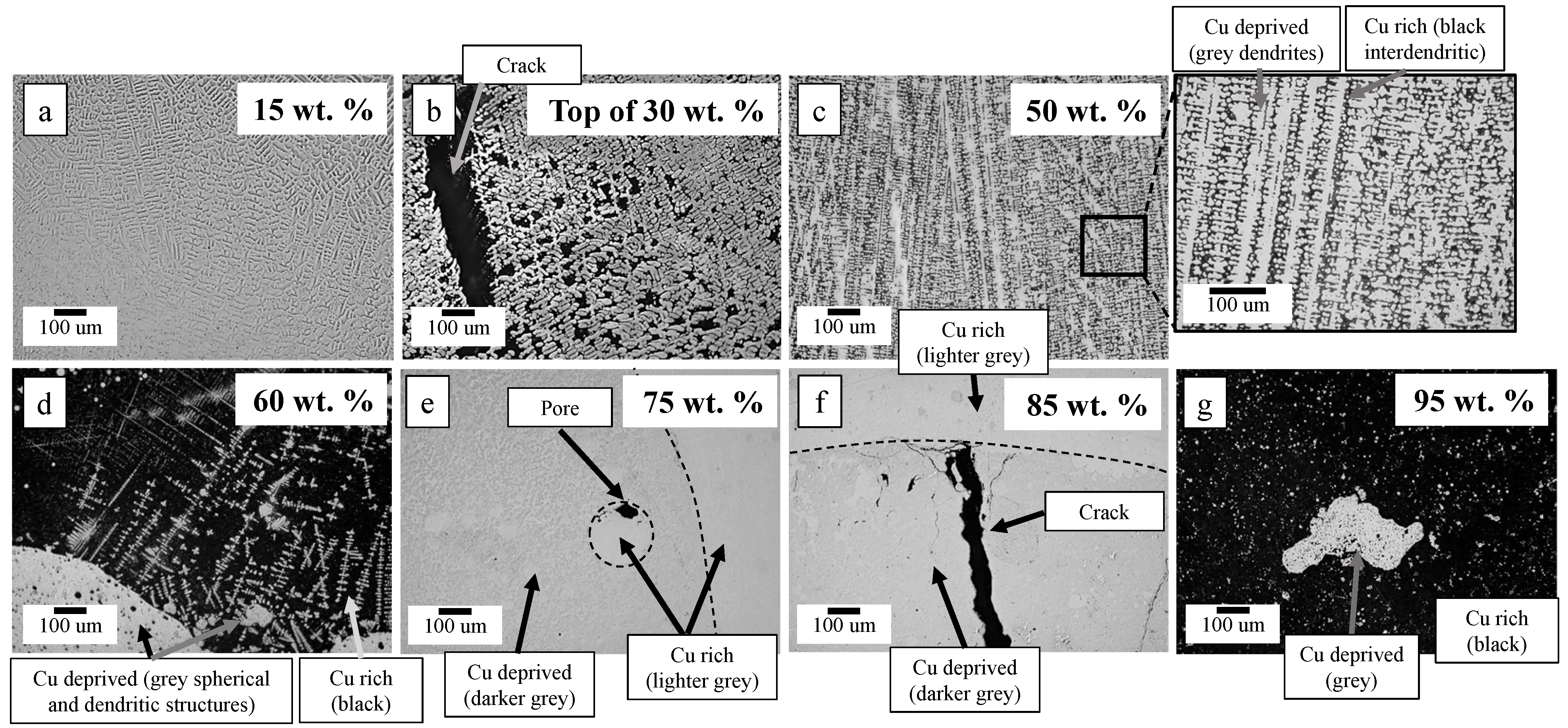
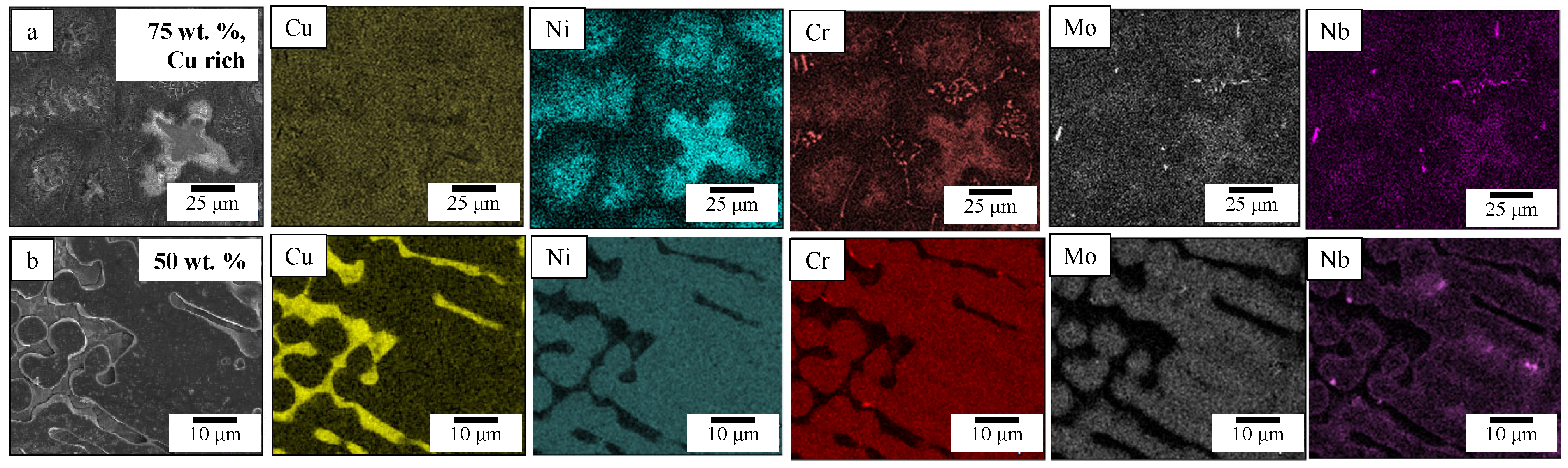
3.1. Microstructure Morphology and Composition
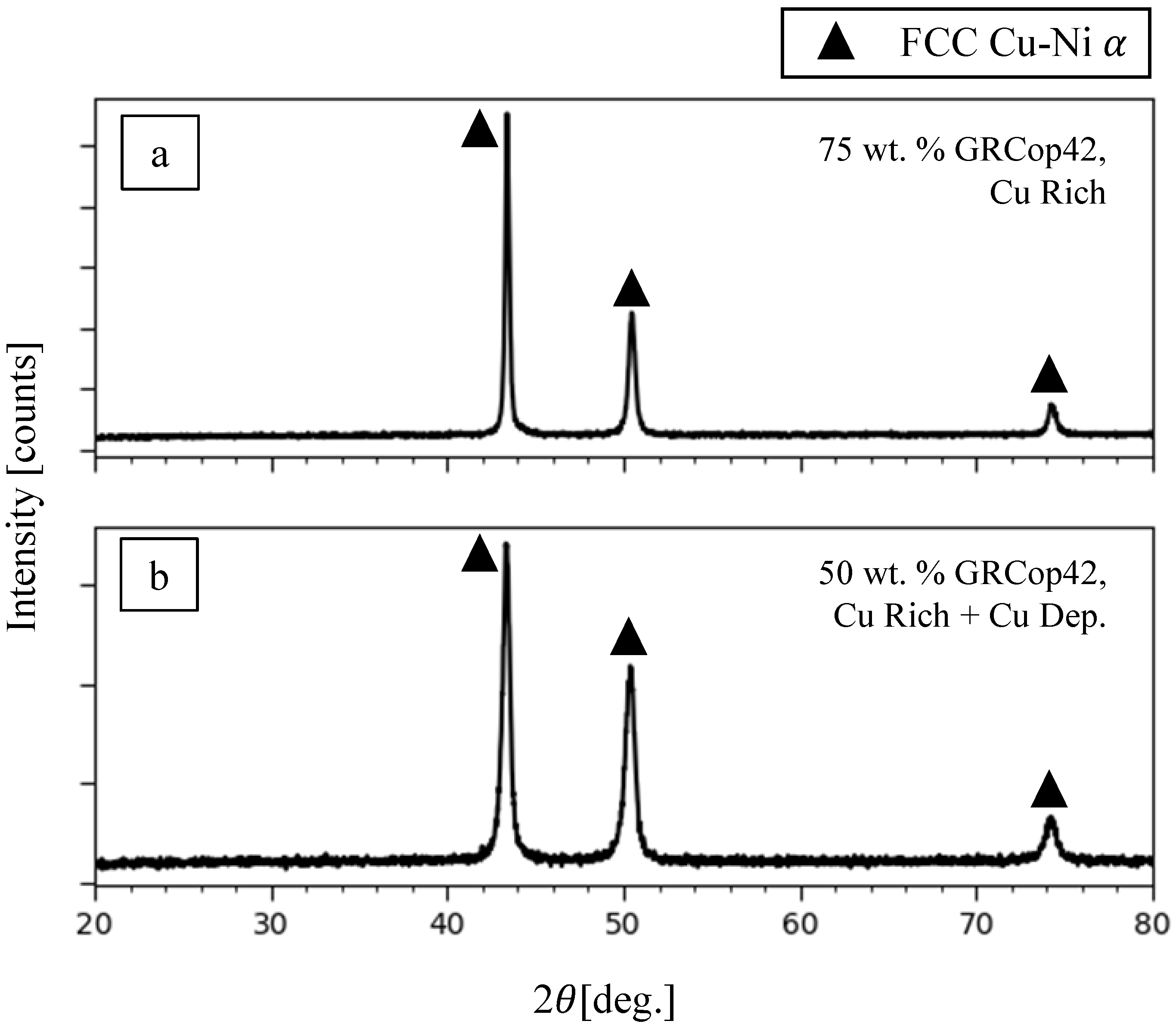
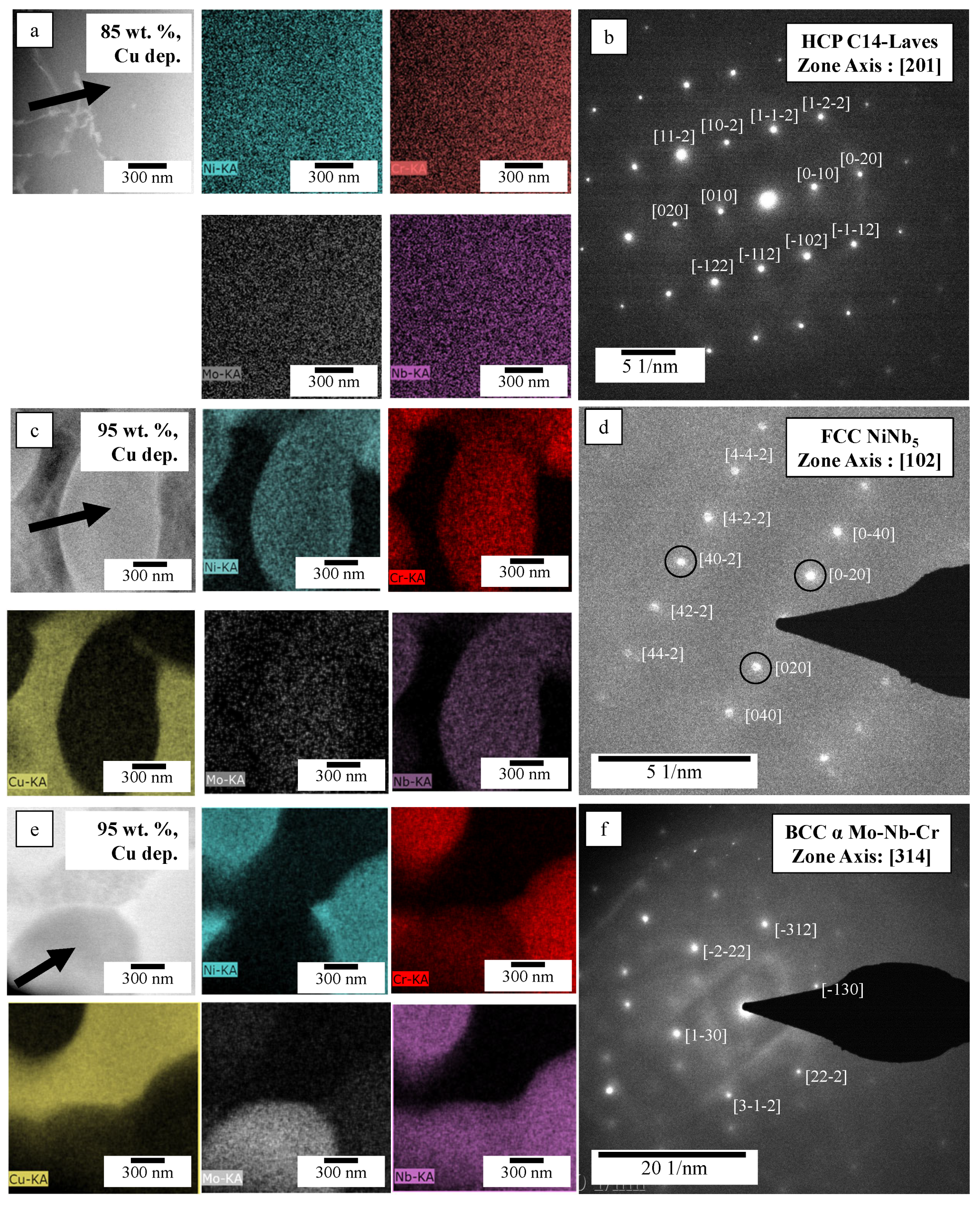
3.2. Phase Analysis
3.2.1. Cu-Rich Regions
3.2.2. 15–50 wt.% GRCop42 Cu-Deprived Regions
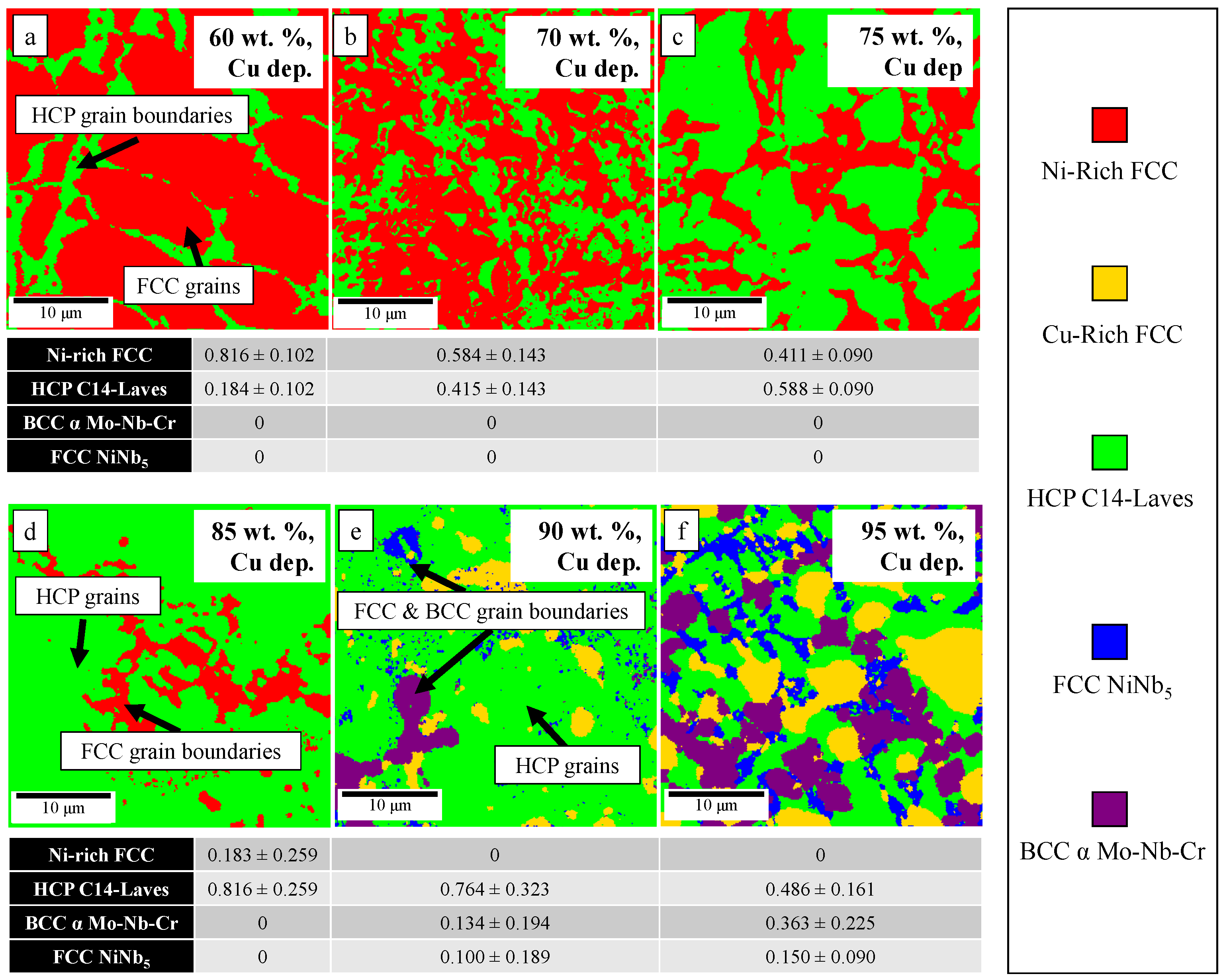
3.2.3. 60–95 wt.% GRCop42 Cu-Deprived Regions
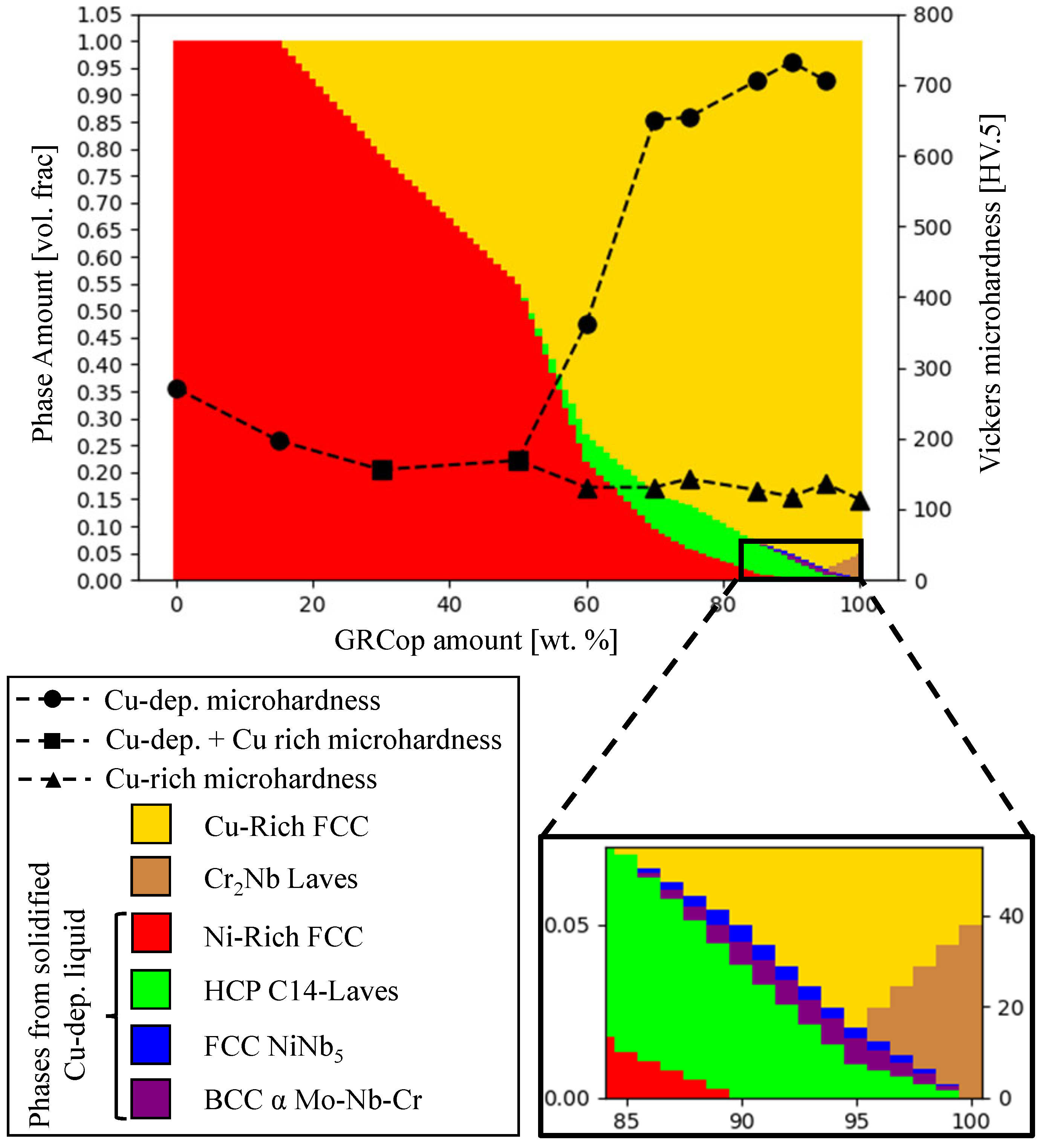
3.2.4. Microhardness
4. Discussion
4.1. A Liquid Miscibility Gap in Inconel 625–GRCop42 Mixtures and Formation of Cu-Deprived and Cu-Rich Regions
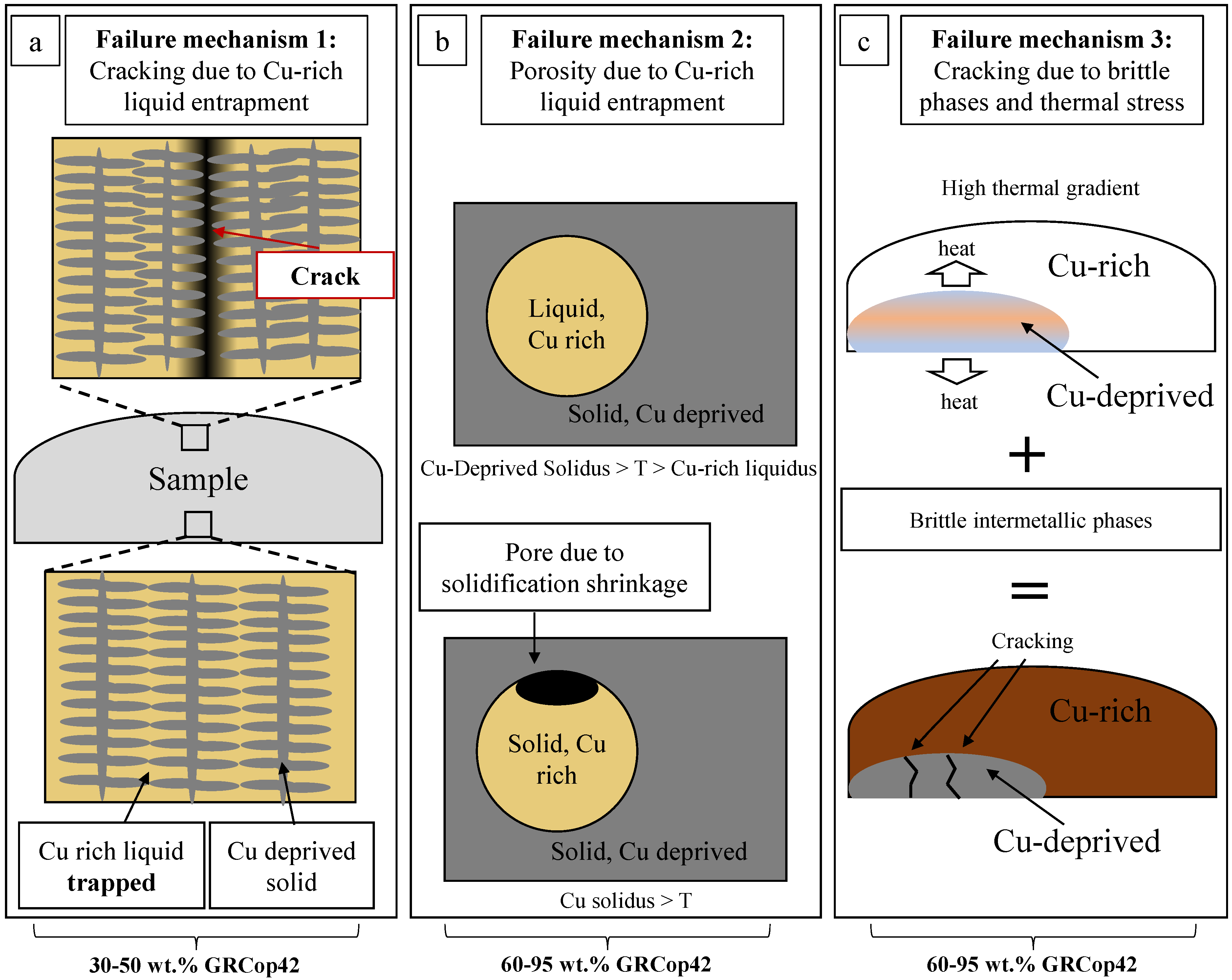
4.2. Failure Mechanisms in Inconel 625–GRCop42 Alloys
5. Conclusions
- At 30–50 wt.% GRCop42, Cu-rich liquid is entrapped within interdendritic boundaries, resulting in a Cu-rich liquid deficiency and cracking at the last region of solidification.
- At 60–95 wt.% Cu-rich liquid can become entrapped within Cu-deprived regions, leading to porosity due to solidification shrinkage.
- At 60–95 wt.% GRCop42, cracking occurs in Cu-deprived regions due to a combination of a brittle intermetallic phases and high thermal strain induced by arc melting.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Cu | Ni | Cr | Mo | Nb |
|---|---|---|---|---|---|
| wt.% GRCop42 | wt.% | wt.% | wt.% | wt.% | wt.% |
| 30 | 16.6 ± 2.0 | 52.3 ± 1.9 | 19.6 ± 1.7 | 7.6 ± 1.2 | 3.7 ± 2.5 |
| 50 | 16.5 ± 2.0 | 50.9 ± 1.3 | 19.9 ± 2.7 | 7.9 ± 2.7 | 4.7 ± 3.8 |
