Separation of Multi-Material Polymer Combinations Produced by Joining Using Pin-like Structures
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
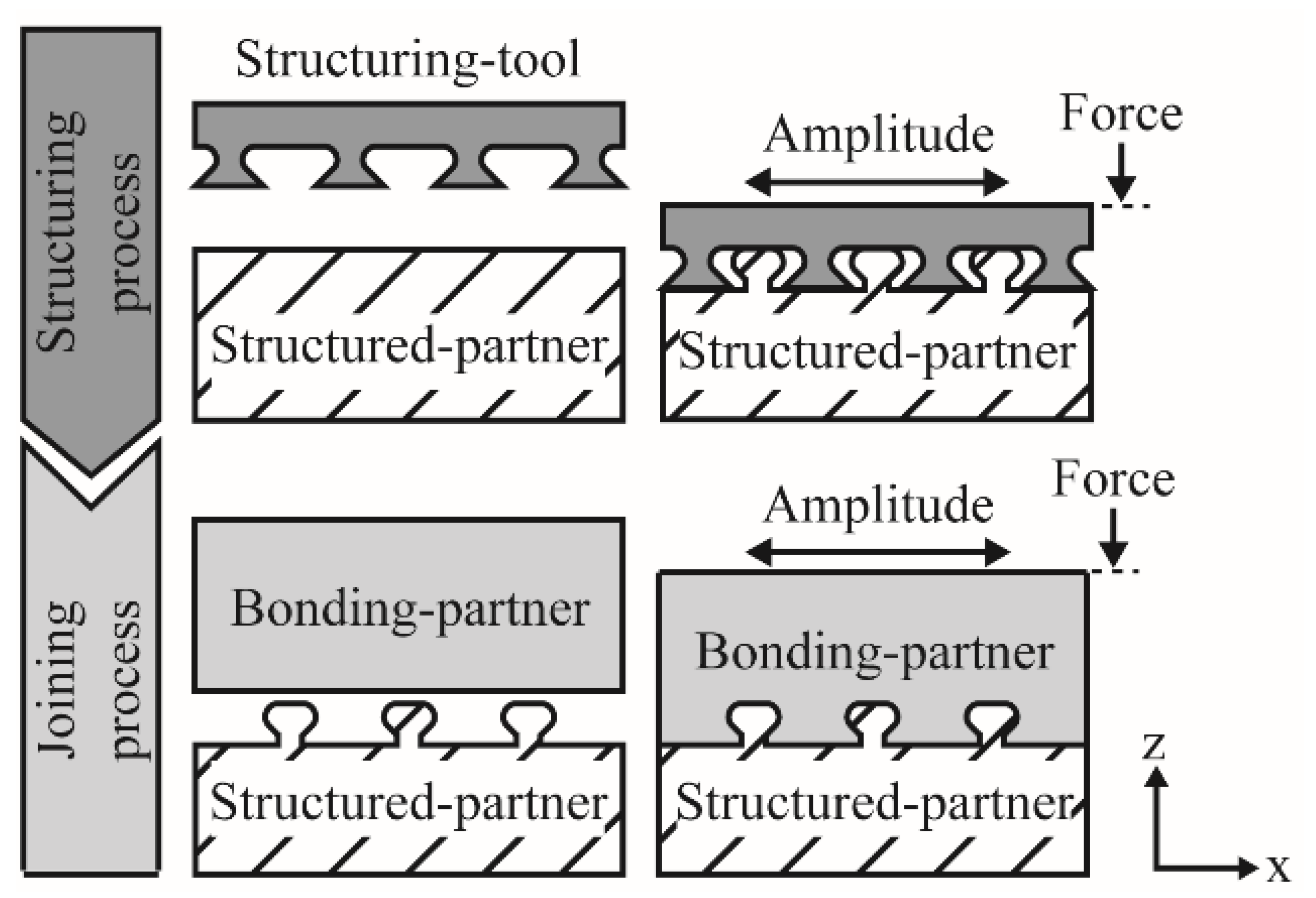
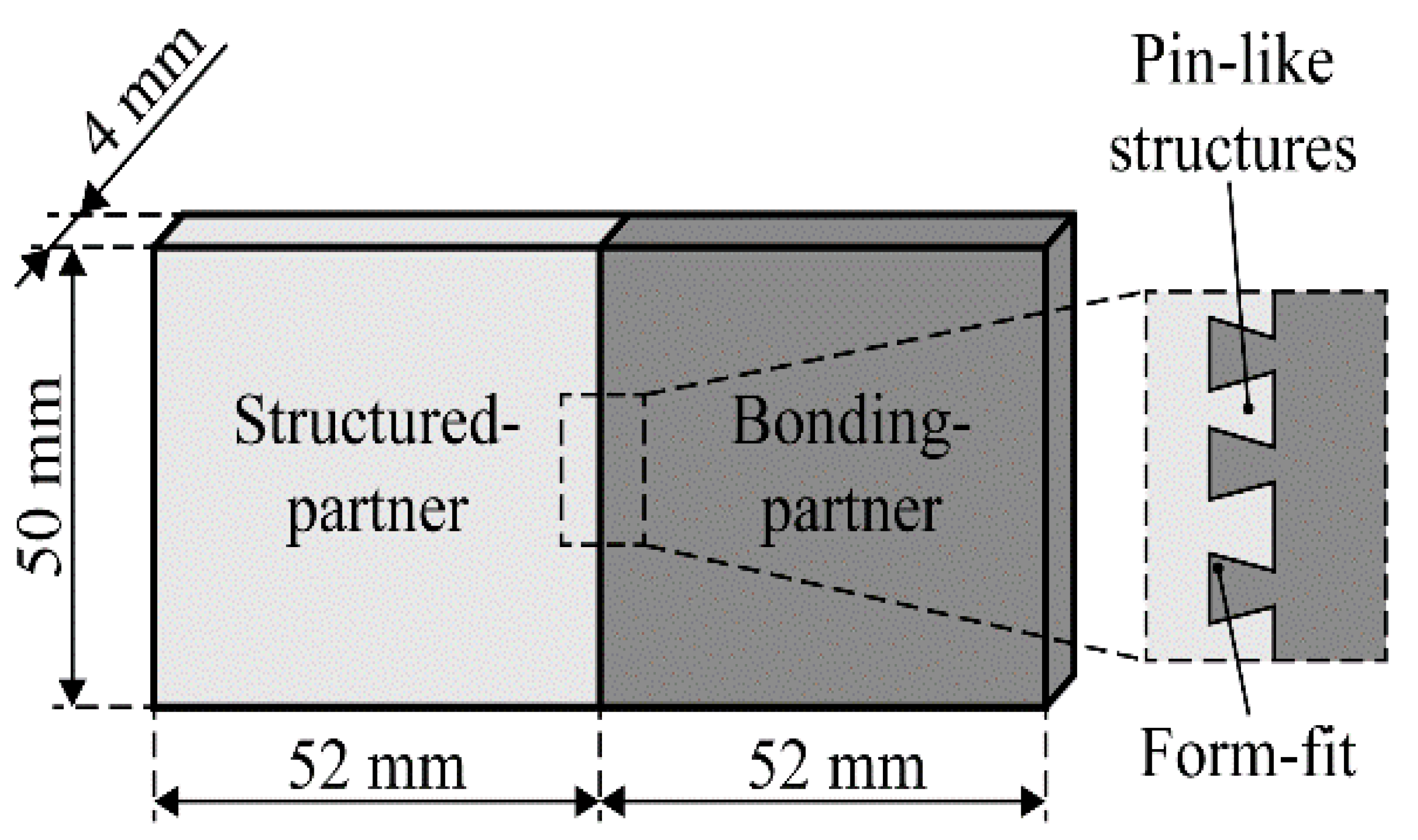
2.2. Joining by Use of Pin-Like Structures
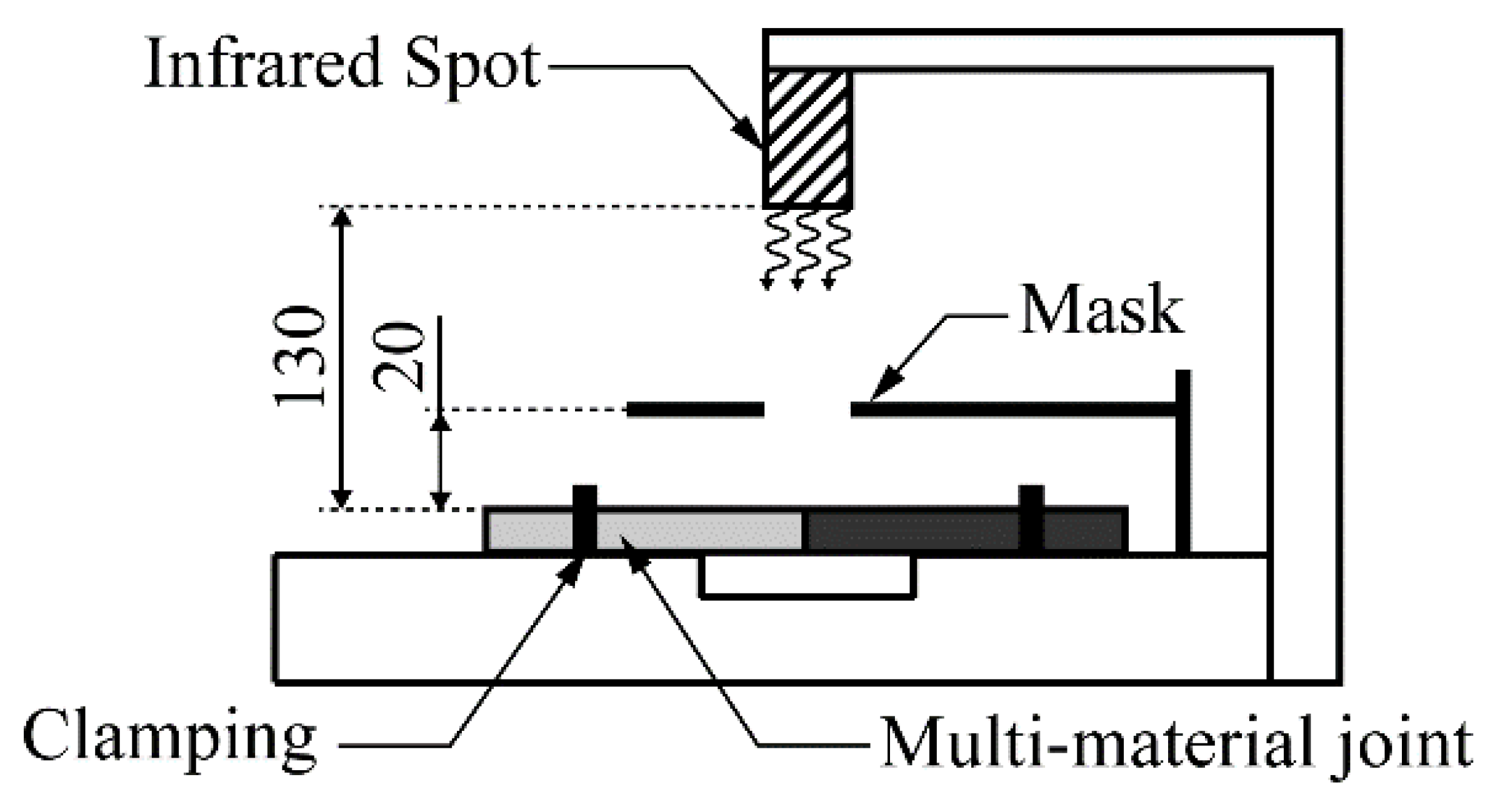
2.3. Separation Processes
2.4. Analyzing Methods
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Rotational Rheometry
2.4.3. Surface Tension
2.4.4. Viscosity Number
2.4.5. Single Material Dissolution
2.4.6. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Result and Discussion
