Vacuum Hot Pressing of Oxide Dispersion Strengthened Ferritic Stainless Steels: Effect of Al Addition on the Microstructure and Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
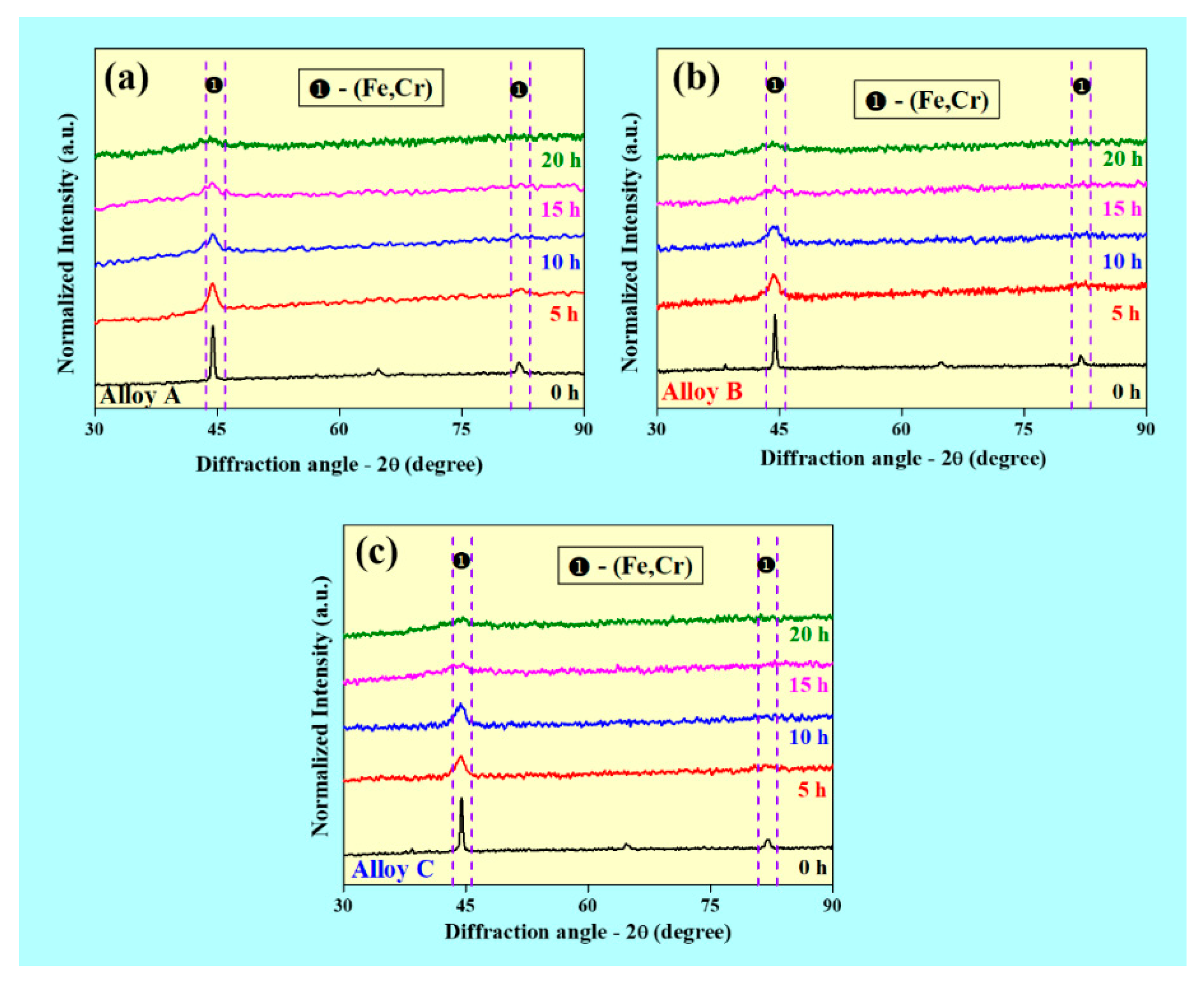
3.1. Structural Characterization of the Powders by XRD