| 60 | 11.8 ± 1.4 | 44.3 ± 1.8 | 22.2 ± 1.2 | 12.5 ± 1.5 | 9.3 ± 0.9 |
| 70 | 9.3 ± 2.6 | 38.9 ± 1.9 | 21.4 ± 0.9 | 16.2 ± 0.3 | 14.2 ± 0.3 |
| 75 | 6.4 ± 1.8 | 38.4 ± 1.0 | 23.0 ± 1.3 | 15.5 ± 2.5 | 16.6 ± 0.9 |
| 85 | 2.8 ± 1.2 | 28.3 ± 4.6 | 22.0 ± 2.5 | 19.3 ± 1.4 | 27.6 ± 2.4 |
| 90 | 4.4 ± 3.0 | 25.2 ± 7.7 | 17.2 ± 2.5 | 19.4 ± 13.4 | 34.0 ± 4.5 |
| 95 | 5.1 ± 1.2 | 14.1 ± 2.3 | 13.7 ± 1.2 | 19.2 ± 4.5 | 47.9 ± 1.9 |
| Sample | Cu-dep. Amount | Cu-dep. Amount |
|---|---|---|
| wt.% GRCop42 | wt. frac. | vol. frac |
| 30 | 0.79 ± 0.126 | 0.79 ± 0.124 |
| 50 | 0.55 ± 0.189 | 0.55 ± 0.188 |
| 60 | 0.27 ± 0.034 | 0.27 ± 0.034 |
| 70 | 0.16 ± 0.003 | 0.16 ± 0.003 |
| 75 | 0.14 ± 0.024 | 0.14 ± 0.024 |
| 85 | 0.07 ± 0.005 | 0.07 ± 0.005 |
| 90 | 0.05 ± 0.032 | 0.05 ± 0.034 |
| 95 | 0.02 ± 0.005 | 0.02 ± 0.005 |
| Sample | Cu-Rich Density | Cu-Deprived Density |
|---|---|---|
| wt.% GRCop42 | g/cm3 | g/cm3 |
| 30 | 8.73 | 8.50 |
| 50 | 8.75 | 8.49 |
| 60 | 8.71 | 8.49 |
| 70 | 8.71 | 8.51 |
| 75 | 8.73 | 8.45 |
| 85 | 8.73 | 8.29 |
| 90 | 8.74 | 8.33 |
| 95 | 8.74 | 8.48 |

References
- Ellis, D.L.; Michal, G.M. Mechanical and Thermal Properties of Two Cu-Cr-Nb Alloys and NARloy-Z; NASA Conractor Report 198529; NASA: Cleveland, OH, USA, 1996. [Google Scholar]
- Anam, M.A. Microstructure and Mechanical Properties of Selective Laser Melted Superalloy Inconel 625; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Reed, R.C. The Superalloys Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006; Volume 9780521859042. [Google Scholar] [CrossRef]