3.1. Base Material Characterization
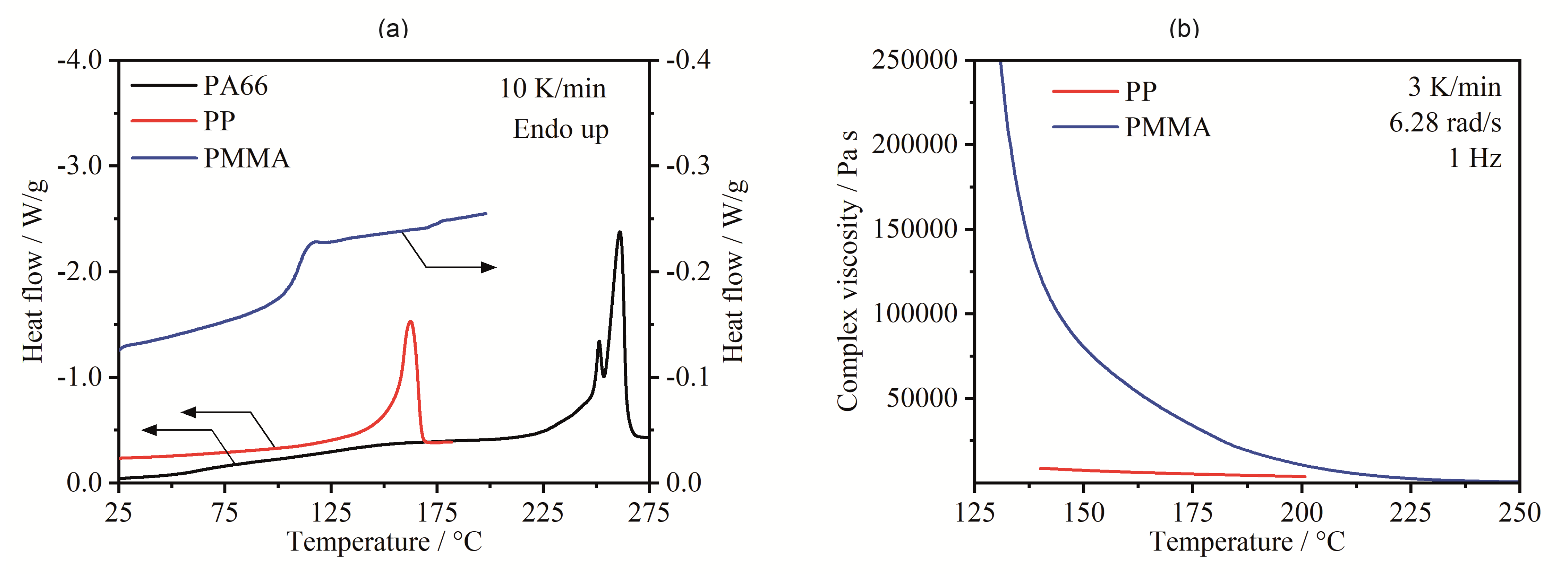
3.1.1. Thermal Properties
3.1.2. Surface Tension
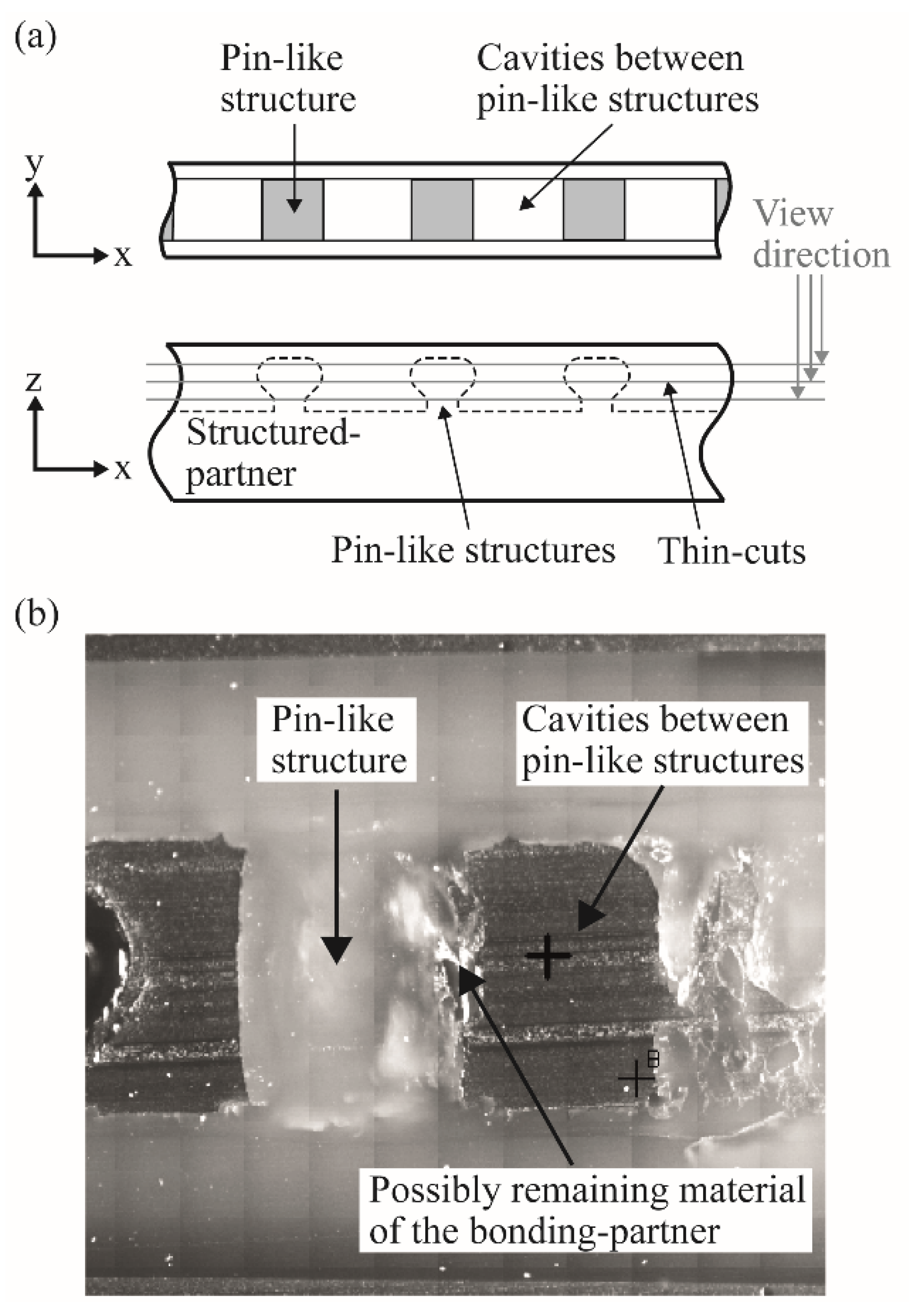
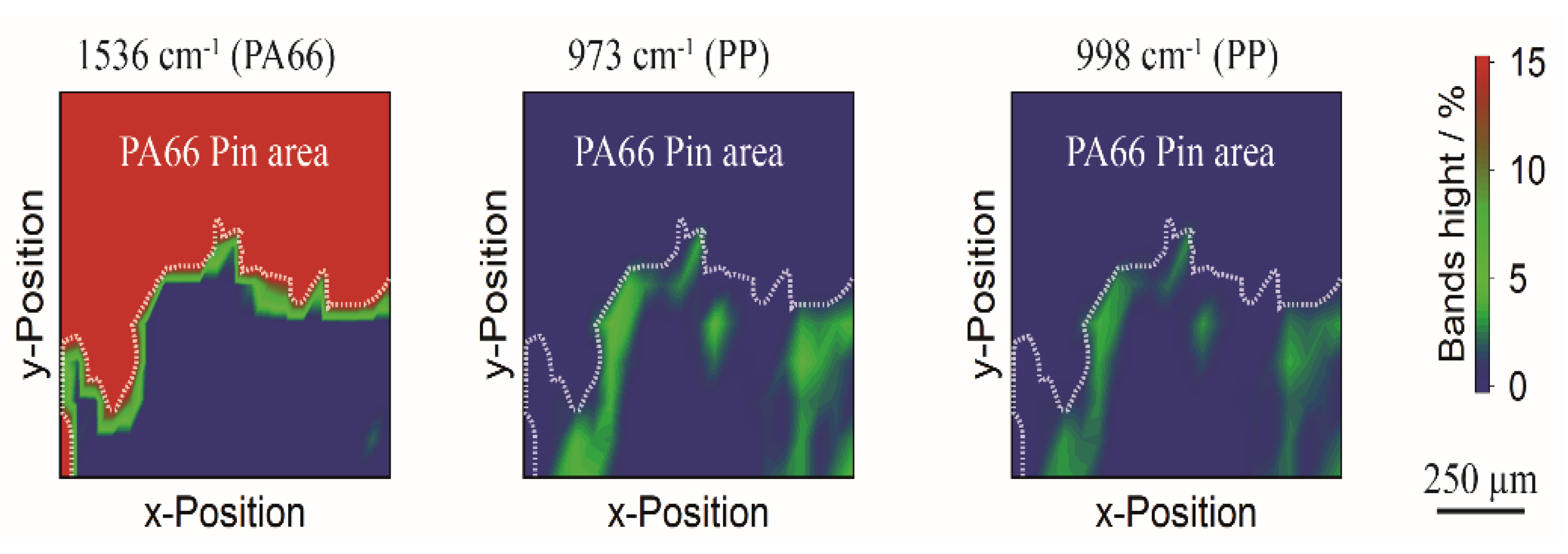
3.2. Separation by Shredding
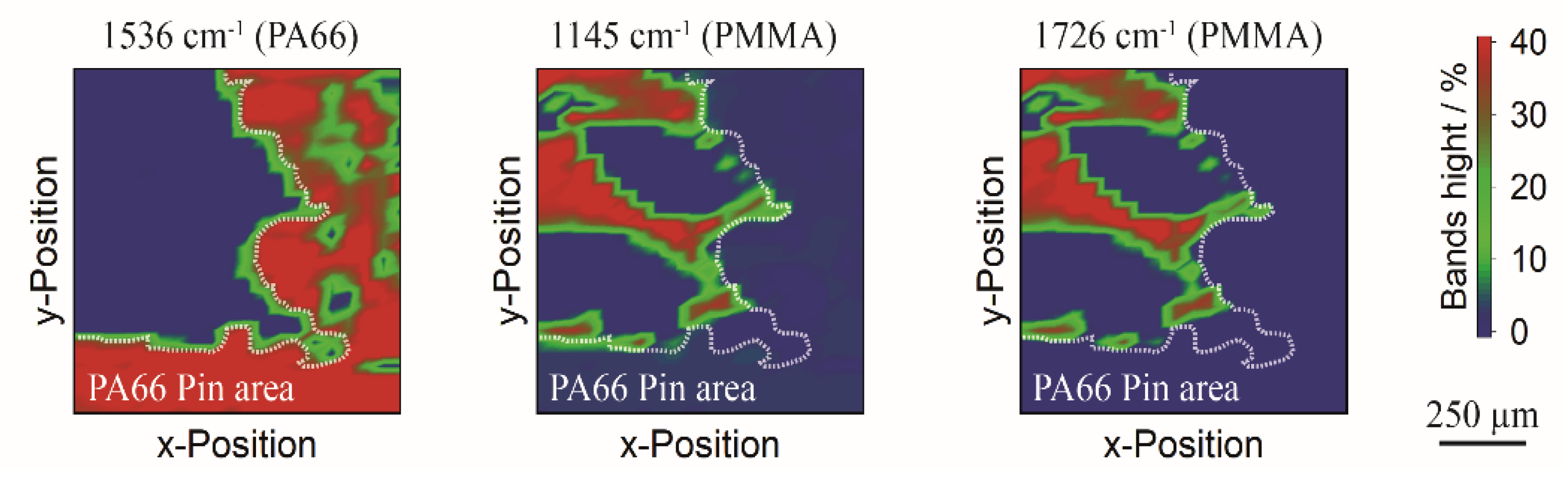
3.3. Thermal Separation
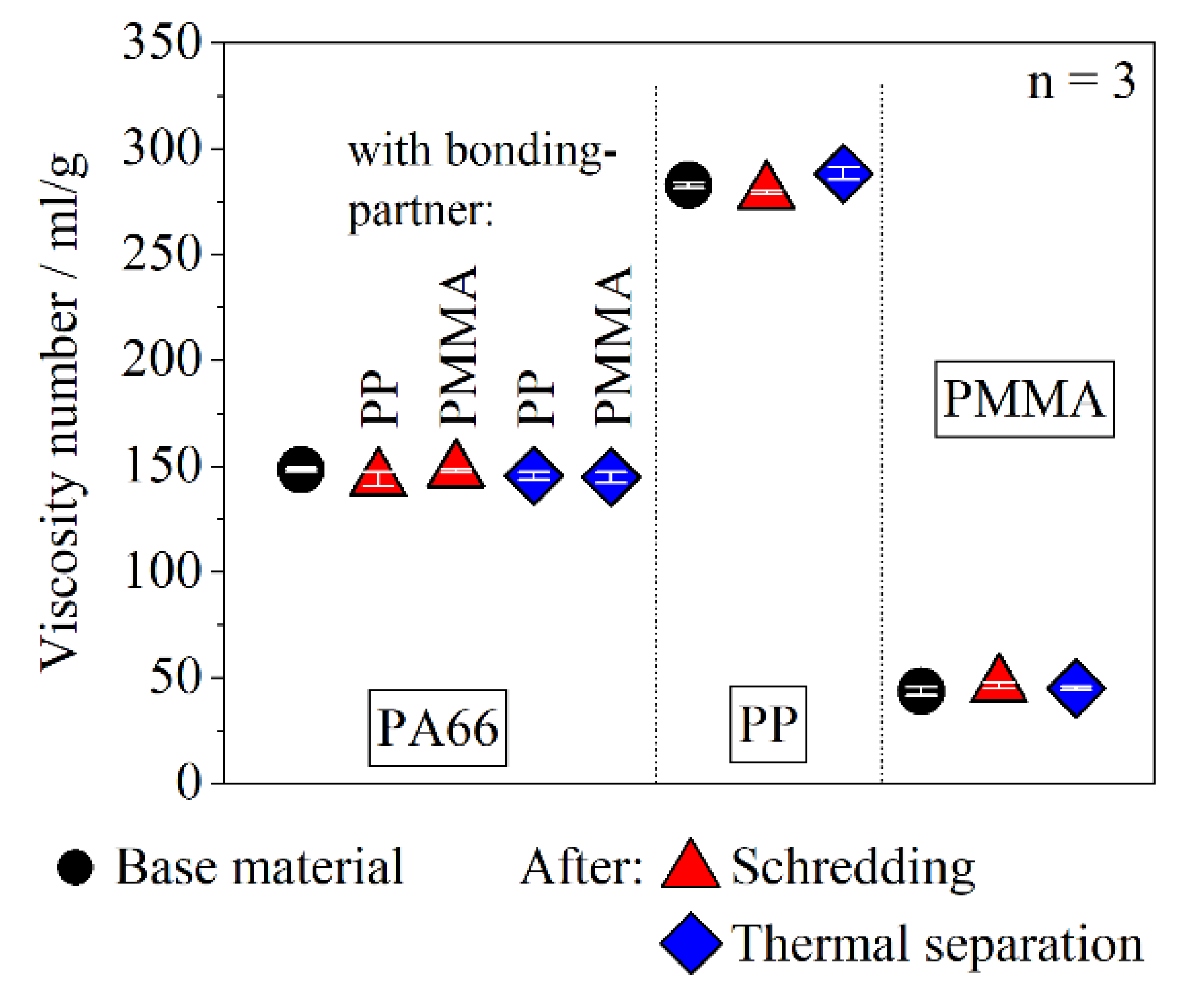
3.4. Material Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westkämper, E.; Löffler, C. Strategien der Produktion-Technologien, Konzepte und Wege in Die Praxis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Concil Directive (EC) 2000/53/EC of the European Parliament and of the Council of 18 September 2000 on End-of Life Vehicles; European Council: Brussels, Belgium, 2000.
- Rotheiser, J. Joining of Plastics-Handbook for Designers and Engineers; Hanser: München, Germany, 1999. [Google Scholar]
- DVS-Deutscher Verband für Schweißen und verwandte Verfahren. Taschenbuch DVS-Merkblätter und -Richtlinien, Band 68/4-Fügen von Kunststoffen, 18, Überarbeitete und Erweiterte Auflage, Fachbuchreihe Schweißtechnik Band 68/IV; DVS Media GmbH: Düsseldorf, Germany, 2020. [Google Scholar]
- Habenicht, G. Kleben. Grundlagen, Technologie, Anwendungen mit 37 Tabellen, 6th ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Ehrenstein, G.W. Handbuch Kunststoff-Verbindungstechnik; Hanser: München, Germany, 2004. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y. An Evaluation and Development of Bonding Technologies for Rapid Disassembly of Automotive Vehicles. Ph.D. Thesis, Oxford Brookes University, Oxford, UK, 2015. [Google Scholar]
- Essig, O.; Hartweg, M.; Keller, M.; Tomaschko, S. Methods and Apparatuses for Detaching Components Adhesively Bonded to One Another. U.S. Patent US6716297B2, 6 April 2004. [Google Scholar]