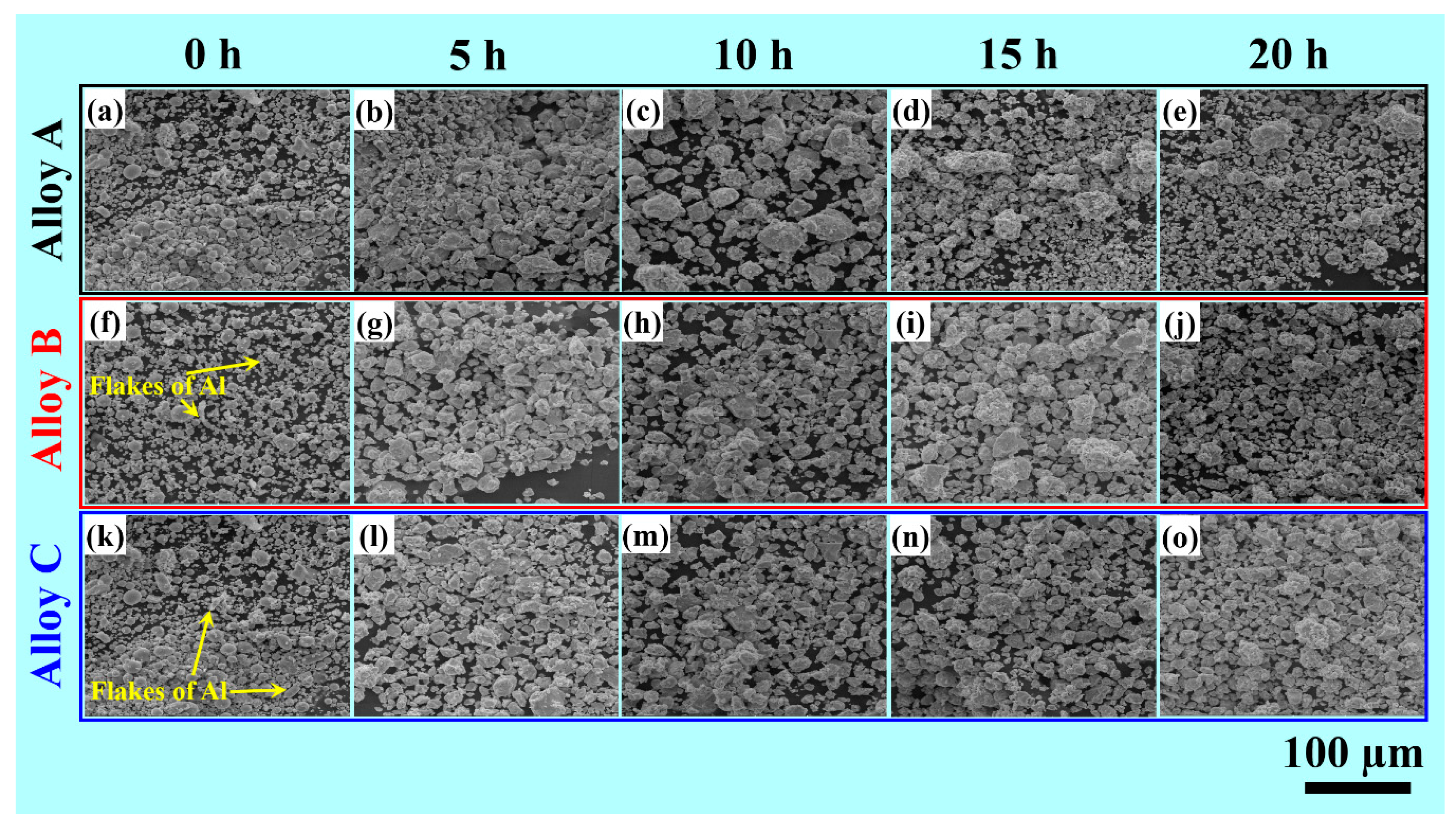
3.2. Morphology of the Mechanically Alloyed Powders
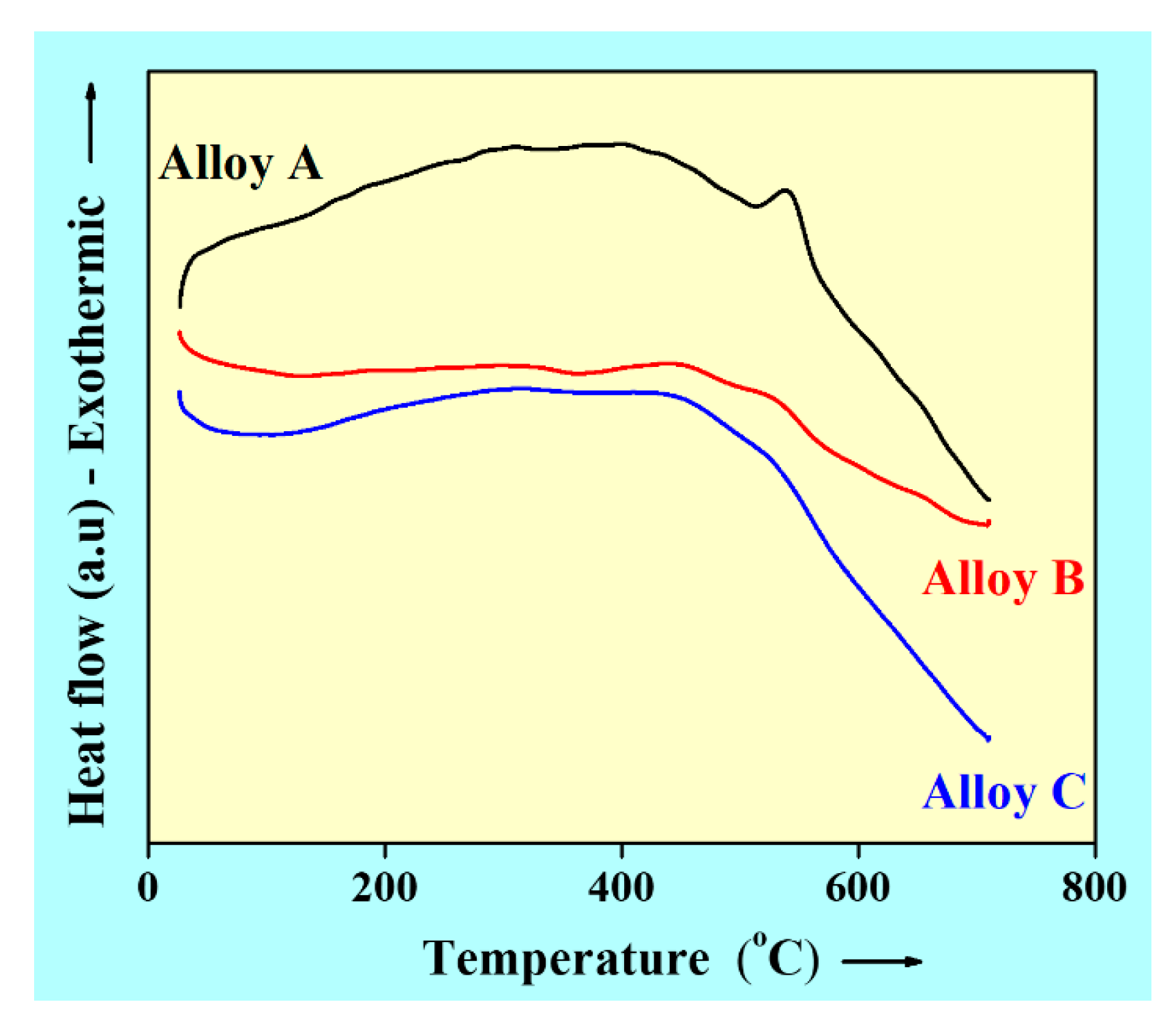
3.3. Thermal Effects on MA Milled Powder Alloys
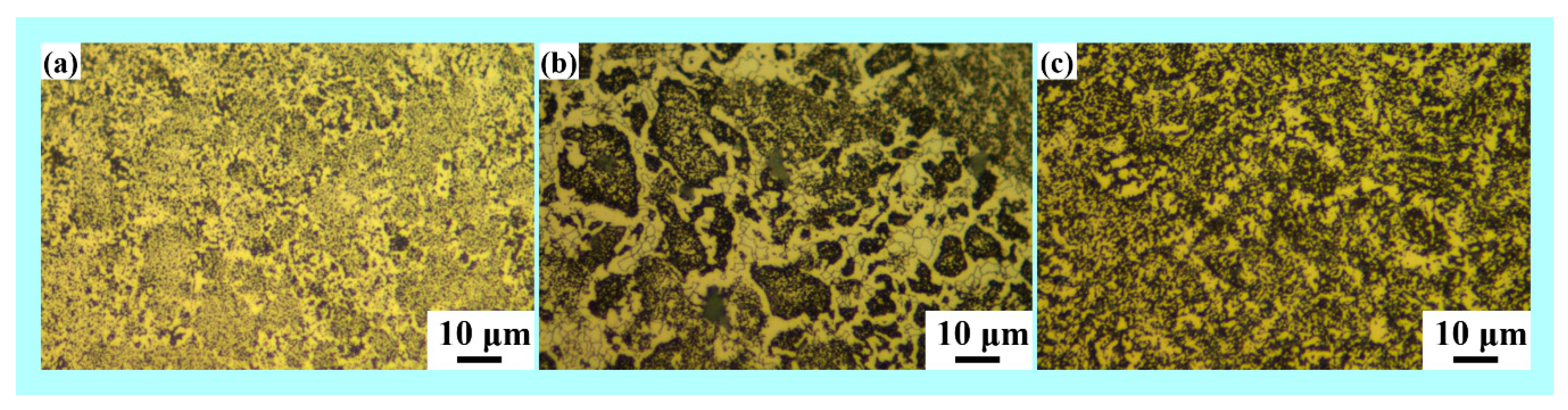
3.4. Density and Microstructure of the Consolidated Samples