- Gradl, P.R.; Protz, C.S.; Ellis, D.L.; Greene, S.E. Progress in additively manufactured copper-alloy GRCOP-84, GRCOP-42, and bimetallic combustion chambers for liquid rocket engines. In Proceedings of the International Astronautical Congress, IAC, 2019, Washington, DC, USA, 21–25 October 2019. [Google Scholar]
- Gradl, P.R.; Protz, C.; Fikes, J.; Clark, A.; Evans, L.; Miller, S.; Ellis, D.; Hudson, T. Lightweight thrust chamber assemblies using multi-alloy additive manufacturing and composite overwrap. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Virtual Event, 24–26 August 2020. [Google Scholar] [CrossRef]
- Hales, S.; Domack, C. Electron Beam Freeform Fabrication of Dissimilar Materials: Cracking in Inconel ® 625 Deposited on GRCop-84; National Aeronautics and Space Administration: Washington, DC, USA, 2020. [Google Scholar]
- Anderson, R.; Terrell, J.; Schneider, J.; Thompson, S.; Gradl, P. Characteristics of Bi-metallic Interfaces Formed During Direct Energy Deposition Additive Manufacturing Processing. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2019, 50, 1921–1930. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, X.; Yamazaki, T.; Sutton, A.; Cui, W.; Li, L.; Liou, F. Characteristics of Inconel 625—Copper bimetallic structure fabricated by directed energy deposition. Int. J. Adv. Manuf. Technol. 2020, 109, 1261–1274. [Google Scholar] [CrossRef]
