Effect of Heat-Treatment on the Thermal and Mechanical Stability of Ni/Al2O3 Nanocrystalline Coatings
Abstract
1. Introduction
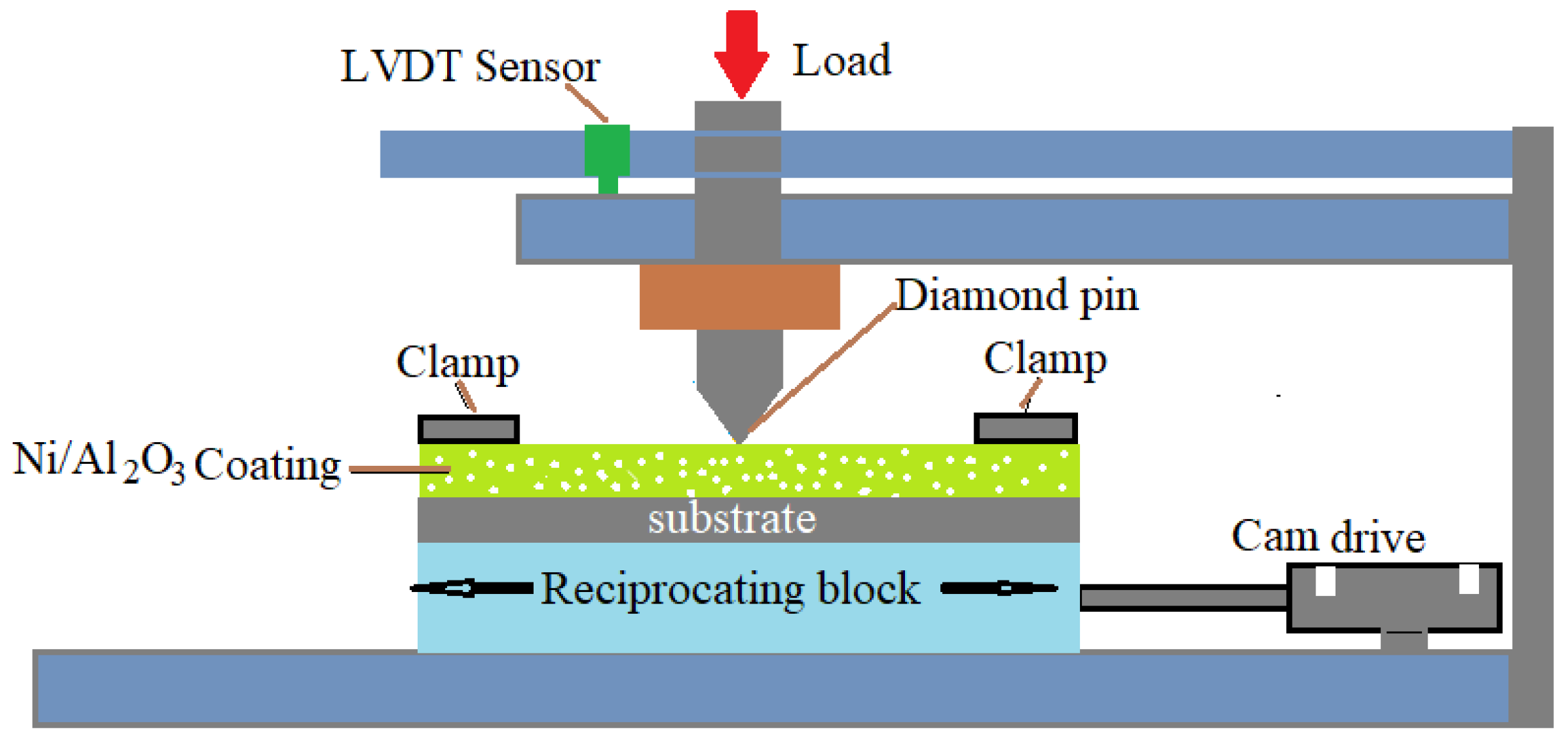
2. Experimental Procedure
3. Results
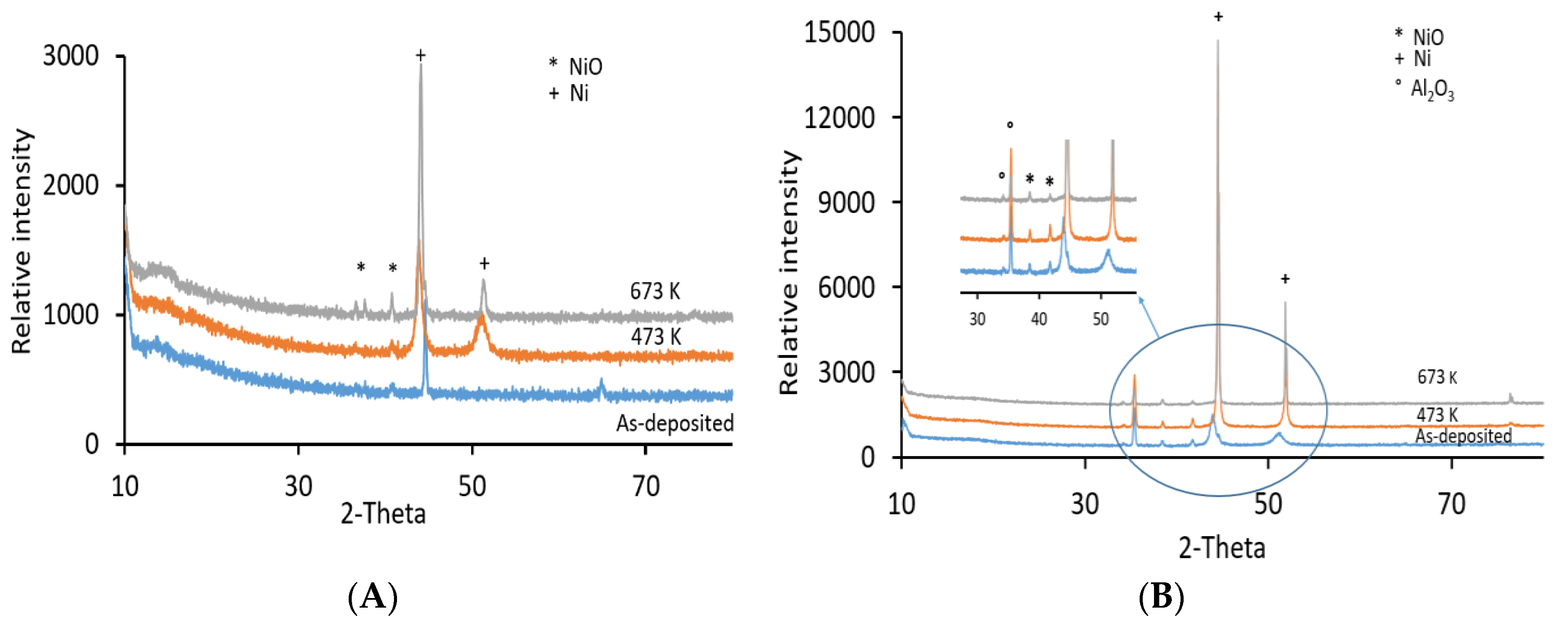
3.1. XRD Analyses
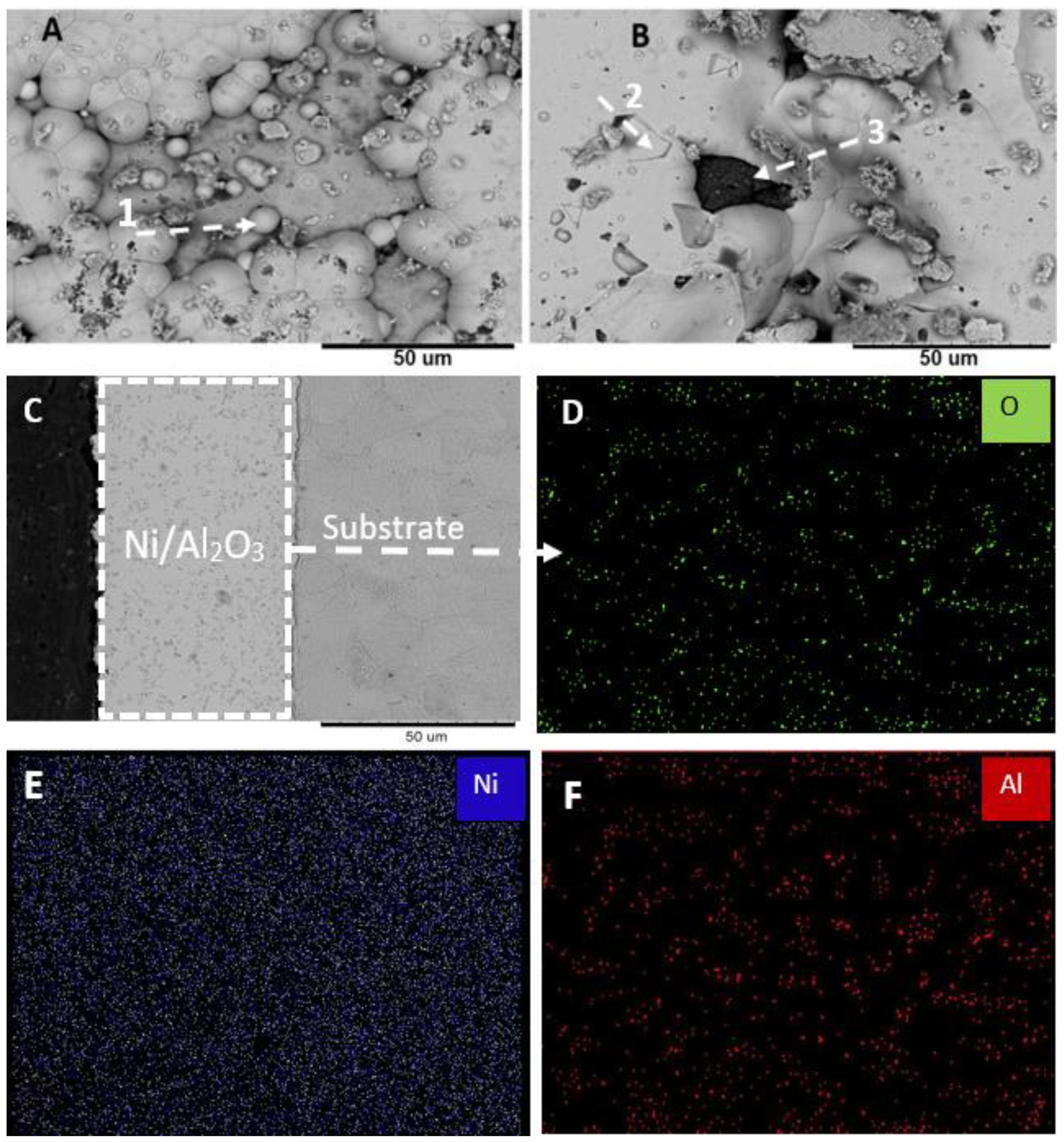
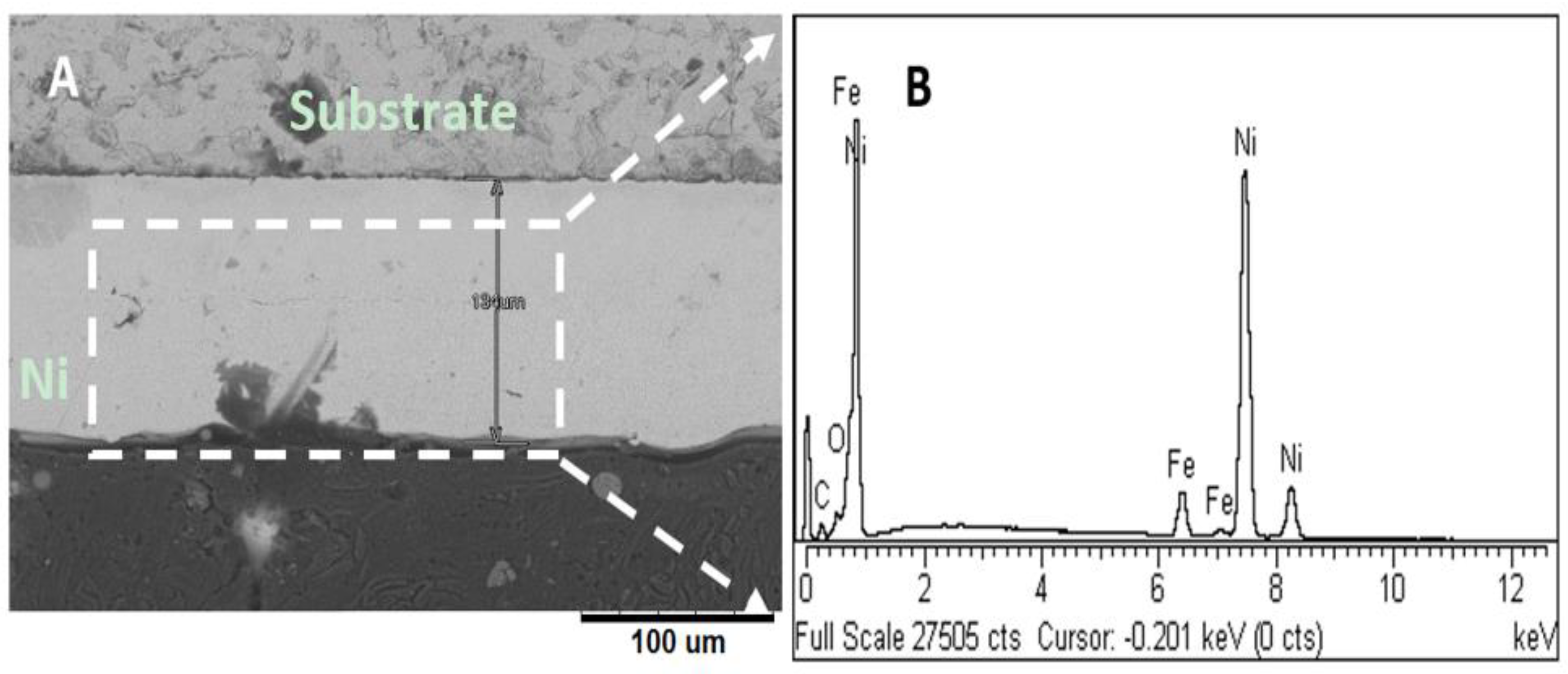
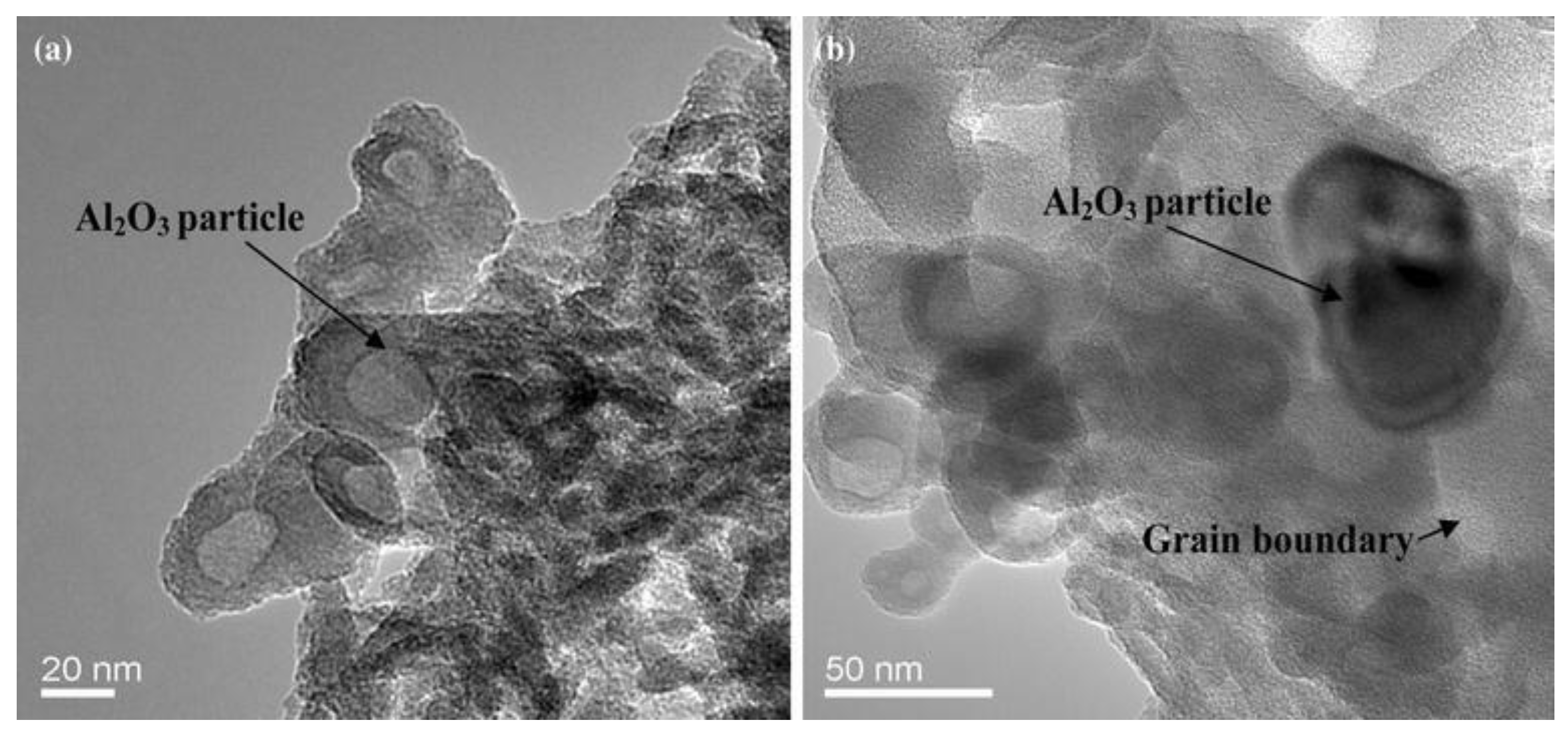
3.2. Microstructural Analyses and Surface Morphology
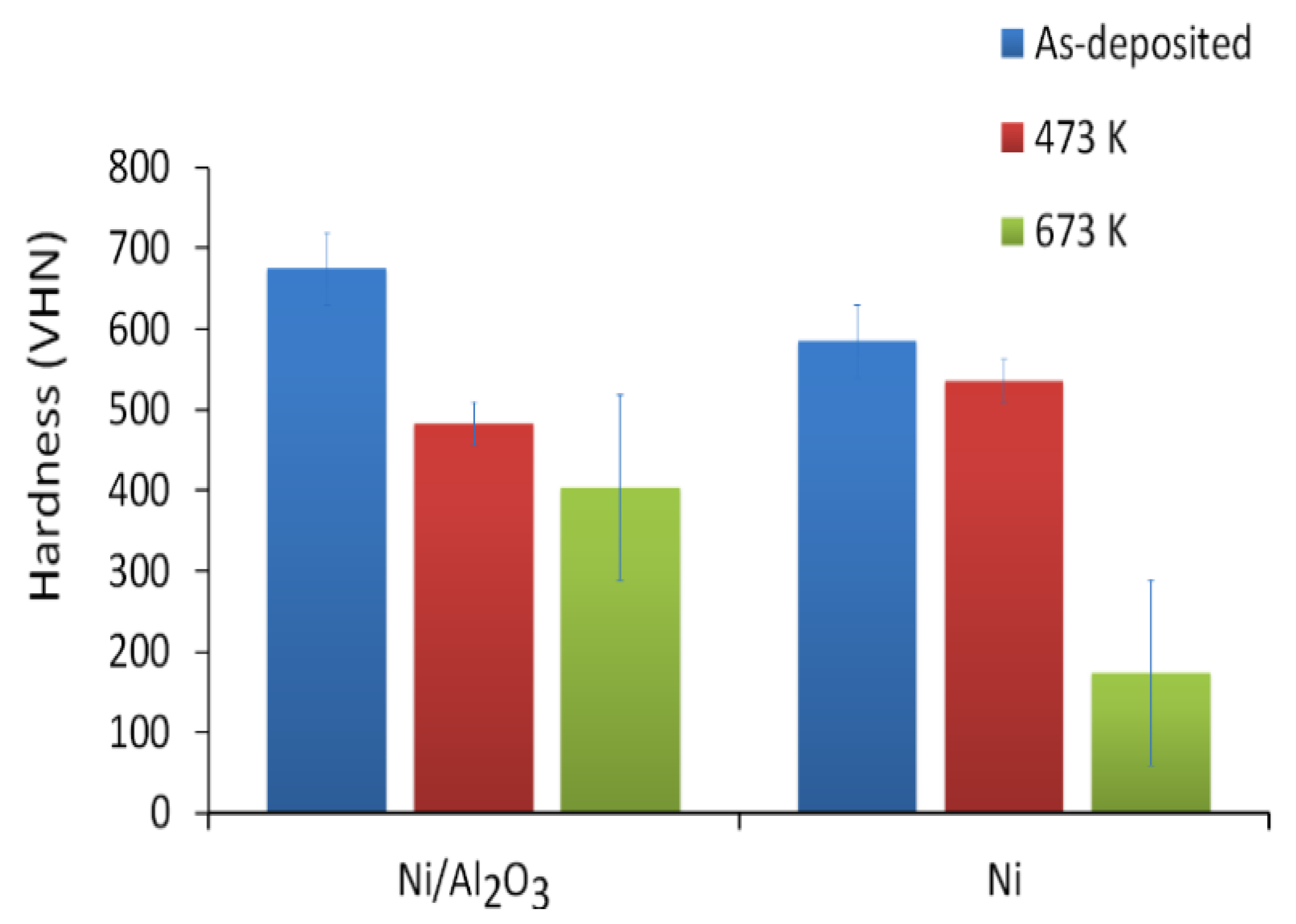
3.3. Hardness
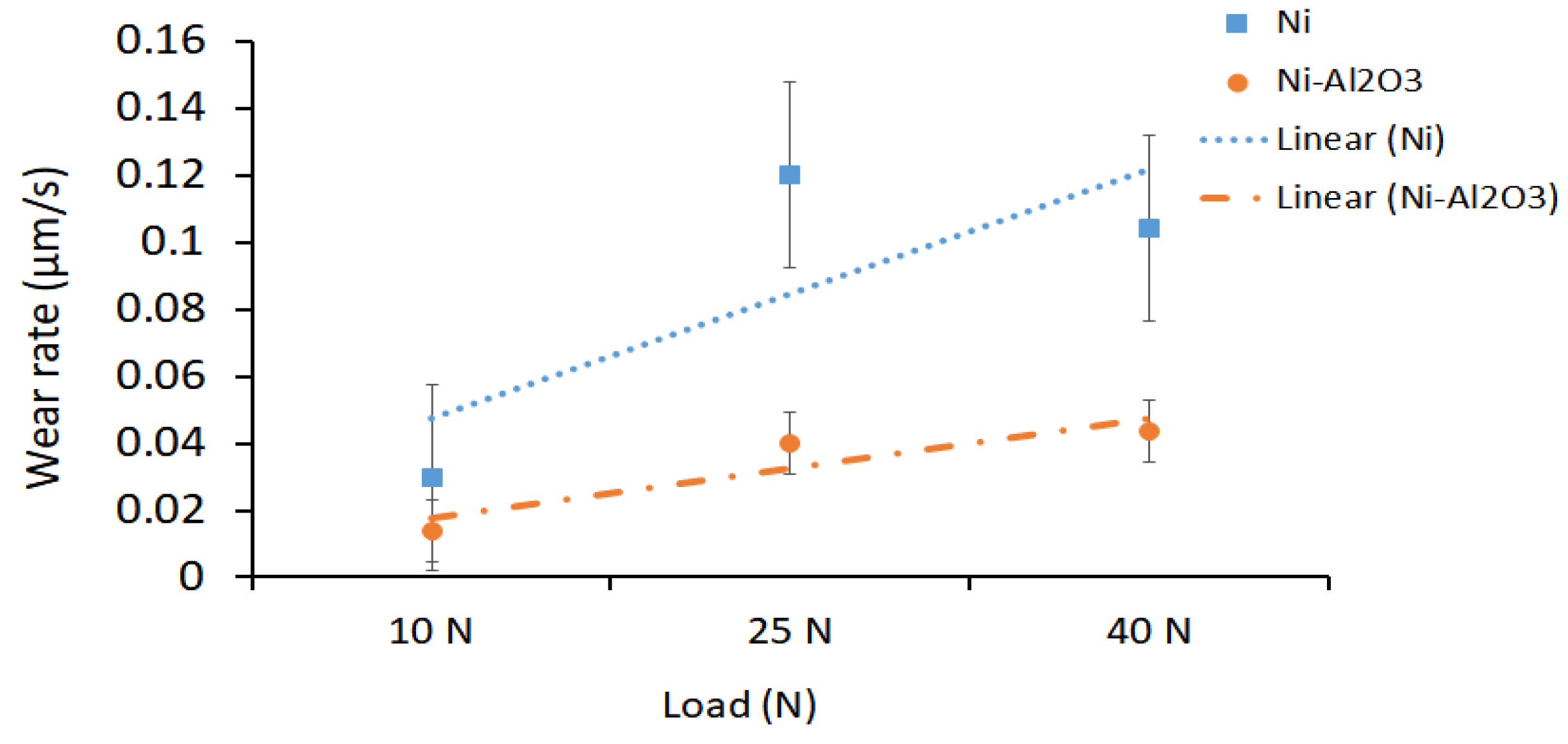
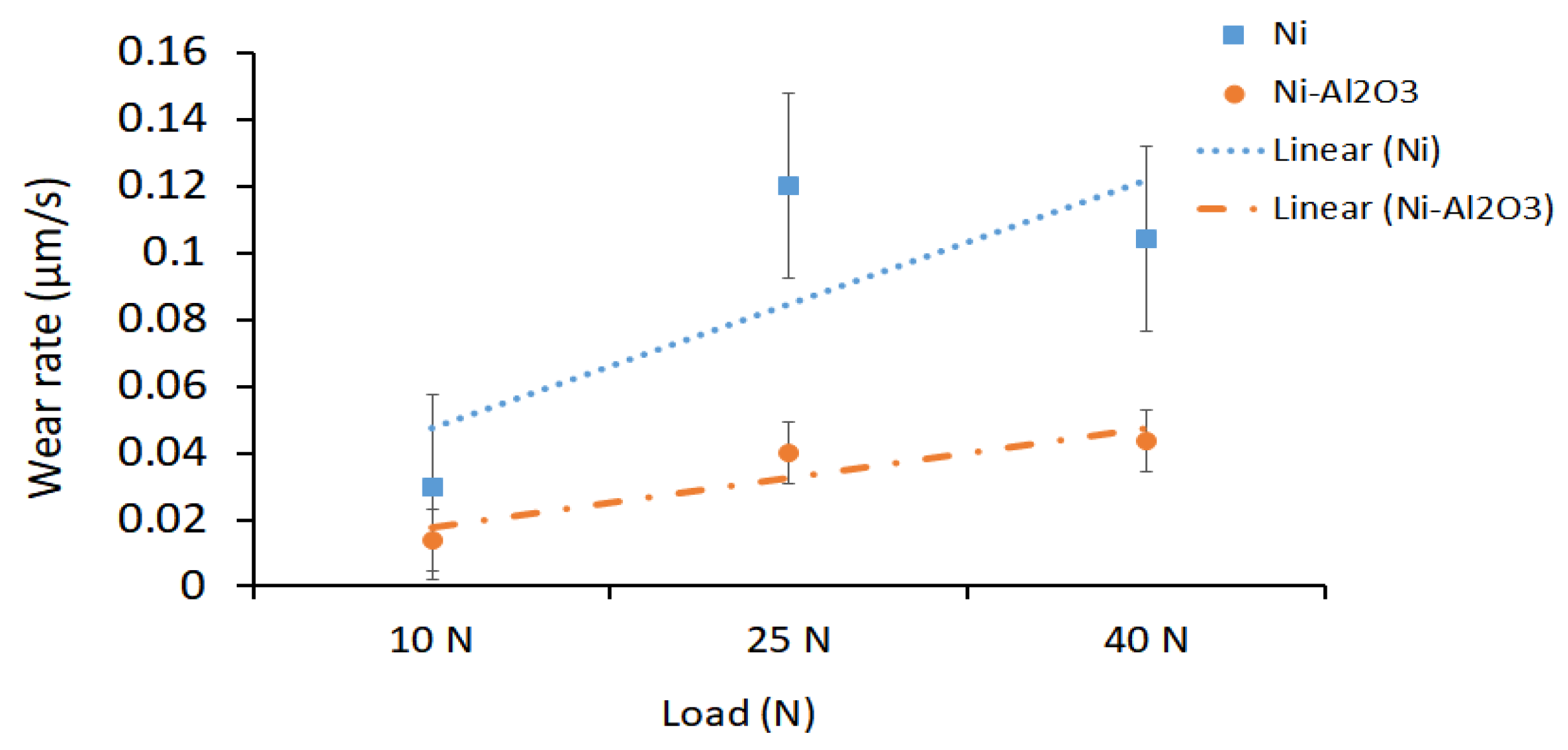
3.4. Sliding Wear Performance of the Coatings
3.4.1. Effect of Load on the Sliding Wear Performance