3.5. Hardness of the Consolidated ODS Specimens
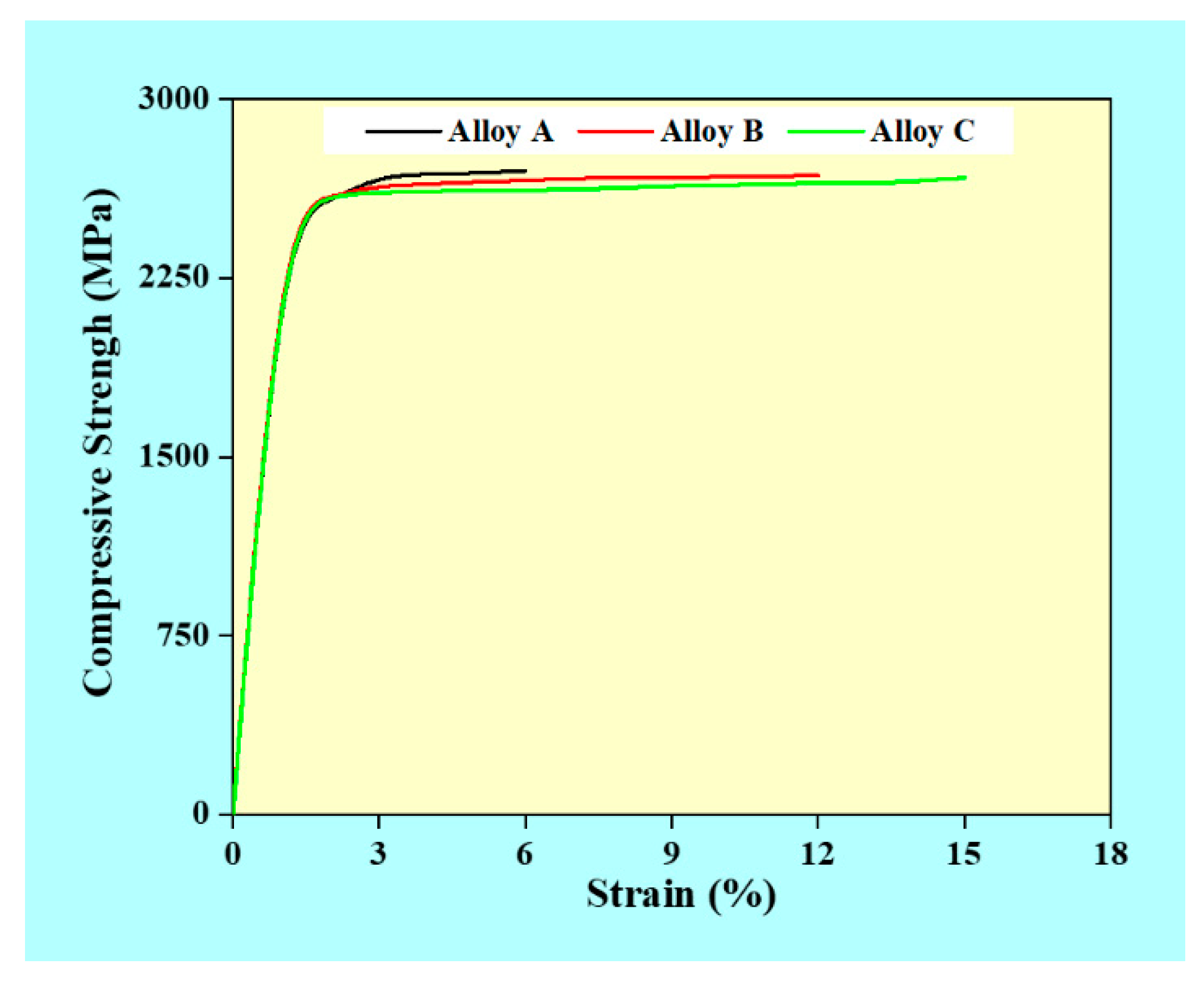
3.6. Compressive Strength of the Consolidated ODS Specimens
4. Conclusions
- ⮚
- The density and hardness of vacuum hot pressed alloys show increasing trend due to higher sintering temperature