- Onuike, B.; Heer, B.; Bandyopadhyay, A. Additive manufacturing of Inconel 718—Copper alloy bimetallic structure using laser engineered net shaping (LENS™). Addit. Manuf. 2018, 21, 133–140. [Google Scholar] [CrossRef]
- Blakey-Milner, B.; Gradl, P.; Snedden, G.; Brooks, M.; Pitot, J.; Lopez, E.; Leary, M.; Berto, F.; du Plessis, A. Metal additive manufacturing in aerospace: A review. Mater. Des. 2021, 209, 110008. [Google Scholar] [CrossRef]
- Gradl, P.R.; Protz, C.S.; Garcia, C.P.; Mireles, O.R.; MartinLeary. Introduction to and Applications of Additive Manufacturing for Propulsion. In Metal Additive Manufacturing for Propulsion Applications; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2023; Chapter 1; pp. 1–48. [Google Scholar] [CrossRef]
- Gradl, P.R.; Protz, C.; Zagorski, K.; Doshi, V.; McCallum, H. Additive manufacturing and hot-fire testing of bimetallic grcop-84 and c-18150 channel-cooled combustion chambers using powder bed fusion and inconel 625 hybrid directed energy deposition. In Proceedings of the AIAA Propulsion and Energy Forum and Exposition, 2019, Indianapolis, IN, USA, 19 August 2019. [Google Scholar] [CrossRef]
- Gradl, P.R.; Protz, C.; Cooper, K.; Garcia, C.; Ellis, D.; Evans, L. Grcop-42 development and hot-fire testing using additive manufacturing powder bed fusion for channel-cooled combustion chambers. In Proceedings of the AIAA Propulsion and Energy Forum and Exposition, 2019, Indianapolis, IN, USA, 19 August 2019; 2019. [Google Scholar] [CrossRef]