3.4.2. Effect of Heat Treatment Temperature on the Sliding Wear Performance
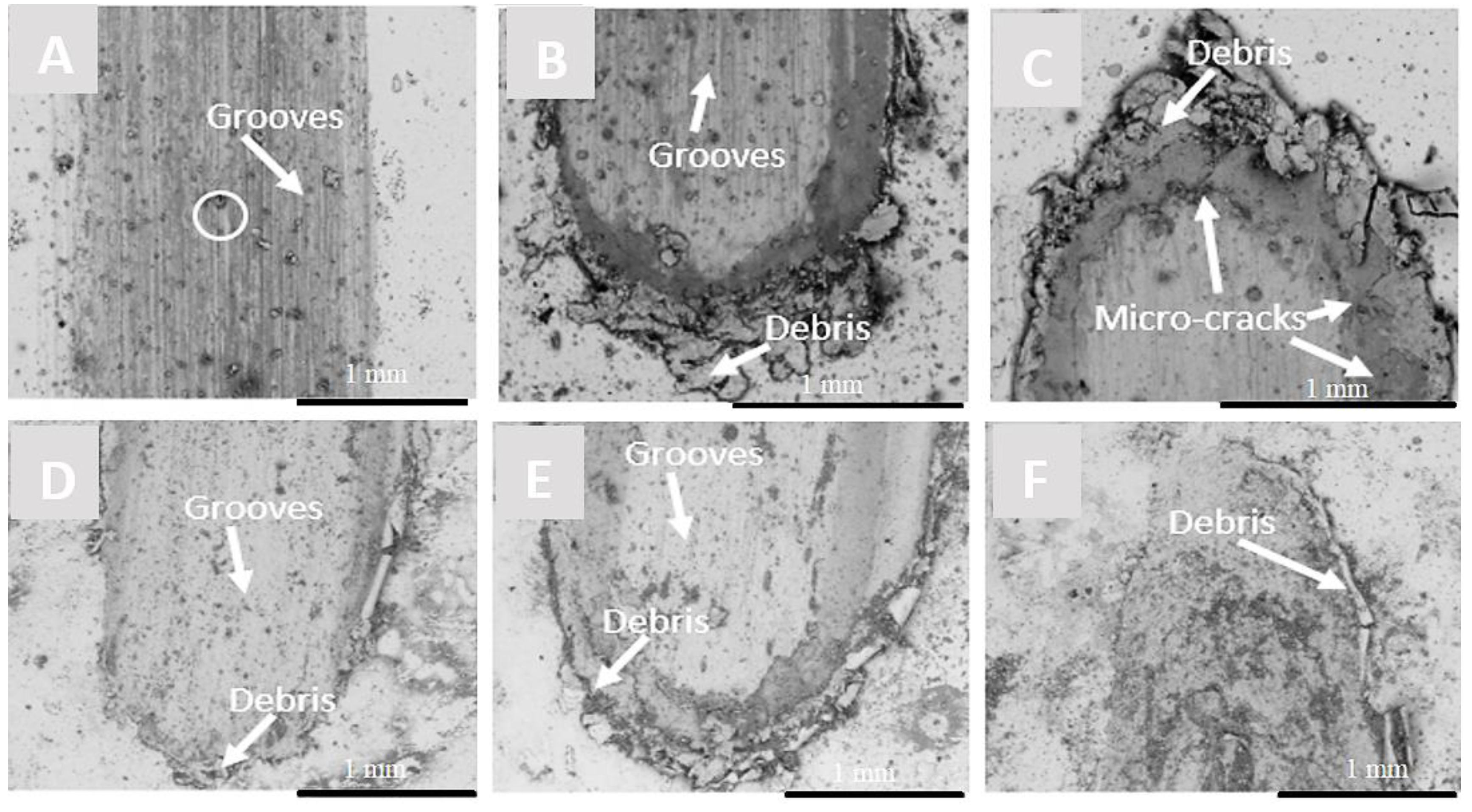
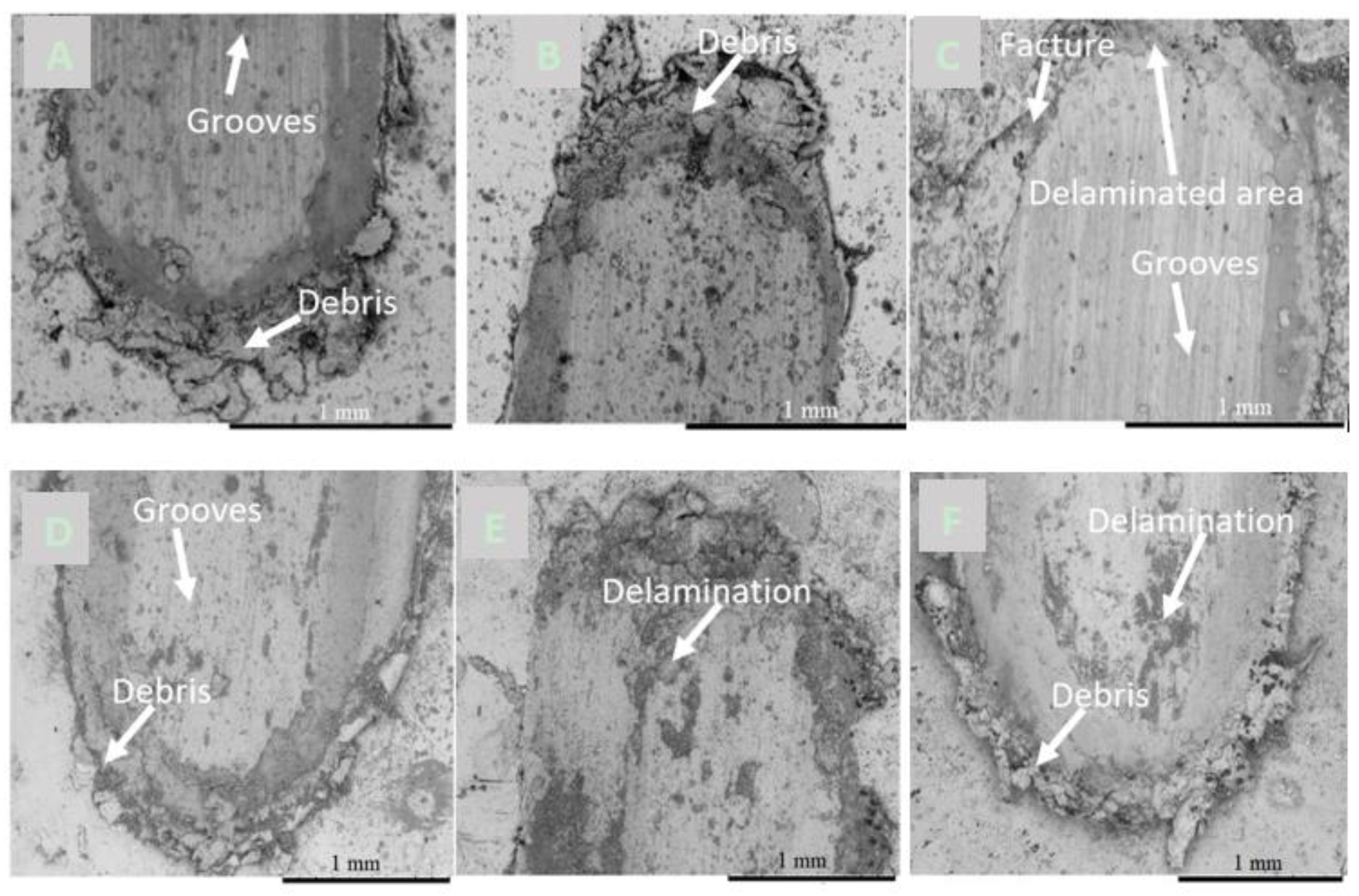
3.4.3. Analysis of Wear Track
4. Discussion
4.1. Hardening Effect of Nanoparticles
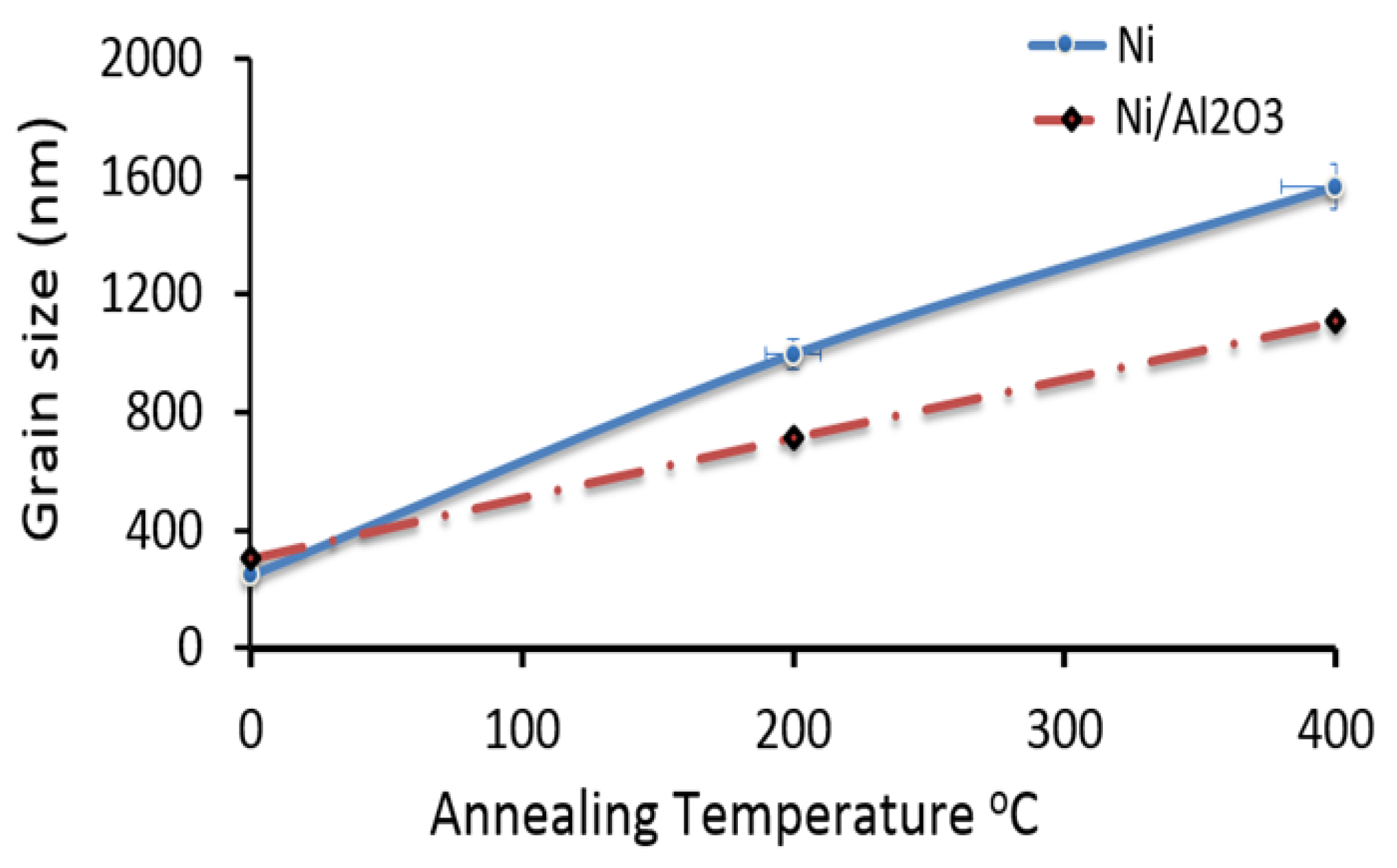
4.2. Grain Growth Kinetics
4.3. Wear Performance of the Coatings
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Riccardis, M.F. Ceramic Coatings Obtained by Electrophoretic Deposition: Fundamentals, Models, Post-Deposition Processes and Applications. Ceram. Coat. Appl. Eng. 2012, 2, 43–68. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Moritz, T.; Eiselt, W.; Moritz, K. Electrophoretic