1. Introduction
Structural elements used for offshore facilities, e.g., wind turbines and oil rigs, are exposed to various loads such as mechanical stresses and corrosive environments. Nevertheless, the components, which are mostly built from structural steel due to the conditions of use, weldability, and relatively low costs, must endure these loads over decades, as replacement or maintenance is difficult and costly in most cases. A combination of static and dynamic mechanical stresses generated by wind, waves, tide, biofouling, and floating ice, as well as corrosive stress from seawater, mist, and weather, acts on the components and leads, e.g., to crack formation and crack corrosion [
1]. Thus, suitable protection of the components is necessary, and this can be achieved by, e.g., cathodic protection, coatings, and post-treatment of the components [
2,
3].
For corrosion protection, combinations of anti-corrosion coatings with additional organic coatings such as paintings as well as sacrificial anode protection and impressed current cathodic protection are mainly used [
4]. Especially in the atmospheric and splash zones, where the materials are exposed to both maritime atmosphere and seawater, protective coatings are generally applied [
5]. For this, ZnAl-based coatings are often used [
6]. This kind of coating counteracts surface corrosion in two ways. First, the electropositive zinc acts as a sacrificial anode. Second, the formation of aluminum oxide effectuates passivation of the workpiece surface [
7].
ZnAl-based corrosion protection can be applied by either galvanization or thermal-spraying processes. The electroplating bath used for galvanization processes limits the size of components. In addition, the intense heating during this process can lead to the formation of brittle Fe-Zn intermetallic phases [
8], which can negatively influence the corrosion fatigue behavior of the components. Thermal spraying such as Twin Wire Arc Spraying (TWAS), on the other hand, introduces significantly less heat into the components, imposes minor restrictions on the dimensions of components, and can be applied on-site [
9]. Compared to other spraying techniques like cold spraying, flame spraying or plasma spraying, TWAS allows the highest deposition rates and, therefore, the lowest process times and costs [
10].
Disadvantages of thermally sprayed coatings compared to galvanic coatings include often-higher porosities [
11], heterogeneous compositions, lamellar layered microstructures, and thermally or kinetically induced residual stresses. Depending on the inherent process characteristics, the induced residual stresses include tensile residual stresses [
12] in the case of higher temperatures, and compressive residual stresses [
13] in case of higher kinetic energy process types. Especially for ZnAl coatings, due to their low melting point, compressive residual stresses have been observed. The reason for this is that the kinetic energy effect, caused by the larger and not completely melted particles at impact during the TWAS process, is higher than the thermal effect [
14]. Pores as well as tensile residual stresses in coatings can facilitate the formation and propagation of fatigue cracks [
15] and cause the coating to delaminate and flake, which can lead to local corrosion attacks. Thus, ZnAl-based coatings used in maritime environments often require additional protection like organic coatings [
16]. These multi-layer coatings are difficult to apply and restore. Additionally, these protective systems have a non-negligible environmental impact. The degradation of the sacrificial anodes [
17,
18] or organic compounds from paints [
19], for example, could have toxic effects that have not yet been sufficiently investigated [
20].
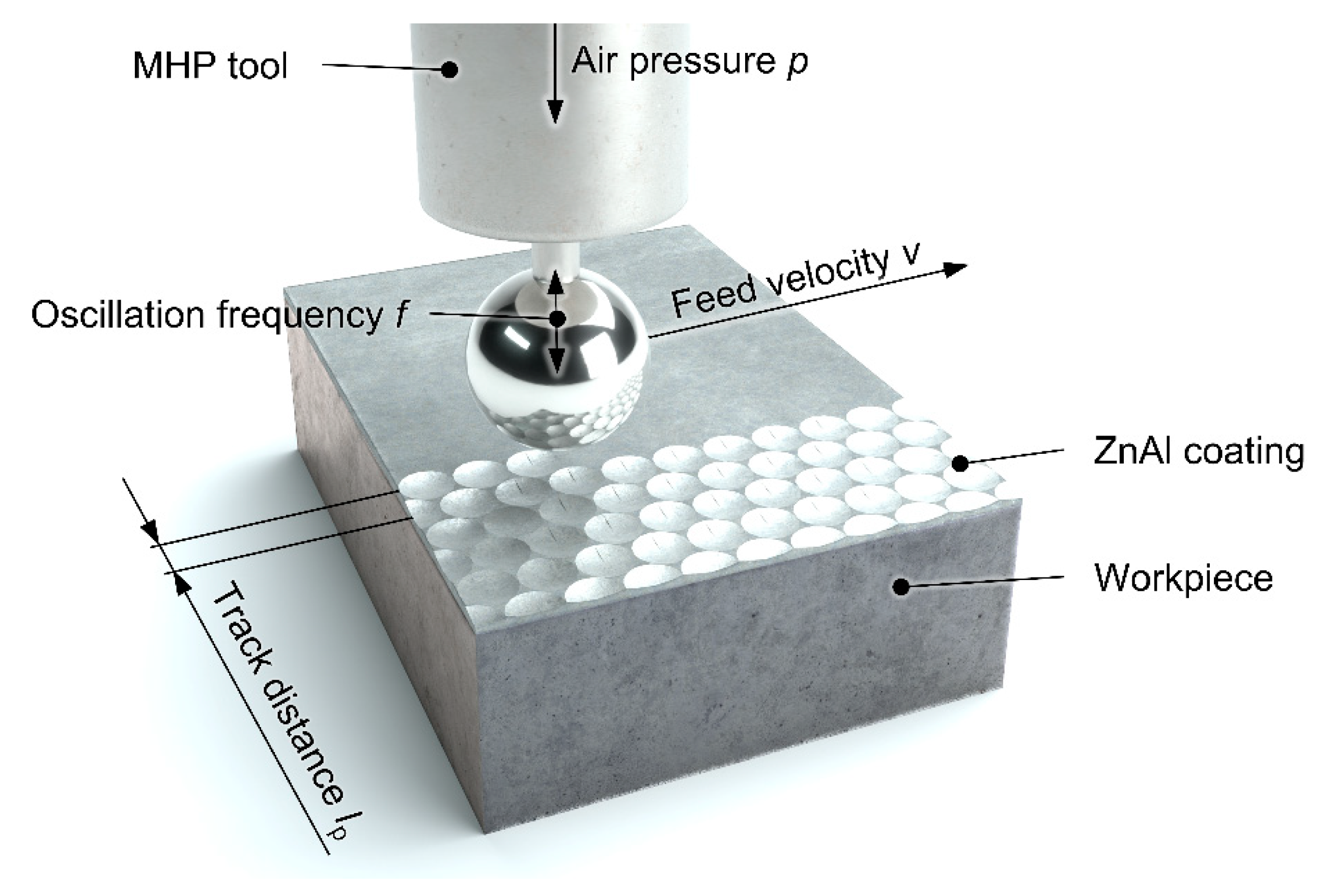
A different method of adapting the properties of functional surfaces, such as thermally sprayed coating systems, to the requirements involves mechanical compacting post-treatment by means of machine hammer peening (MHP) (
Figure 1).