- Gradl, P.R.; Teasley, T.; Protz, C.; Garcia, M.; Kantzos, C.; Ellis, D. Advancing GRCop-based Bimetallic Additive Manufacturing to Optimize Component Design and Applications for Liquid Rocket Engines. In Proceedings of the AIAA Propulsion and Energy Forum, Virtual Event, 9–11 August 2021. [Google Scholar] [CrossRef]
- Kadoi, K.; Shinozaki, K. Effect of Chemical Composition on Susceptibility to Weld Solidification Cracking in Austenitic Weld Metal. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5860–5869. [Google Scholar] [CrossRef]
- Kah, P.; Vimalraj, C.; Martikainen, J.; Suoranta, R. Factors influencing Al-Cu weld properties by intermetallic compound formation. Int. J. Mech. Mater. Eng. 2015, 10, 10. [Google Scholar] [CrossRef]
- Atabaki, M.M.; Nikodinovski, M.; Chenier, P.; Ma, J.; Harooni, M.; Kovacevic, R. Welding of Aluminum Alloys to Steels: An Overview. J. Manuf. Sci. Prod. 2014, 14, 2. [Google Scholar] [CrossRef]
- Abdul Karim, M.; Park, Y.D. A Review on Welding of Dissimilar Metals in Car Body Manufacturing. J. Weld. Join. 2020, 38, 8–23. [Google Scholar] [CrossRef]
- Liu, J.; Kou, S. Crack susceptibility of binary aluminum alloys during solidification. Acta Mater. 2016, 110, 84–94. [Google Scholar] [CrossRef]
- Kou, S. A simple index for predicting the susceptibility to solidification cracking. Weld. J. 2015, 94, 374–388. [Google Scholar]