deposition applied to ceramic dental crowns and bridges. J. Mater. Sci. 2006, 41, 8123–8129. [Google Scholar] [CrossRef]
- Garcia, I.; Fransaer, J.; Celis, J.-P. Electrodeposition, and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Wang, S.C.; Wei, W.C.J. Characterization of electroplated Ni/SiC and Ni/Al2O3composite coatings bearing nanoparticles. J. Mater. Res. 2003, 18, 1566–1574. [Google Scholar] [CrossRef]
- Bengoa, L.N.; Pary, P.; Egli, W.A. Codeposition of Particles: Role of Adsorption of the Electroactive Species. J. Electrochem. Soc. 2016, 163, 14. [Google Scholar] [CrossRef]
- Stroumbouli, M.; Gyftou, P.; Pavlatou, E.A.; Spyrellis, N. Codeposition of ultrafine WC particles in Ni matrix composite electrocoatings. Surf. Coat. Technol. 2005, 195, 325–332. [Google Scholar] [CrossRef]
- Farrokhzad, M.A.; Khan, T.I. Sliding wear performance of nickel-based cermet coatings composed of WC and Al2O3 nanosized particles. Surf. Coatings Technol. 2016, 304, 401–412. [Google Scholar] [CrossRef]
- Raghavendra, C.R.; Basavarajappa, S.; Sogalad, I. Electrodeposition of Ni-Al2O3 nano composite coating and evaluation of wear characteristics. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012110. [Google Scholar] [CrossRef]
- Gül, H.; Kiliç, F.; Aslan, S.; Alp, A.; Akbulut, H. Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings. Wear 2009, 256, 976–990. [Google Scholar] [CrossRef]