- Banea, M.; da Silva, L.; Campilho, R. An overview of the technologies for adhesive debonding on command. Weld. Equip. Technol. 2013, 24, 11–14. [Google Scholar]
- Malnati, P. Reversible Multi-Material Adhesive Bonds. Available online: https://www.compositesworld.com/articles/reversible-multi-material-adhesive-bonds (accessed on 13 November 2017).
- Haq, M.; Koricho, E.; Khomenko, A.; Gerth, R.; Drzahl, L. Tailorable Adhesives for Multi-material Joining, Facile Repair and Re-assembly. In Proceedings of the American Society for Composites 30th Technical Conference, East Lansing, MI, USA, 28–30 September 2015. [Google Scholar]
- Wolf, M.; Kleffel, T.; Leisen, C.; Drummer, D. Joining of Incompatible Polymer Combinations by Form Fit Using the Vibration Welding Process. Int. J. Polym. Sci. 2017, 2017, 6809469. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.; Drummer, D. Influence of the Structuring-Tool Geometry on Form-Fit Joining by Use of Pin-Like Structures in Vibration Welding. Procedia Manuf. 2020, 47, 375–382. [Google Scholar] [CrossRef]
- Wolf, M.; Drummer, D. Design criteria for the pin-foot ratio for joining adhesion-incompatible polymers using pin-like structures in vibration welding process. J. Polym. Eng. 2021, 41, 873–882. [Google Scholar] [CrossRef]
- Wolf, M.; Hertle, S.; Drummer, D. Influence of the thermomechanical properties on the joining of adhesion incompatible polymers by form-fit using the vibration welding process. Express Polym. Lett. 2019, 13, 365–378. [Google Scholar] [CrossRef]
- BASF SE. Product Information Ultramid®-A3K. Available online: https://documents.basf.com/8c1cc60248e98198abfcbfb4b73d5b78eae0c0c4?response-content-disposition=inline (accessed on 8 April 2021).