of 1170 °C and pressure of 60 MPa than the existing methods.
- ⮚
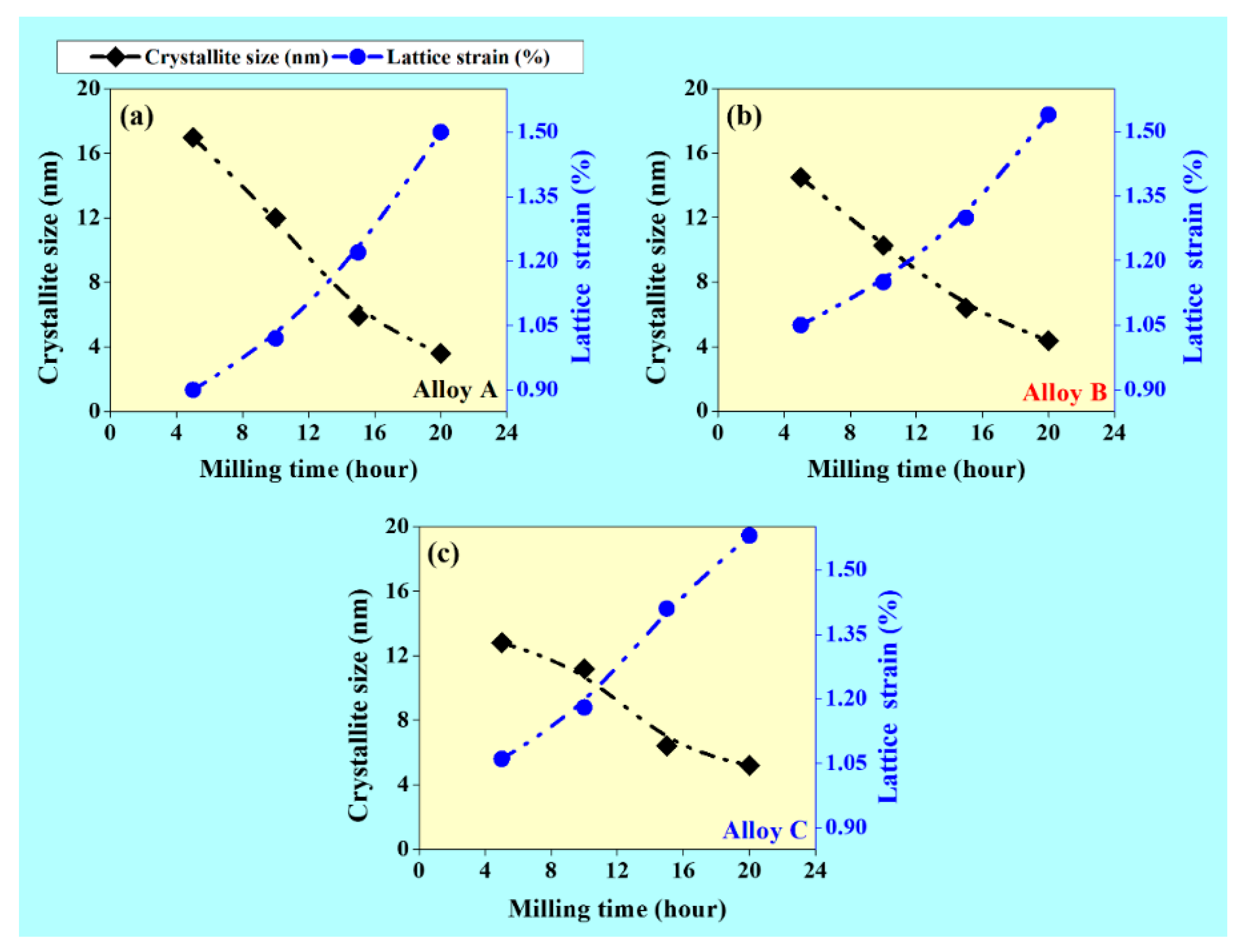
- Nanocrystalline size of the powder particles decrease rapidly with increases in milling time (20 h), whereas the lattice strain increases with increasing milling time.