- Jung, A.; Natter, H.; Hempelmann, R.; Lach, E. Nanocrystalline alumina dispersed in nanocrystalline nickel: Enhanced mechanical properties. J. Mater. Sci. 2009, 44, 2725–2735. [Google Scholar] [CrossRef]
- Bicelli, L.P.; Bozzini, B.; Mele, C.; D’Urzo, L. A review of nanostructural aspects of metal electrodeposition. Int. J. Electrochem. Sci. 2008, 3, 356–408. [Google Scholar]
- Hou, K.H.; Ger, M.D.; Wang, L.M.; Ke, S.T. The wear behaviour of electro-codeposited Ni-SiC composites. Wear 2002, 253, 994–1003. [Google Scholar] [CrossRef]
- Gawad, S.A.A.; Baraka, A.M.; Morsi, M.S.; Eltoum, S.A.A.A. Development of electroless Ni-P-Al2O3 and Ni-P-TiO2 composite coatings from alkaline hypophosphite gluconate baths and their properties. Int. J. Electrochem. Sci. 2013, 8, 1722–1734. [Google Scholar]
- Aruna, S.T.; Selvi, V.E.; Grips, V.K.W.; Rajam, K.S. Corrosion- and wear-resistant properties of Ni-Al2O3 composite coatings containing various forms of alumina. J. Appl. Electrochem. 2011, 41, 461–468. [Google Scholar] [CrossRef]
- Cooke, K.O. A study of the effect of nanosized particles on transient liquid phase diffusion bonding AI6061 metal-matrix composite (MMC) using Ni/Al2O3 Nanocomposite Interlayer, Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 627–634. [Google Scholar] [CrossRef]