Machine hammer peening processes are used to improve the tribological characteristics of surfaces [
21,
22,
23], or the microstructure or mechanical properties of additively manufactured workpieces [
24], e.g., in the mold making industry. It has been shown that MHP results in the introduction of compressive residual stresses as well as a smoothening of the surface, which can replace or reduce manual polishing processes [
14,
21]. An analysis of the peening of low-alloy tempered steel at temperatures between
T = −180 °C and
T = 200 °C by both simulation and measurement indicated higher compressive residual stresses at the surface for high temperatures and a shift of the maxima below the surface for low, cryogenic temperatures [
25]. Prevéy and Cammett analyzed the influence of the mechanical post-treatment on the corrosion fatigue of aluminum EN AW-7075 by means of low plasticity burnishing [
26]. In their studies, the introduction of compressive residual stresses led to a shift of the fatigue origin from the surface to the sub-surface area and a significant increase of the fatigue strength of both the uncorroded, machined surface and the corroded state.
Mechanical compacting has also been applied to influence the microstructure and surface of coatings or functional surfaces to reduce the roughness and porosity, induce compressive residual stresses and increase the surface hardness of ZnAl-based corrosion protection coatings [
2,
27]. Consequently, the disadvantageous properties of TWAS coatings in view of corrosion protection, i.e., high porosity, rough surface, and tensile residual stresses in the surface-near zone, can be reduced or eliminated. Thus, mechanical post-treatment methods such as MHP could improve the corrosion fatigue behavior of thermally sprayed coatings.
In this study, a thermally assisted MHP-process (TaMHP) is presented and analyzed by means of experimental investigation of the influence on a TWAS sprayed ZnAl4-coating. Thermal support of the MHP process can cause a softening of the coating and, thus, incease the densification effect. Additionally, at a temperature of
Tpt = 77 °C, a thermally induced phase transformation from α + η to β + η of the ZnAl4 coating occurs [
28], which can lead to an embrittlement of the layer system, but could also allow deeper compaction of the coating. In the analyses, the MHP process is conducted in a temperature range between room temperature and melting temperature to find an optimum for high compaction and to generate a uniform, dense and non-porous coating. Additionally, the influence of a TaMHP treatment on the residual stresses, surface hardness, and structural composition of the coating is investigated.
2. Materials and Methods
In this section, the experimental setup and procedure for investigating the effect of thermally assisted MHP processes on TWAS ZnAl4 coatings is described. Afterward, the measurement setup is presented.
2.1. Experimental Setup and Procedure
Rectangular specimens of unalloyed structural steel 1.0577 (S355 J2 + N) with the dimensions 70 mm × 50 mm × 10 mm were used. Before coating, the surfaces were sandblasted with corundum EKF 24 (−850 µm + 600 µm) using compressed air at a