- ⮚
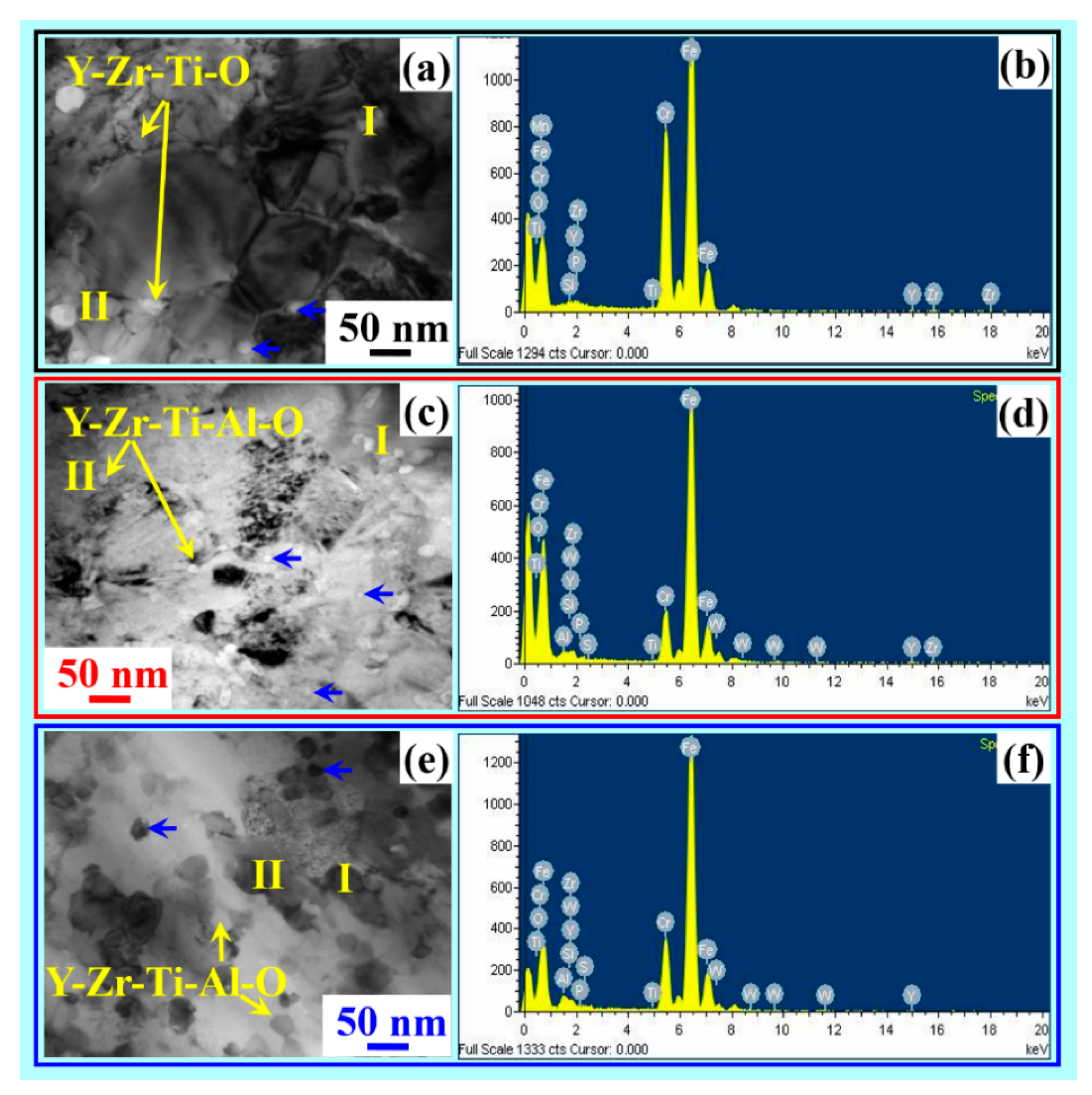
- The microstructure of considered alloys A, B and C shows uniform distribution of nano oxide particles and complex oxides particles of Y-Zr-Ti-O (Alloy A), Y-Zr-Ti-Al-O (Alloy B) and Y-Zr-Ti-Al-O (Alloy C), which is evident from TEM-EDS.