- Kou, S. Welding Metallurgy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Jasthi, B.K.; Kuca, T.S.; Ellingsen, M.D.; Ellis, D.L.; Kandadai, V.A.; Curtis, T.R. Microstructure and mechanical properties of cold spray additive manufactured Cu-Cr-Nb and Fe-Ni-Cr alloys. Addit. Manuf. 2023, 61, 103354. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.; Yeon, S.M.; Aranas, C.; Choi, K.; Yoon, J.; Yang, S.W.; Lee, H. Selective compositional range exclusion via directed energy deposition to produce a defect-free Inconel 718/SS 316L functionally graded material. Addit. Manuf. 2021, 47, 102288. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Kolodziejska, J.; Roberts, S.; Otis, R.; Dillon, R.P.; Suh, J.O.; Liu, Z.K.; Borgonia, J.P. Compositionally graded metals: A new frontier of additive manufacturing. J. Mater. Res. 2014, 29, 1899–1910. [Google Scholar] [CrossRef]
- Reichardt, A.; Shapiro, A.A.; Otis, R.; Dillon, R.P.; Borgonia, J.P.; McEnerney, B.W.; Hosemann, P.; Beese, A.M. Advances in additive manufacturing of metal-based functionally graded materials. Int. Mater. Rev. 2021, 66, 1–29. [Google Scholar] [CrossRef]
- Feenstra, D.R.; Banerjee, R.; Fraser, H.L.; Huang, A.; Molotnikov, A.; Birbilis, N. Critical review of the state of the art in multi-material fabrication via directed energy deposition. Curr. Opin. Solid State Mater. Sci. 2021, 25, 1899–1910. [Google Scholar] [CrossRef]
- Liu, Z.K. First-principles calculations and CALPHAD modeling of thermodynamics. J. Phase Equilibria Diffus. 2009, 30, 517–534. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, Q. Effects of Cr content and electromagnetic stirring on the phase separation of Cu-Cr alloy. Mater. Res. Express 2022, 9, 5. [Google Scholar] [CrossRef]