p = 4 bar pressure at a blasting angle of approximately 45° to ensure sufficient coating adhesion. Cleaning of sandblasted surfaces was conducted in an ultrasonic ethanol bath to remove residues of oil and dust. The surfaces were coated by TWAS using a Durum Duraspray 450 wire arc spraying system with ZnAl4 wires with the chemical composition given in
Table 1. For the coating process dry and compressed air was used as an atomization gas in all experiments. The process parameters wire federate, arc voltage, and atomization gas pressure were varied according to a full-factorial plan in three stages each. The center point was repeated twice (
CA3 and
CA6), see
Table 2. The coating process was carried out with a spray distance between gun and substrate surface of
d = 120 mm with an axial gun velocity vs. = 18,000 mm/min and meander spacing of
s = 4 mm in two passes using an industrial robot ABB IRB 4600.
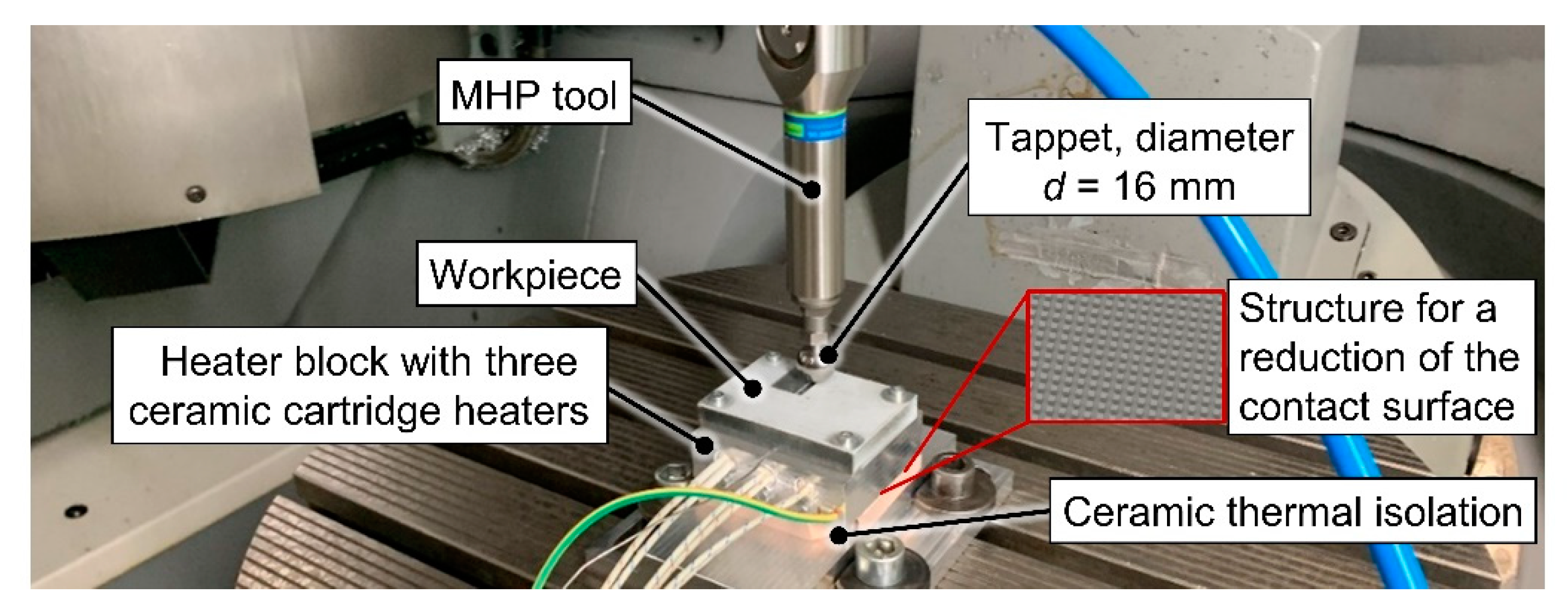
For the MHP process, the specimens were mounted on a setup designed for heating and insulation to adjust the temperature of the specimen in a range of
T = 20–365 °C, see
Figure 2. Heating was carried out using three ceramic cartridge heaters Hewid G13M with a rated power
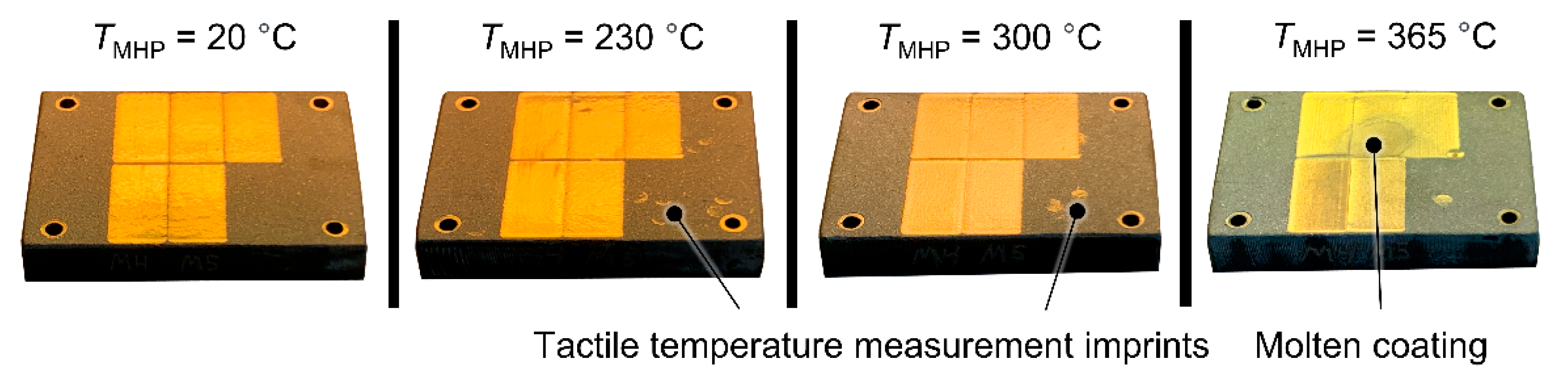
P = 180 W each, mounted in an aluminum EN AW-7075 block used for spreading the thermal energy and distributing it to the specimen. The temperature was controlled by a temperature controller Hillesheim HT41 using a thermocouple mounted directly under the specimen as a reference signal. Additionally, temperature measurements were conducted on the coating surface with a temperature meter Testo 925 and a hand-held thermocouple temperature probe type K in order to accomplish a maximum temperature deviation of Δ
T = 2 K during the experiments. For the insulation of the heated specimen and heater block against the base and clamping device, a block of fired industrial ceramics type 9020 (aluminum silicate) by Kager with a thickness
s = 10 mm was used. Heating and cooling of the specimens was performed on the device. To reduce the introduction of tensile residual stresses during cooling, this process was carried out slowly in open air.
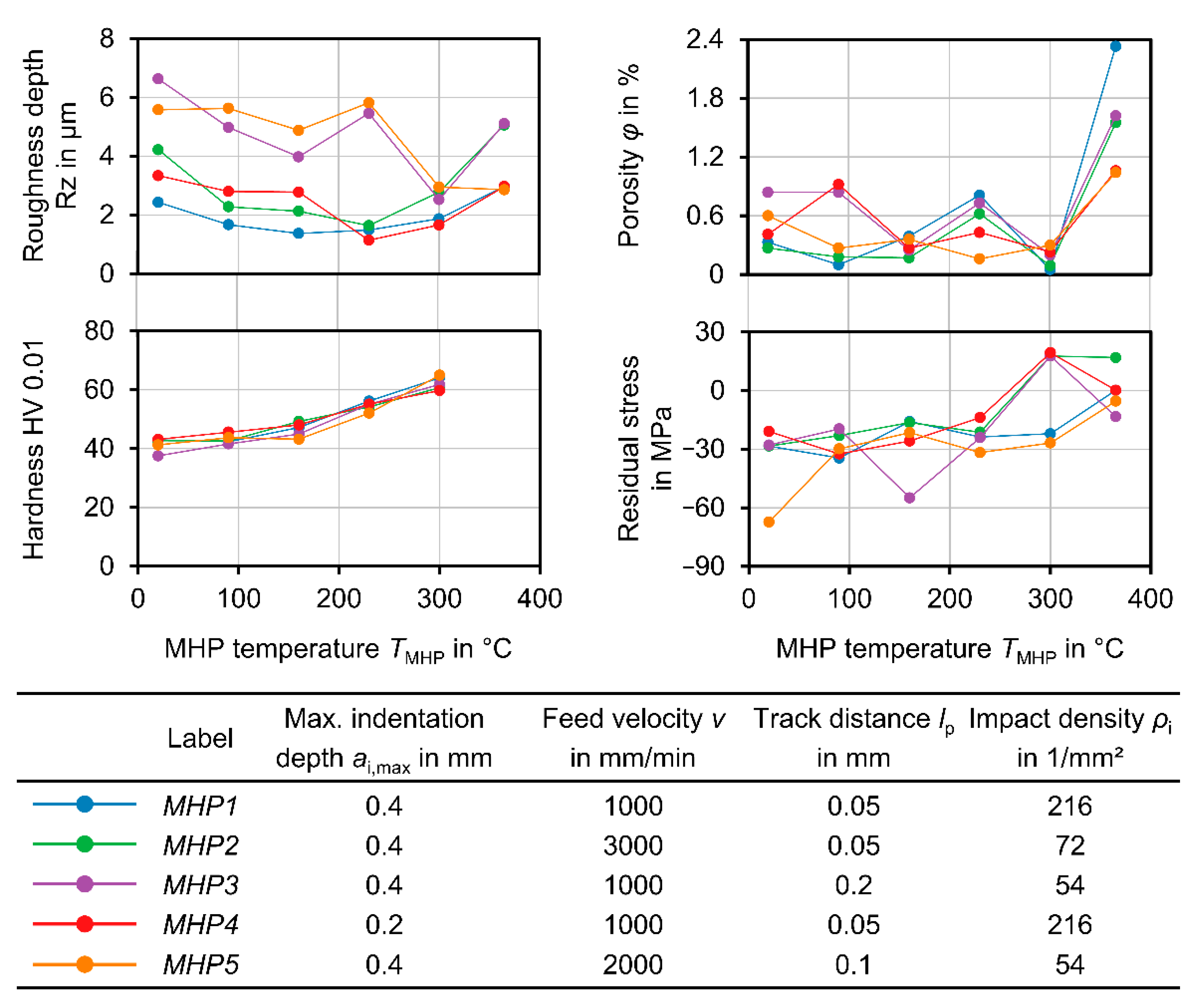
The thermally assisted MHP processes were carried out with five process parameter settings for the center point ZnAl4 coating (A6), varying the maximal indentation depth
ai,max, feed velocity
v, and track distance
lp according to
Table 3. For the other coatings, the process parameter set
MHP1 was used, which was identified as the best parameter set for generating a low-roughness surface in previous studies. The processing temperature
TMHP was varied in steps of 70 K between room temperature,
Tmin = 20 °C and
Tmax = 300 °C. Each MHP process was carried out in a rectangular area with dimensions between 16 mm × 26 mm and 18 mm × 30 mm. For the center point coating
CA6, the MHP process was additionally carried out for a temperature
TMHP = 365 °C, slightly below the melting temperature of ZnAl4,
Tmelt = 381 °C [
28].