- ⮚
- Aluminum free Alloy A has higher density and hardness than the aluminum containing Alloy B and C, due to the presence of a refined microstructure
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masuda, H.; Tobe, H.; Sato, E.; Sugino, Y.; Ukai, S. Transgranular dislocation activities and substructural evolutions accommodating two-dimensional grain boundary sliding in ODF ferritic steel. Acta Mater. 2017, 132, 245–254. [Google Scholar] [CrossRef]
- Chen, J.; Jung, P.; Hoffelner, W.; Ullmaier, H. Dislocation loops and bubbles in oxide dispersion strengthened ferritic steel after helium implantation under stress. Acta Mater. 2008, 56, 250–258. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Long, F.; Jia, H.; Guo, N.; Sun, Y.; Yao, Z.; Daymond, M.R. Influence of Al Addition Strategy on the Microstructure of a Low--Cr Oxide Dispersion--Strengthened Ferritic Steel. Adv. Eng. Mater. 2020, 22, 1900879. [Google Scholar] [CrossRef]
- Sanctis, M.D.; Fava, A.; Lovicu, G.; Montanari, R.; Richetta, M.; Testani, C.; Varone, A. Mechanical characterization of a nano-ODS steel prepared by low-energy mechanical alloying. Metals 2017, 7, 283. [Google Scholar] [CrossRef]
- Svoboda, J.; Horník, V.; Stratil, L.; Hadraba, H.; Mašek, B.; Khalaj, O.; Jirková, H. Microstructure Evolution in ODS Alloys with a High-Volume Fraction of Nano Oxides. Metals 2018, 8, 1079. [Google Scholar] [CrossRef]
- Zhao, Q.; Qiao, Z.; Liu, Y.; Yu, L.; Huang, Y.; Guo, Q.; Li, H. Characterizatino of 14Cr ODS Steel fabricated by spark plasma sintering. Metals 2019, 9, 200. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, Z.; Yu, L.; Li, H.; Wang, Z.; Liu, Y. Inhibition effect of Ti on the formation of martensite lath in 14Cr oxide dispersion strengthened steel. Metals 2018, 8, 802. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Karak, S.K.; Chudoba, T.; Witczak, Z.; Lojkowski, W.; Manna, I. Development of ultra-high strength nano-Y2O3 dispersed ferritic steel by mechanical alloying and hot isostatic pressing. Mater. Sci. Eng. A 2011, 528, 7475–7483. [Google Scholar] [CrossRef]
- Miller, M.K.; Kenik, E.A.; Russell, K.F.; Heatherly, L.; Hoelzer, D.T.; Maziasz, P.J. Atom probe tomography of nanoscale particles in ODS ferritic alloys. Mater. Sci. Eng. A 2003, 353, 140–145. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, O.Y.; Jang, J.; Hong, S.H. Modification of anisotripic mechanical properties in recrystallized oxide dispersion strengthened ferritic alloy. Scr. Mater. 2006, 54, 1703–1707. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Zheng, W.; Jia, H. Mechanical properties evaluation and plastic instabilities of Fe-9%Cr ODS steels. Fusion Eng. Des. 2019, 149, 111335. [Google Scholar] [CrossRef]
- Das, A.; Chekhonin, P.; Altstadt, E.; Bergner, F.; Heintze, C.; Lindau, R. Microstructural characterization of inhomogeneity in 9Cr ODS EUROFER steel. J. Nucl. Mater. 2020, 533, 152083. [Google Scholar] [CrossRef]
- Song, L.; Yanf, X.; Zhao, Y.; Wang, W.; Mao, X. Si-containing 9Cr ODS steel designed for high temperature application in lead-cooled fast rector. J. Nucl. Mater. 2019, 519, 22–29. [Google Scholar] [CrossRef]
- Henry, J.; Averty, X.; Dai, Y.; Pizzanelli, J.; Espinas, J. Tensile properties of ODS-14%Cr ferritic alloy irradiated in a spallation environment. J. Nucl. Mater. 2009, 386, 345–348. [Google Scholar] [CrossRef]
- McClintock, D.A.; Sokolov, M.A.; Hoelzer, D.T.; Nanstad, R.K. Mechanical properties of irradiated ODS-EUROFER and nanocluster strengthened 14YWT. J. Nucl. Mater. 2009, 392, 353–359. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODF steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Xia, Y.P.; Wang, X.P.; Zhuang, Z.; Sun, Q.X.; Zhang, T.; Fang, Q.F.; Hao, T.; Liu, C.S. Microstructure and oxidation properties of 16Cr-5Al-ODS steel prepared by sol-gel and spark plasma sintering methods. J. Nucl. Mater. 2013, 432, 198–204. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Shen, H.; Chou, W.; Iwata, N.Y.; Kimura, A. The effects of Cr and Al concentrations on the oxidation behavior of oxide dispersion strengthened ferritic alloys. Corr. Sci. 2013, 76, 310–316. [Google Scholar] [CrossRef]