- Munitz, A.; Venkert, A.; Landau, P.; Kaufman, M.J.; Abbaschian, R. Microstructure and phase selection in supercooled copper alloys exhibiting metastable liquid miscibility gaps. J. Mater. Sci. 2012, 47, 7955–7970. [Google Scholar] [CrossRef]
- Koutsky, J.; Kletečka, Z. Vacuum arc re-melting and its influence on mechanical properties of steels. Vacuum 1969, 19, 187–189. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, X.J.; Takaku, Y.; Ohnuma, I.; Kainuma, R.; Ishida, K. Formation of core-type macroscopic morphologies in Cu-Fe base alloys with liquid miscibility gap. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35, 1243–1253. [Google Scholar] [CrossRef]
- Li, S.; Li, J.Y.; Jiang, Z.W.; Cheng, Y.; Li, Y.Z.; Tang, S.; Leng, J.Z.; Chen, H.X.; Zou, Y.; Zhao, Y.H.; et al. Controlling the columnar-to-equiaxed transition during Directed Energy Deposition of Inconel 625. Addit. Manuf. 2022, 57, 102958. [Google Scholar] [CrossRef]
- Kaufman, L.; Ågren, J. CALPHAD, first and second generation—Birth of the materials genome. Scr. Mater. 2014, 70, 3–6. [Google Scholar] [CrossRef]
- Kaufman, L.; Bernstein, H. Computer calculation of phase diagrams. With special reference to refractory metals. Met. Powder Rep. 2000, 55, 2004171. [Google Scholar]
- Thermo-Calc Software, TCCU Cu-Alloys Database. version 4. Thermo-Calc Software: Stockholm, Sweden, 2023.