The MHP processes were carried out with a FORGEFix Air Tool by 3S engineering equipped with a carbide ball tip with a diameter of dp = 16 mm mounted in a 5-axis CNC machining center Deckel Maho DMU 50 eVolution. The compressed air pressure used to power the MHP tool was kept constant at p = 6 bar during all experiments to achieve the maximum possible impact energy and, thus, maximum coating compaction.
2.2. Process Force Measurements
The process forces applied to the workpiece surface and coating system were measured by MHP trials conducted on uncoated specimens of S235 JR structural steel (1.0037). This allowed a measurement of the process forces without being affected by the high plastic compliance of the coating. The process parameters were individually varied in a range suitable for the process according to past studies [
27]: the compressed air pressure was varied between
p = 4 bar and
p = 6 bar, the feed velocity between
v = 500 mm/min and
v = 4000 mm/min, and the maximal indentation depth between
ai,max = 0.1 mm and
ai,max = 0.4 mm. For the force measurements, a multicomponent dynamometer Kistler type 9255C with charge amplifier Kistler 5070A was used in a 5-axis machining center Deckel Maho DMU 50 eVolution. The process forces were measured with a measuring frequency of
fmeas = 10 kHz and filtered with a fourth-order Bessel low-pass filter with a cut-off frequency of
fcut-off = 2 kHz. Afterward, arithmetic mean force amplitudes were calculated from 500 automatically detected peak values for each process configuration.
2.3. Coating Specification Measurements
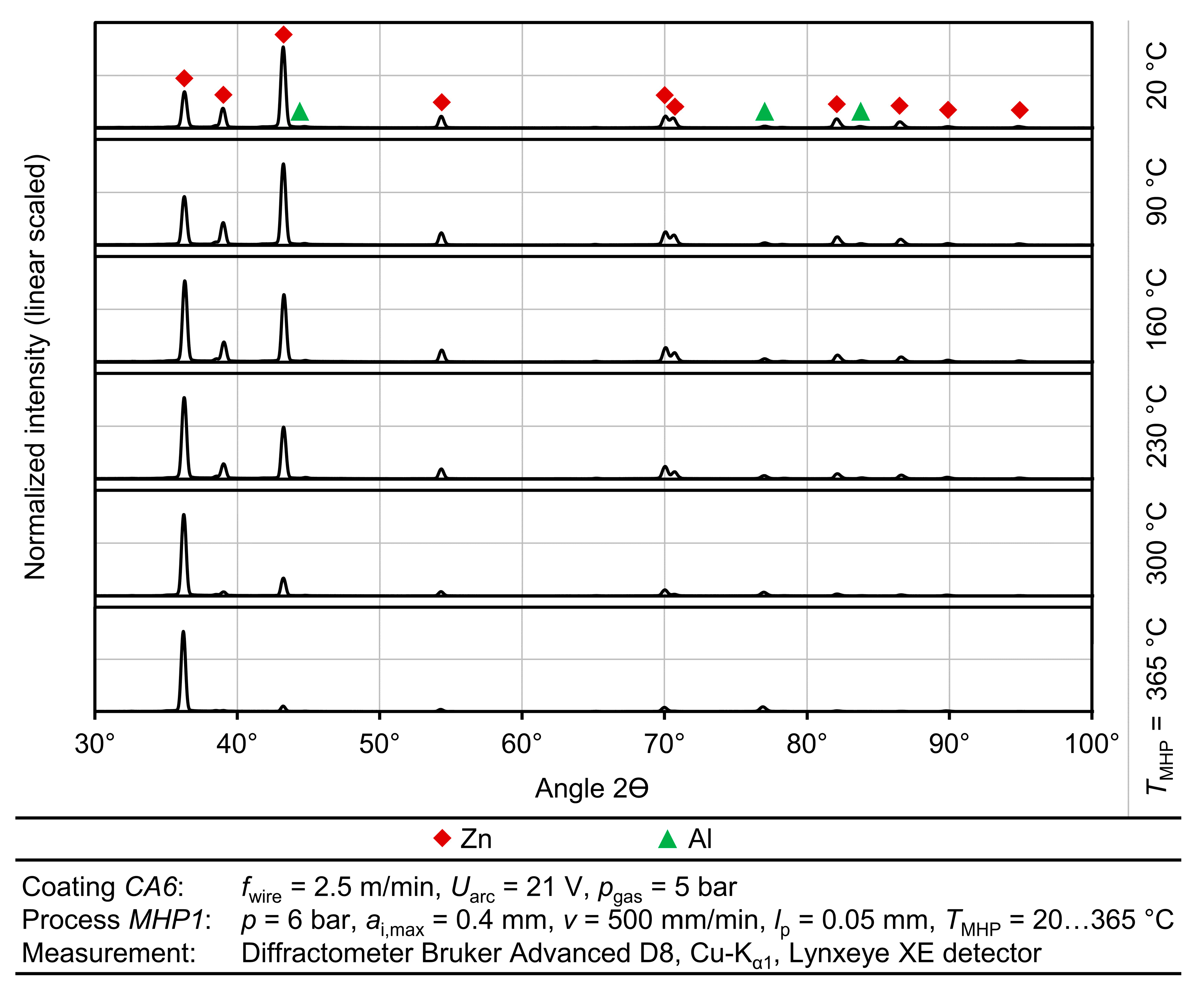
To evaluate the effect of thermally assisted MHP on the ZnAl4 layer system, the coating thickness, porosity, averaged roughness depth Rz, mean roughness Ra, residual stress, and the coating hardness were evaluated before and after the MHP treatment. The roughness values, Rz and Ra, were determined by measuring the surface using a confocal white light microscope Nanofocus µsurf with a 20× short objective and calculation with a robust Gaussian filter with a cutoff wavelength of λc = 2.5 mm. Cross-sections were metallurgically prepared to determine the layer morphology. The layer thickness and porosity were determined as an average of ten traverse micrographs of the layers imaged with an Olympus BX51 optical microscope and analyzed using an Olympus stream motion software. The residual stresses in the sprayed and machine hammer peened coatings were measured by X-ray diffraction (XRD) using Cu-Kα1 with a Lynxeye XE detector Bruker Advanced D8 diffractometer.