- Macía, E.; García-Junceda, A.; Serrano, M.; Hernández-Mayoral, M.; Diaz, L.A.; Campos, M. Effect of the heating rate on the microstructure of a ferritic ODS steel with four oxide formers (Y-Ti-Al-Zr) consolidated by spark plasma sintering (SPS). J. Nucl. Mater. M 2019, 518, 190–201. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Scudino, S.; Prashanth, K.G.; Eckert, J. Microstructure and mechanical properties of Mg-Al based alloy modified with cerium. Mater. Sci. Eng. A 2015, 625, 46–49. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; Sun, B.A.; Scudino, S.; Prashanth, K.G.; Zhang, W.W.; Li, Y.Y.; Eckert, J. Fabrication and mechanical properties of Al-based metal matrix composites reinforced with Mg65Cu20Zn5Y10 metallic glass particles. Mater. Sci. Eng. A 2014, 600, 53–58. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Scudino, S.; Samadi Khoshkhoo, M.; Prashanth, K.G.; Mukhopadhyay, N.K.; Mishra, B.K.; Eckert, J. High-strength ultrafine grain Mg-7.4%Al alloy synthesized by consolidation of mechanically alloyed powders. J. Alloys Compd. 2014, 610, 456–461. [Google Scholar] [CrossRef]
- Ali, F.; Scudino, S.; Liu, G.; Srivastava, V.C.; Mukhopadhyay, N.K.; Khoshkhoo, M.S.; Prashanth, K.G.; Uhlenwinkel, V.; Calin, M.; Eckert, J. Modeling the strengthening effect of Al–Cu–Fe quasicrystalline particles in Al-based metal matrix composites. Alloys Compd. 2012, 536, S130–S133. [Google Scholar] [CrossRef]
- Marko, D.; Prashanth, K.G.; Scudino, S.; Wang, Z.; Ellendt, N.; Uhlenwinkel, V.; Eckert, J. Al-based metal matrix composites reinforced with Fe49.9Co35.1Nb7.7B4.5Si2.8 glassy powder: Mechanical behavior under tensile loading. J. Alloys Compd. 2014, 615, S382–S385. [Google Scholar] [CrossRef]
- Lu, Z.-G.; Wu, J.; Xu, L.; Yang, R. Powder size infouence on tensile properties and porosity for PM Ti2AlNb alloy prepared by hot isostatic pressing. Acta Metal. Sin. 2019, 3, 1329–1336. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; Scudino, S.; Sun, B.A.; Qu, R.T.; He, J.; Prashanth, K.G.; Zhang, W.W.; Li, Y.Y.; Eckert, J. Mechanical behavior of Al-based matrix composites reinforced with Mg58Cu28.5Gd11Ag2.5 metallic glasses. Adv. Powder Technol. 2014, 25, 635–659. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Murty, B.S. Production, kinetic study and properties of Fe-based glass and its composites. Mater. Manuf. Process. 2010, 25, 592–597. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Murty, B.S.; Eckert, J. Fabrication, mechanical and tribological properties of Al70Y16Ni10Co4 glass reinforced metal matrix composites. Mater. Manuf. Process. 2011, 26, 1242–1247. [Google Scholar] [CrossRef]
- Scudino, S.; Liu, G.; Prashanth, K.G.; Bartusch, B.; Surreddi, K.B.; Murty, B.S.; Eckert, J. Mechanical properties of Al-based metal matrix composites reinforced with Zr-based glassy particles produced by powder metallurgy. Acta Mater. 2009, 57, 2029–2039. [Google Scholar] [CrossRef]
- Prashanth, K.G. Influence of mechanical activation on decomposition of titanium hydride. Mater. Manuf. Process. 2010, 25, 974–977. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Murty, B.S.; Eckert, J. Crystallization kinetics and consolidation of mechanically alloyed Al70Y16Ni10Co4 glassy powders. J. Alloys Compd. 2009, 477, 171–177. [Google Scholar] [CrossRef]
- Surreddi, K.B.; Scudino, S.; Sakaliyska, M.; Prashanth, K.G.; Sordelet, D.J.; Eckert, J. Crystallization behavior and consolidation of gas-atomized Al84Gd6Ni7Co3 glassy powder. J. Alloy Compd. 2010, 491, 137–142. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallize size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Micropor. Mesopor. Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Deb, A.K.; Chatterjee, P. Estimation of lattice strain in alumina-zirconia nanocomposites by X-ray diffraction peak profile analysis. J. Theor. Appl. Phys. 2019, 13, 221–229. [Google Scholar] [CrossRef]
- Rajulapati, S.K.; Prakash, U.; Laha, K.; Dabhade, V.V. Studies on alloying process of a ferritic/martensituc oxide dispersion strengthened (ODS) steel prepared by mechanical alloying of elements. Powder Metall. 2016, 59, 350–358. [Google Scholar] [CrossRef]