- Grüner, D.; Ormeci, A.; Kreiner, G. Crystal structure of niobium chromium nickel, Nb(Cr1-xNix)2 (x = 0.49). New Cryst. Struct. 2014, 221, 269–270. [Google Scholar] [CrossRef]
- Iams, A.; Lienert, T.; Otazu, D.; Ramoni, M. Effects of deposition sequence on microstructural evolution in additively manufactured Cu-Cr-Nb alloy/superalloy bimetallic structures. Addit. Manuf. Lett. 2023, 6, 100151. [Google Scholar] [CrossRef]
- Šlapáková, M.; Zendegani, A.; Liebscher, C.H.; Hickel, T.; Neugebauer, J.; Hammerschmidt, T.; Ormeci, A.; Grin, J.; Dehm, G.; Kumar, K.S.; et al. Atomic scale configuration of planar defects in the Nb-rich C14 Laves phase NbFe2. Acta Mater. 2020, 183, 362–376. [Google Scholar] [CrossRef]
- Du, Y.; Liu, S.; Chang, Y.A.; Yang, Y. A thermodynamic modeling of the Cr–Nb–Ni system. Calphad 2005, 29, 140–148. [Google Scholar] [CrossRef]
- Kripyakevich, P.I.; Pylaeva, E.N. Crystalline Structures of Some Compounds in the Nb- Ni and Ta-Ni Systems. Sov. Phys. Crystallogr. 1967, 12, 294–296. [Google Scholar]
- Nash, P.; Nash, A. The Nb-Ni (Niobium-Nickel) system. Bull. Alloy. Phase Diagrams 1986, 7, 124–130. [Google Scholar] [CrossRef]
- Goldschmidt, H.J.; Brand, J.A. The constitution of the chromium-niobium-molybdenum system. J. Less Common Met. 1961, 3, 44–61. [Google Scholar] [CrossRef]
- Ohnuma, I.; Saegusa, T.; Takaku, Y.; Wang, C.P.; Liu, X.J.; Kainuma, R.; Ishida, K. Microstructural evolution of alloy powder for electronic materials with liquid miscibility gap. J. Electron. Mater. 2009, 38, 2–9. [Google Scholar] [CrossRef]
- Gor, M.; Soni, H.; Wankhede, V.; Sahlot, P.; Grzelak, K.; Szachgluchowicz, I.; Kluczyński, J. A critical review on effect of process parameters on mechanical and microstructural properties of powder-bed fusion additive manufacturing of ss316l. Materials 2021, 14, 6527. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.g.; Gao, H.j.; Zhang, Y.d.; Wu, Q.; Gao, Z.h.; Zhou, X. Review on residual stresses in metal additive manufacturing: Formation mechanisms, parameter dependencies, prediction and control approaches. J. Mater. Res. Technol. 2022, 17, 2950–2974. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams for Binary Alloys; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]

| Alloy | Ni | Cr | Nb | Ta | Mo | Cu | Ti | Fe | Al | Si | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inconel 625 | 64.8 | 22.2 | 3.49 | 8.6 | 0.016 | 0.21 | 0.26 | 0.14 | 0.09 | 0.194 | |
| GRCop42 | - | 3.3 | 2.7 | - | - | Bal | - | - | - | - | - |
| Sample | Microstructure Morphology | Macro Defects | Cu-Dep. Amount |
|---|---|---|---|
| wt.% GRCop42 | vol. frac. | ||
| 15 | Dendritic solid solution | None | 1 |
| 30 | Cu-deprived dendrites with Cu-rich interdendritic regions | Cracking at top of part | 0.79 |
| 50 | " | " | 0.55 |
| 60 | Cu-deprived spherical region and Cu-deprived dendrites in Cu-rich matrix | Porosity in Cu-deprived spherical regions | 0.27 |
| 70 | Cu-deprived spherical region in Cu-rich matrix | Porosity and cracking in Cu-deprived region | 0.16 |
| 75 | " | " | 0.14 |
| 85 | " | " | 0.07 |
| 90 | " | " | 0.05 |
| 95 | " | " | 0.02 |
| Sample | Phase | Cu | Ni | Cr | Mo | Nb |
|---|---|---|---|---|---|---|
| wt.% GRCop42 | wt.% | wt.% | wt.% | wt.% | wt.% | |
| 85 | HCP C14-Laves | - | 34.9 ± 3.1 | 19.9 ± 1.8 | 15.3 ± 4.6 | 29.8 ± 2.7 |
| 95 | HCP C14-Laves | - | 22.6 ± 2.1 | 18.2 ± 1.7 | 5.9 ± 1.84 | 53.2 ± 5.3 |
| 95 | FCC NiNb5 | - | 18.2 ± 1.8 | 13.1 ± 1.3 | - | 68.7 ± 6.8 |
| 95 | BCC Mo-Nb-Cr | - | 1.0 ± 0.1 | 4.5 ± 0.4 | 49.9 ± 4.6 | 44.6 ± 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preis, J.; Xu, D.; Paul, B.K.; Eschbach, P.A.; Pasebani, S. Effect of Liquid Miscibility Gap on Defects in Inconel 625–GRCop42 Joints through Analysis of Gradient Composition Microstructure. J. Manuf. Mater. Process. 2024, 8, 42. https://doi.org/10.3390/jmmp8010042
Preis J, Xu D, Paul BK, Eschbach PA, Pasebani S. Effect of Liquid Miscibility Gap on Defects in Inconel 625–GRCop42 Joints through Analysis of Gradient Composition Microstructure. Journal of Manufacturing and Materials Processing. 2024; 8(1):42. https://doi.org/10.3390/jmmp8010042
Chicago/Turabian StylePreis, Jakub, Donghua Xu, Brian K. Paul, Peter A. Eschbach, and Somayeh Pasebani. 2024. "Effect of Liquid Miscibility Gap on Defects in Inconel 625–GRCop42 Joints through Analysis of Gradient Composition Microstructure" Journal of Manufacturing and Materials Processing 8, no. 1: 42. https://doi.org/10.3390/jmmp8010042
APA StylePreis, J., Xu, D., Paul, B. K., Eschbach, P. A., & Pasebani, S. (2024). Effect of Liquid Miscibility Gap on Defects in Inconel 625–GRCop42 Joints through Analysis of Gradient Composition Microstructure. Journal of Manufacturing and Materials Processing, 8(1), 42. https://doi.org/10.3390/jmmp8010042