The measurements were conducted for 2θ 86.56° (Zn (201)) in an investigated range of high diffraction angle 2θ of between 85.7° and 87.5° at a step size of 0.1° and a measurement time of
t = 3.5 s. Diffracted beams were measured for a Phi angle between 0° and 180°, while Chi was varied between 0° and 60° in 8 steps. The residual stresses were calculated using the software LEPTOS 7.03 by Bruker based on the sin
2ψ method according to [
18,
19]. The material constants were assumed to be as follows: Young’s modulus
E = 96 GPa, Poisson’s ratio
ν = 0.29, and elastic constants
S1 = −3.021 × 10
−6 and ½
S2 = 1.344 × 10
−6.
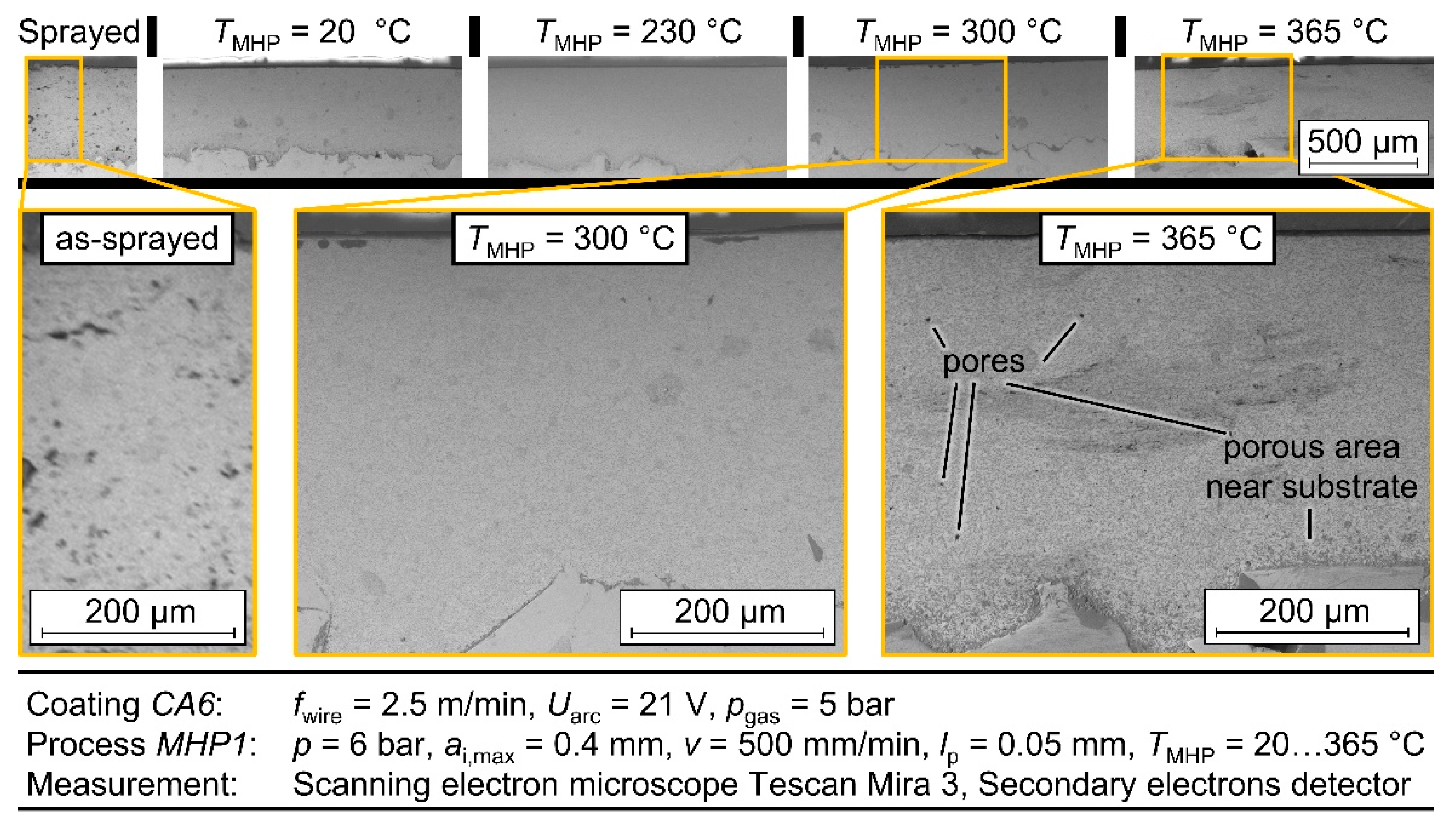
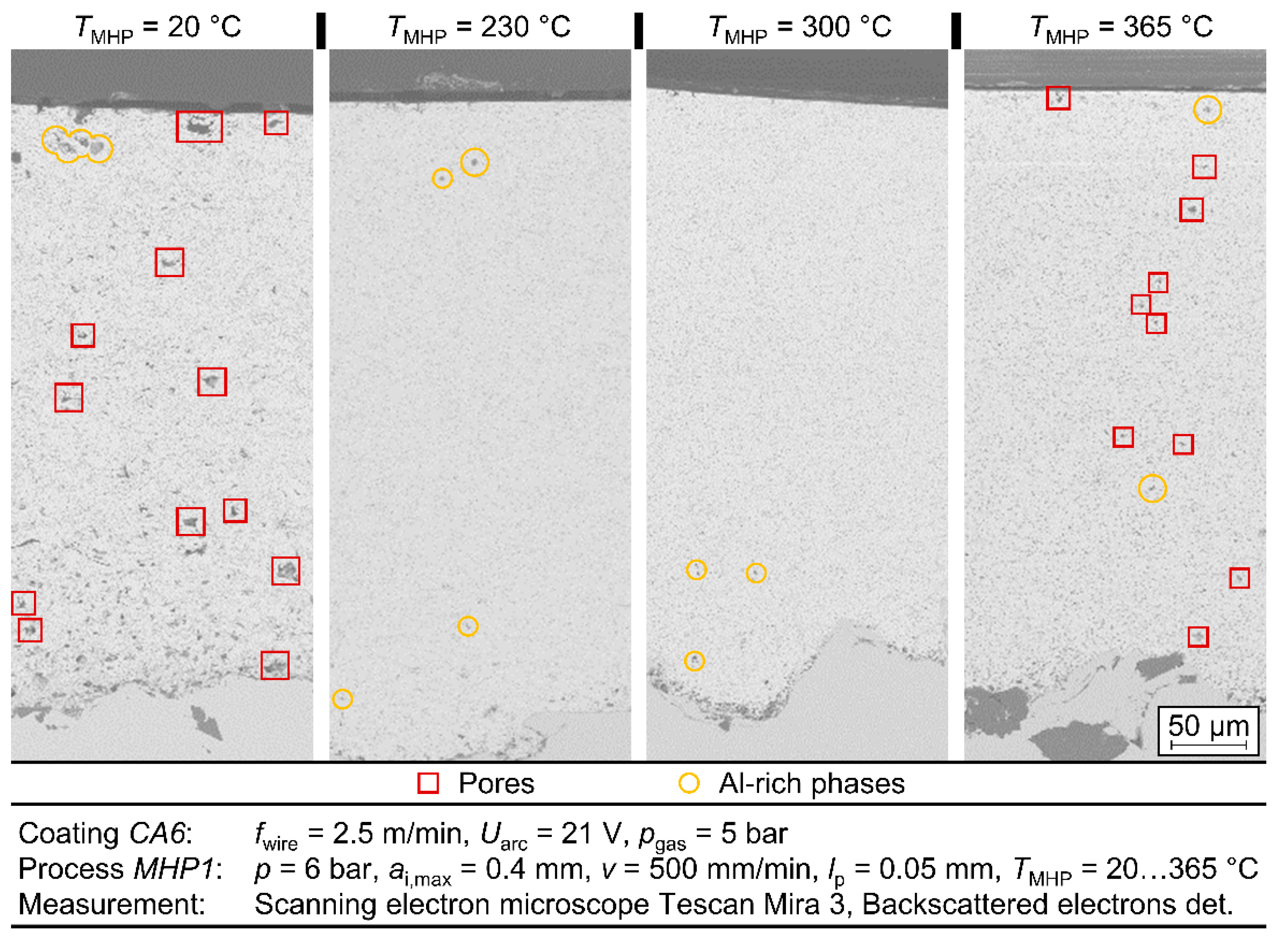
To examine the metallographic layer structure of the TaMHP samples in more detail, selected samples were examined by scanning electron microscopy (SEM). The cross-sections of the samples made for this purpose were polished using ethanol to prevent possible oxidation. The images were taken using a TESCAN Mira 3 scanning electron microscope, whereby the secondary electrons (SE) and backscattered electrons (BSE) were detected to visualize a topographic