- Karak, S.K.; Majumdar, J.D.; Witczak, Z.; Lojkowski, W.; Manna, I. Microstructure and mechanical properties of nano-Y2O3 dispersed ferritic alloys synthesized by mechanical alloying and consolidated by hydrostatic extrusion. Mater. Sci. Eng. A 2013, 580, 231–241. [Google Scholar] [CrossRef]
- Karak, S.K.; Majumdar, J.D.; Witczak, Z.; Lojkowski, W.; Ciupiński, Ł.; Kurzydłowski, K.J.; Manna, I. Evaluation of microstructure and mechanical properties of nano-Y2O3-dispersed ferritic alloy synthesized by mechanical alloying and consolidated by high-pressure sintering. Metall. Mater. Transact. A 2013, 44, 2884–2894. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- García Junceda, A.; García Rodríguez, N.; Campos, M.; Cartón Cordero, M.; Torralba, J.M. Effect of Zr addition on the microstructure and mechanical properties of an Al-alloyed ODS steel consolidated by FAHP. J. Am. Ceram. Soc. 2015, 98, 3582–3587. [Google Scholar] [CrossRef]
- García Junceda, A.; Macía, E.; Garbiec, D.; Serrano, M.; Torralba, J.M.; Campos, M. Effect of small variations in Zr content on the microstructure and properties of ferritic ODS steels consolidated by SPS. Metals 2020, 10, 348. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Sha, X.; Meng, J.; Wang, Z. Microstructure and mechanical properties of 14Cr-ODS steels with Zr addition. High Temp. Mater. Process. 2019, 38, 404–410. [Google Scholar] [CrossRef]
- Karak, S.K.; Majumdar, J.D.; Lojkowski, W.; Michalski, A.; Ciupinski, L.; Kurzydłowski, K.J.; Manna, I. Microstructure and mechanical properties of nano-Y2O3 dispersed ferritic steel synthesized by mechanical alloying and consolidated by pulse plasma sintering. Phil. Mag. 2012, 92, 516–534. [Google Scholar] [CrossRef]
| Specification | Cr (wt.%) | Mn (wt.%) | Si (wt.%) | C (wt.%) | P (Wt.%) | S (wt.%) | Fe (wt.%) | Theoretical Density (g/cc) |
|---|---|---|---|---|---|---|---|---|
| 430L | 17 | 0.89 | 0.89 | 0.02 | 0.01 | 0.01 | Bal. | 7.70 |
| Alloy A | (430L+0.3Y2O3+0.5ZrO2+0.1Ti) wt.% | 7.67 | ||||||
| Alloy B | (430L+0.3Y2O3+0.5ZrO2+0.1Ti+1W+2Al) wt.% | 7.68 | ||||||
| Alloy C | (430L+0.3Y2O3+0.5ZrO2+0.1Ti+1W+4Al) wt.% | 7.59 | ||||||
| Alloy/Element | Morphology | Mean Particle Size |
|---|---|---|
| 430L | Plate shaped | Apprx. 22 μm (length) |
| ZrO2 | Irregular shaped | Apprx. 12 μm dia. |
| Y2O3 | Irregular shaped | Apprx. 10 μm dia. |
| W | Irregular shaped | Apprx. 30 μm dia. |
| Al | Spherical | 67 μm |
| Ti | Spherical | 50 μm |
| Composition | ASTM Grain Size Number | Average Grain Diameter (μm) |
|---|---|---|
| Alloy A | 16 | 1.8 |
| Alloy B | 6 | 8 |
| Alloy C | 8 | 10 |
| Specification | Alloy A | Alloy B | Alloy C | |||
|---|---|---|---|---|---|---|
| Wt. % | At. % | Wt. % | At. % | Wt. % | At. % | |
| O K | 44.13 | 80.20 | 45.06 | 80.42 | 43.96 | 78.42 |
| Ti K | 2.40 | 0.24 | 2.06 | 0.24 | 2.08 | 0.16 |
| Cr K | 0.70 | 0.45 | 0.80 | 0.29 | 0.80 | 0.28 |
| Fe K | 1.50 | 0.47 | 1.62 | 0.90 | 1.64 | 0.80 |
| Y K | 17.20 | 5.15 | 18.01 | 5.33 | 17.71 | 5.41 |
| Zr K | 34.07 | 13.49 | 30.03 | 10.58 | 30.94 | 10.68 |
| Al K | - | - | 2.18 | 2.25 | 3.18 | 3.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, D.; Sellamuthu, P.; Prashanth, K.G. Vacuum Hot Pressing of Oxide Dispersion Strengthened Ferritic Stainless Steels: Effect of Al Addition on the Microstructure and Properties. J. Manuf. Mater. Process. 2020, 4, 93. https://doi.org/10.3390/jmmp4030093
Ganesan D, Sellamuthu P, Prashanth KG. Vacuum Hot Pressing of Oxide Dispersion Strengthened Ferritic Stainless Steels: Effect of Al Addition on the Microstructure and Properties. Journal of Manufacturing and Materials Processing. 2020; 4(3):93. https://doi.org/10.3390/jmmp4030093
Chicago/Turabian StyleGanesan, Dharmalingam, Prabhukumar Sellamuthu, and Konda Gokuldoss Prashanth. 2020. "Vacuum Hot Pressing of Oxide Dispersion Strengthened Ferritic Stainless Steels: Effect of Al Addition on the Microstructure and Properties" Journal of Manufacturing and Materials Processing 4, no. 3: 93. https://doi.org/10.3390/jmmp4030093
APA StyleGanesan, D., Sellamuthu, P., & Prashanth, K. G. (2020). Vacuum Hot Pressing of Oxide Dispersion Strengthened Ferritic Stainless Steels: Effect of Al Addition on the Microstructure and Properties. Journal of Manufacturing and Materials Processing, 4(3), 93. https://doi.org/10.3390/jmmp4030093