- Sabic. Sabic® PP 505P. Available online: https://www.sabic.com/en/products/documents/sabic-pp_505p_global_technical_data_sheet/en (accessed on 8 April 2021).
- Röhm GmbH. Product Informatio—Plexiglas® 7N. Available online: https://www.plexiglas-polymers.com/en/plexiglas-7n?file=files/assets/04-molding_compounds/plexiglas-polymers/downloads/en/product-information/PLEXIGLAS/7N/PLEXIGLAS%207N_EN.pdf (accessed on 8 April 2021).
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Deutsches Institut für Normung (DIN). Kunststoffe-Bestimmung der Viskosität von Polymeren in Verdünnter Lösung Durch ein Kapillarviskosimeter-Teil 3: Polyethylen und Polypropylen (DIN EN ISO 1628-3:2010); Deutsches Institut für Normung (DIN): Berlin, Germany, 2010. [Google Scholar]
- Deutsches Institut für Normung (DIN). Kunststoffe; Bestimmung der Viskositätszahl und der Grenzviskositätszahl; Teil 6: Methylmethacrylatpolymere (DIN EN ISO 1628-6:1990-02); Deutsches Institut für Normung (DIN): Berlin, Germany, 1990. [Google Scholar]
- Deutsches Institut für Normung (DIN). Kunststoffe-Polyamide-Bestimmung der Viskositätszahl (DIN EN ISO 307:2019); Deutsches Institut für Normung (DIN): Berlin, Germany, 2019. [Google Scholar]

| Material | PA66 | PP | PMMA |
|---|---|---|---|
| Melt temperature [°C] | 290 | 250 | 230 |
| Mold temperature [°C] | 70 | 60 | 60 |
| Injection speed [mm/s] | 50 | 60 | 60 |
| Holding pressure [bar] | 450 | 250 | 450 |
| Holding time [s] | 30 | 40 | 25 |
| Process Steps | Structuring | Joining |
|---|---|---|
| Frequency [Hz] | 235 | 235 |
| Amplitude [mm] | 0.7 | 0.7 |
| Force [N] | 600 | 400 |
| Path [mm] | 1.2 | 1.5 |
| Materials | Solid Materials (Contact Angle Measurement) | Melt (Pendant Drop Method) | |||
|---|---|---|---|---|---|
| Wu | OWRK | ||||
| Surface Energy | Polar Fraction | Surface Energy | Polar Fraction | Surface Energy | |
| PA66 | 38.1 | 18.6 | 34.9 | 16.8 | - |
| PP | 26.9 | 10.6 | 23.3 | 6.1 | 19.2 ± 0.4 (at 170 °C) |
| PMMA | 40.1 | 15.1 | 36.4 | 12.8 | 24.6 ± 0.5 (at 230 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, M.; Drummer, D. Separation of Multi-Material Polymer Combinations Produced by Joining Using Pin-like Structures. J. Manuf. Mater. Process. 2022, 6, 13. https://doi.org/10.3390/jmmp6010013
Wolf M, Drummer D. Separation of Multi-Material Polymer Combinations Produced by Joining Using Pin-like Structures. Journal of Manufacturing and Materials Processing. 2022; 6(1):13. https://doi.org/10.3390/jmmp6010013
Chicago/Turabian StyleWolf, Michael, and Dietmar Drummer. 2022. "Separation of Multi-Material Polymer Combinations Produced by Joining Using Pin-like Structures" Journal of Manufacturing and Materials Processing 6, no. 1: 13. https://doi.org/10.3390/jmmp6010013
APA StyleWolf, M., & Drummer, D. (2022). Separation of Multi-Material Polymer Combinations Produced by Joining Using Pin-like Structures. Journal of Manufacturing and Materials Processing, 6(1), 13. https://doi.org/10.3390/jmmp6010013