contrast and a material contrast, respectively. SEM imaging was focused on the near-surface boundary zone, where the TaMHP treatment was expected to have the most significant impact.
4. Discussion and Conclusions
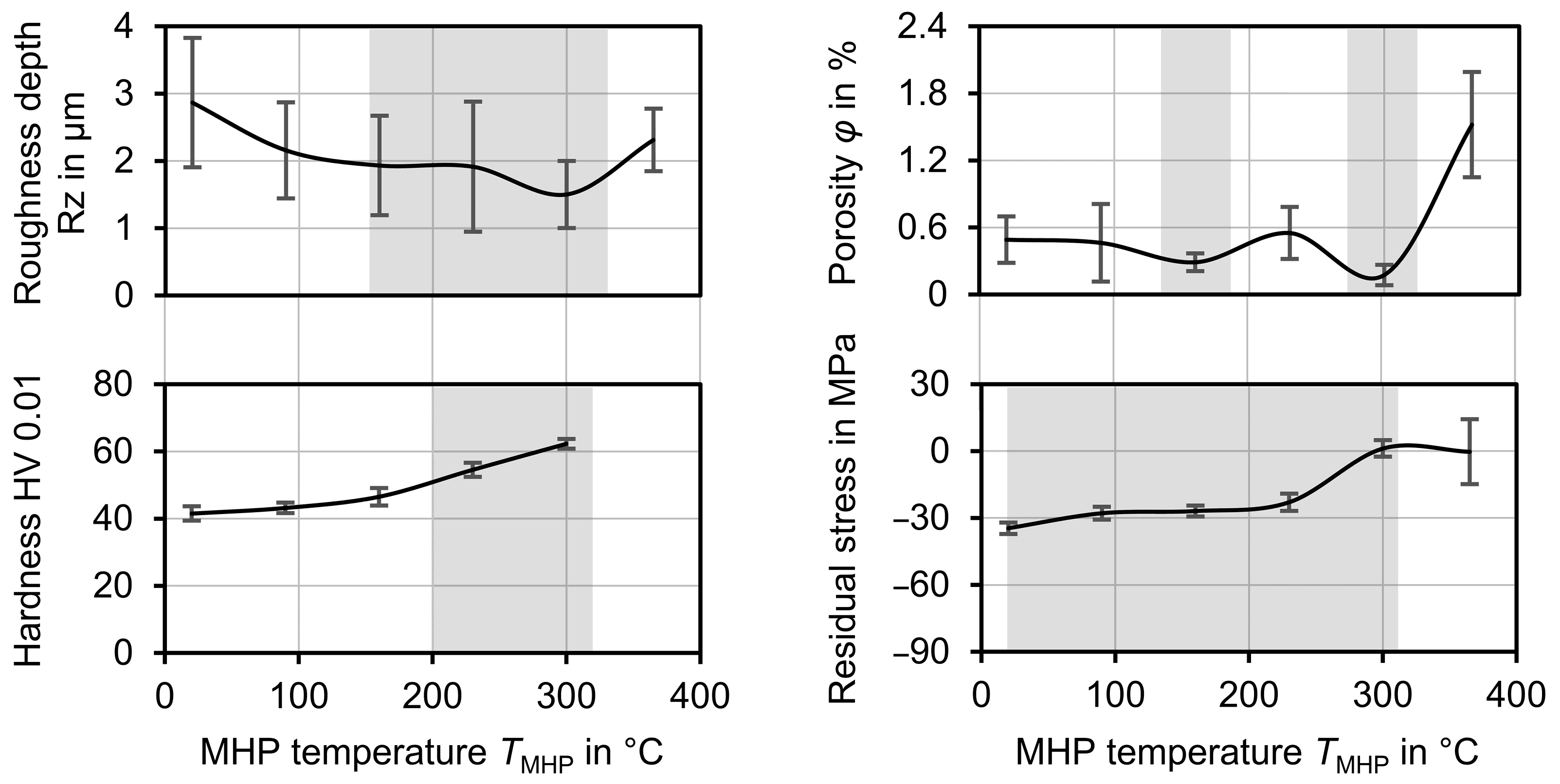
A thermally assisted MHP process of a ZnAl4-based, TWAS-sprayed, corrosion protection coating was investigated in the presented work. The purpose of the compaction peening is to selectively adjust the coating properties. To improve the corrosion fatigue behavior, the objective was to achieve a pore-free coating with low roughness depth, to reduce the area for corrosion to attack. Additionally, compressive residual stresses and a high hardness should be introduced to increase the fatigue strength, suppress cracks and microcracks, and increase wear resistance. In contrast to MHP at room temperature, which already allows improvements in the aforementioned coating properties, these effects could be further enhanced by heating the samples during the processing. The results for different MHP process parameter settings and suitable temperature areas to achieve good results are summarized in
Figure 12.
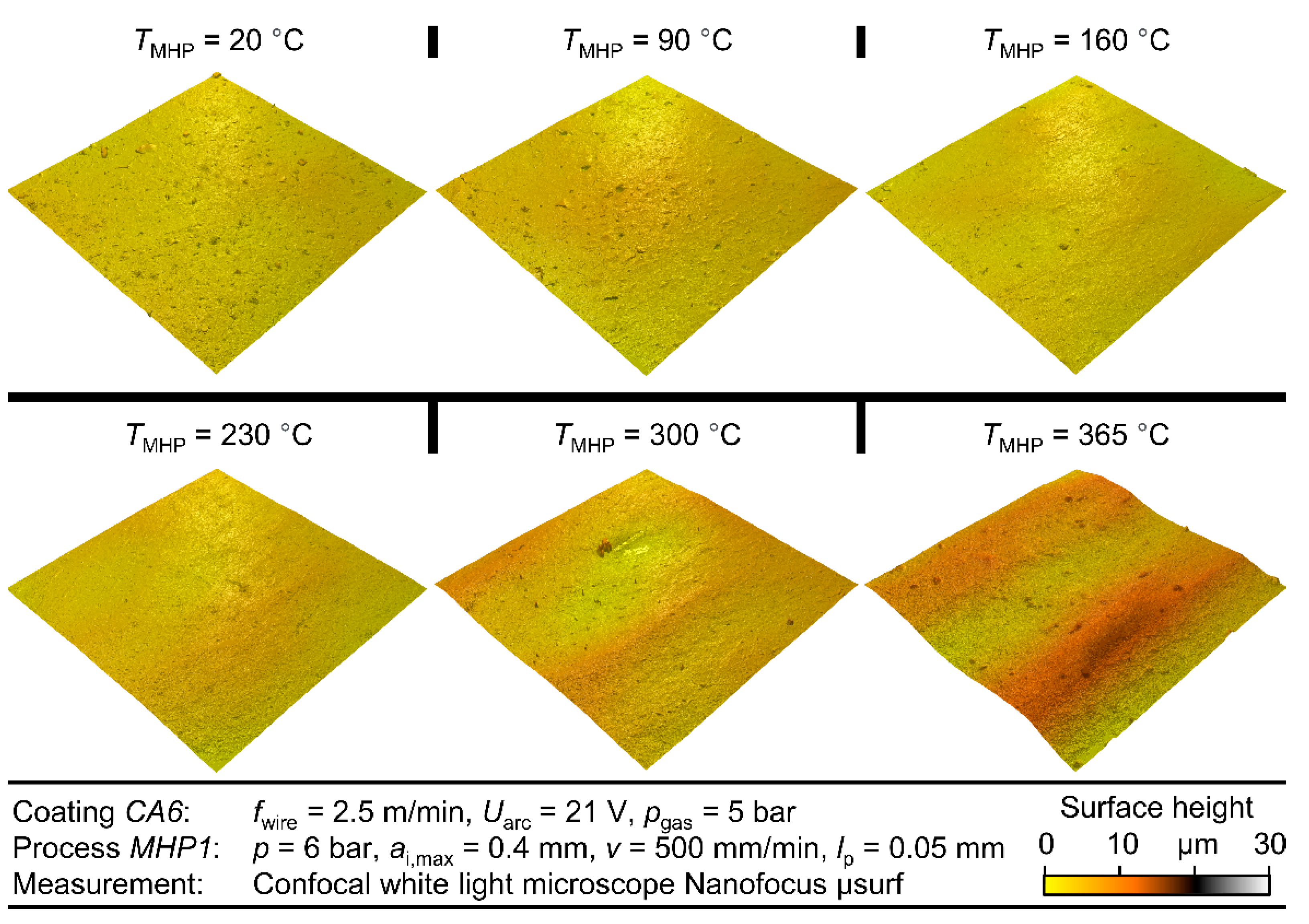
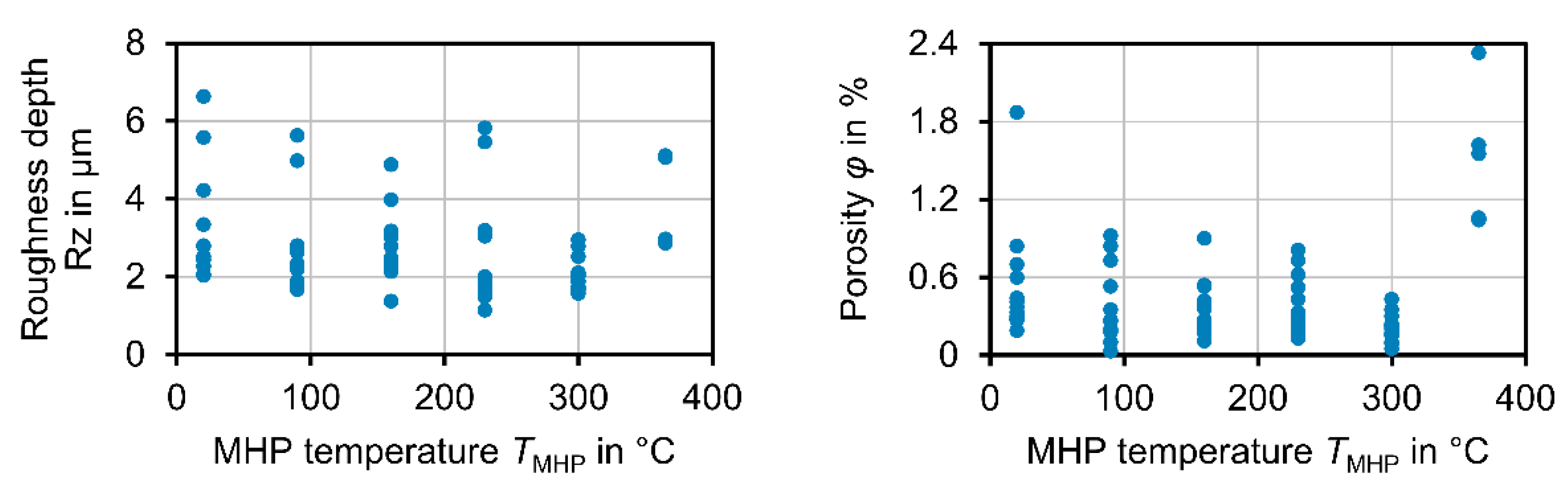
The best results in view of roughness and porosity could be achieved at a process temperature of TMHP = 300 °C. At this temperature, a roughness depth Rz = 1.50 µm ± 0.50 µm and a porosity φ = 0.174% ± 0.92% were achieved for different coatings and different MHP parameter values. Compared to MHP at room temperature TMHP = 20 °C, where Rz = 0.29 µm ± 0.96 µm and φ = 0.49% ± 0.21% were achieved; these values are significantly lower in terms of both arithmetic mean value and standard deviation, which indicates an improvement in the coating properties and the repeatability of the process. At temperatures above TMHP = 300 °C, the roughness depth and porosity were significantly increased, as locally limited melting effects occurred at TMHP = 365 °C. As for MHP at room temperature, the MHP setting MHP1 and coating CA6 were again identified as the best options, as a roughness depth RzMHP1 = 2.43 µm ± 0.53 µm and porosity φ = 0.33% were achieved. In comparison, the differences in roughness and porosity between the various MHP process parameter settings became lower at higher temperatures. This indicates a higher compaction with each impact, which is caused by the softening of the coating.
In terms of the generation of compressive residual stresses, the best results were achieved at room temperature and
TMHP = 160 °C. At a higher temperature, tensile residual stresses counteracting the compressive residual stresses could have been generated during the cooling phase due to the different thermal expansion coefficients of the substrate (
αsteel = 12.0 × 10
−6 K
−1) and coating (
αZnAl4 ≈
αzinc = 25.0 × 10
−6 K
−1) [
29] and due to the phase transformation of the coating at
T = 279 °C. By means of slow cooling of the specimens in mid-air, this effect was reduced. Nevertheless, the tensile residual stresses generated at a temperature of
TMHP = 365 °C could have a strong negative effect on corrosion fatigue behavior. To avoid the negative effect of the tensile residual stresses generated at
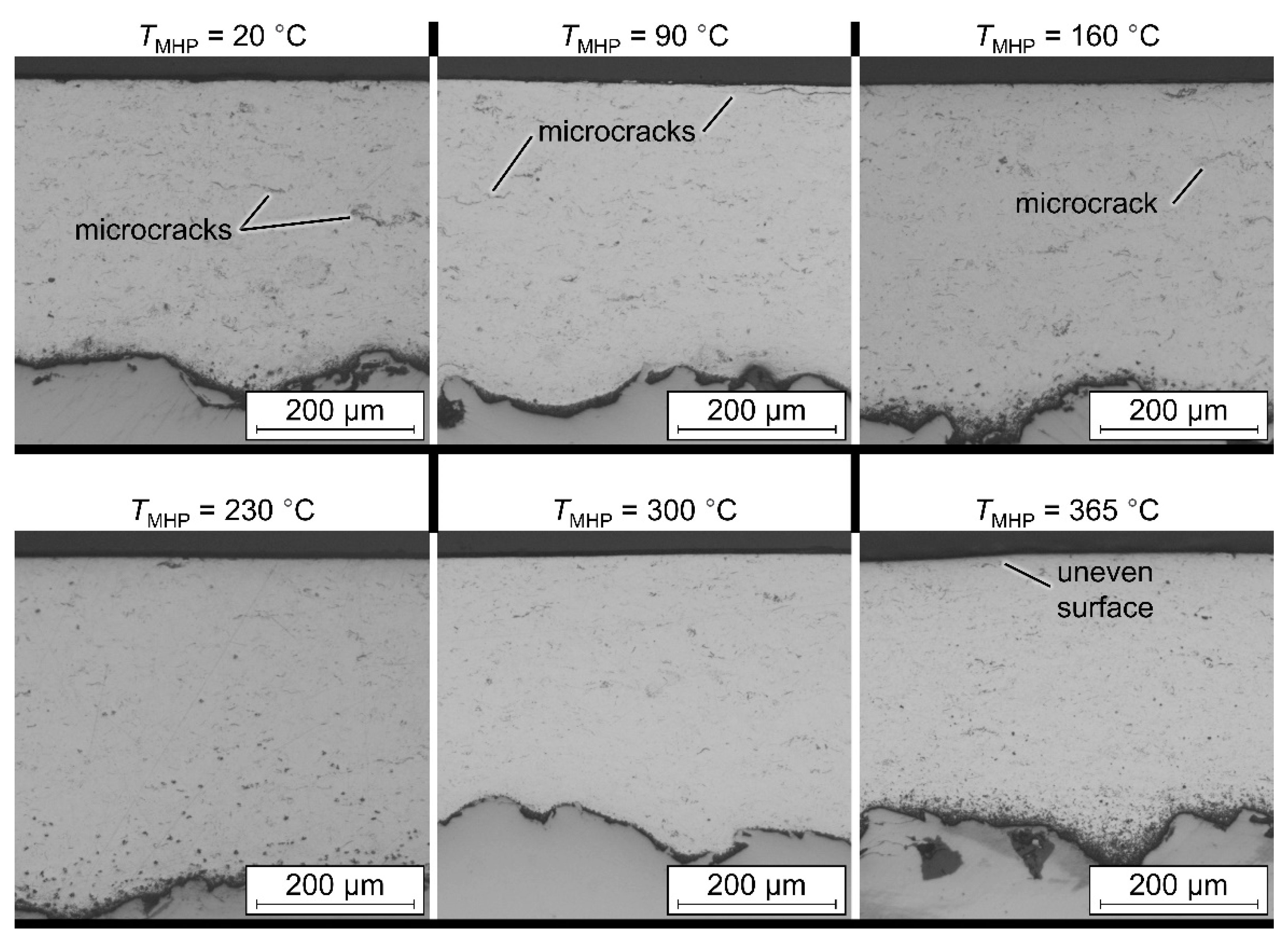
TMHP = 365 °C, a second MHP process could be carried out afterwards using a lower process temperature. The influence of the angle between the NC paths in the successive processing steps should also be taken into account here. A second machining step at a different angle or with a different machining strategy could further improve the surface finish, especially with regard to the roughness depth. The surface hardness of the coating, on the other hand, was steadily increased with rising temperature. For this reason, a subsequent MHP processing in two process steps at different temperatures could lead to a further improvement of the coating properties.
In conclusion, by TaMHP, a thermally induced softening of the coating and, consequently, a better smoothing of the layer could be achieved for the ZnAl4 coatings. In the investigations, a temperature of up to TMHP = 300 °C led to a significant improvement in the coating properties. Furthermore, no more microcracks could be found in the coating system after TaMHP at TMHP = 300 °C, which may improve the corrosion properties and corrosion fatigue behavior of the ZnAl4 coating. In contrast, at higher temperatures, the coatings were negatively affected in terms of the generation of tensile residual stresses and a higher porosity and roughness depth. Thus, a sufficiently accurate temperature control must ensure a homogeneous temperature distribution and prevent a melting of the coating during the TaMHP process. The effect of these enhancements on the corrosion and corrosion fatigue behavior of a coated system will be investigated in future research works. These will feature potentiodynamic polarization tests and corrosion fatigue testing. In the presented investigations, good results were achieved on a laboratory scale, but a sufficiently accurate temperature control could be a considerable difficulty in the post-treatment of large structural components, e.g., for offshore facilities.