- Gadhari, P.; Sahoo, P. Effect of Process Parameters on Microhardness of Ni-P-Al2O3 Composite Coatings. Procedia Mater. Sci. 2014, 6, 623–632. [Google Scholar] [CrossRef]
- Cooke, K.O. Electrodeposited Nanocomposite Coatings: Principles and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2014; ISBN 978-1-62948-569-0. [Google Scholar]
- Cooke, K.O. Effect of Thermal Processing on the Tribology of Nanocrystalline Ni/TiO2 Coatings. J. Emergent Mater. 2018, 1, 165–173. [Google Scholar] [CrossRef]
- Cooke, K.O. Parametric Analysis of Electrodeposited Nano- composite Coatings for Abrasive Wear Resistance. Intech Open 2014, 2, 64. [Google Scholar] [CrossRef]
- Pavlov, I.; Benea, L.; Celis, J.P.; Vazquez, L. Influence of NANO-TiO2 CO-deposition on the morphology, microtopography, and crystallinity of Ni/NANO-TiO2 electrosynthesized nanocomposite coatings. Dig. J. Nanomater. Biostructures 2013, 8, 1043–1050. [Google Scholar]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five Decades of the Zener Equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Kim, B.; Hiraga, K.; Morita, K. Kinetics of Normal Grain Growth Depending on the Size Distribution of Small Grains. J. Mater. Trans. 2003, 44, 2239–2244. [Google Scholar] [CrossRef][Green Version]
- Kato, K. Wear in relation to friction—A review. Wear 2000, 241, 151–157. [Google Scholar] [CrossRef]
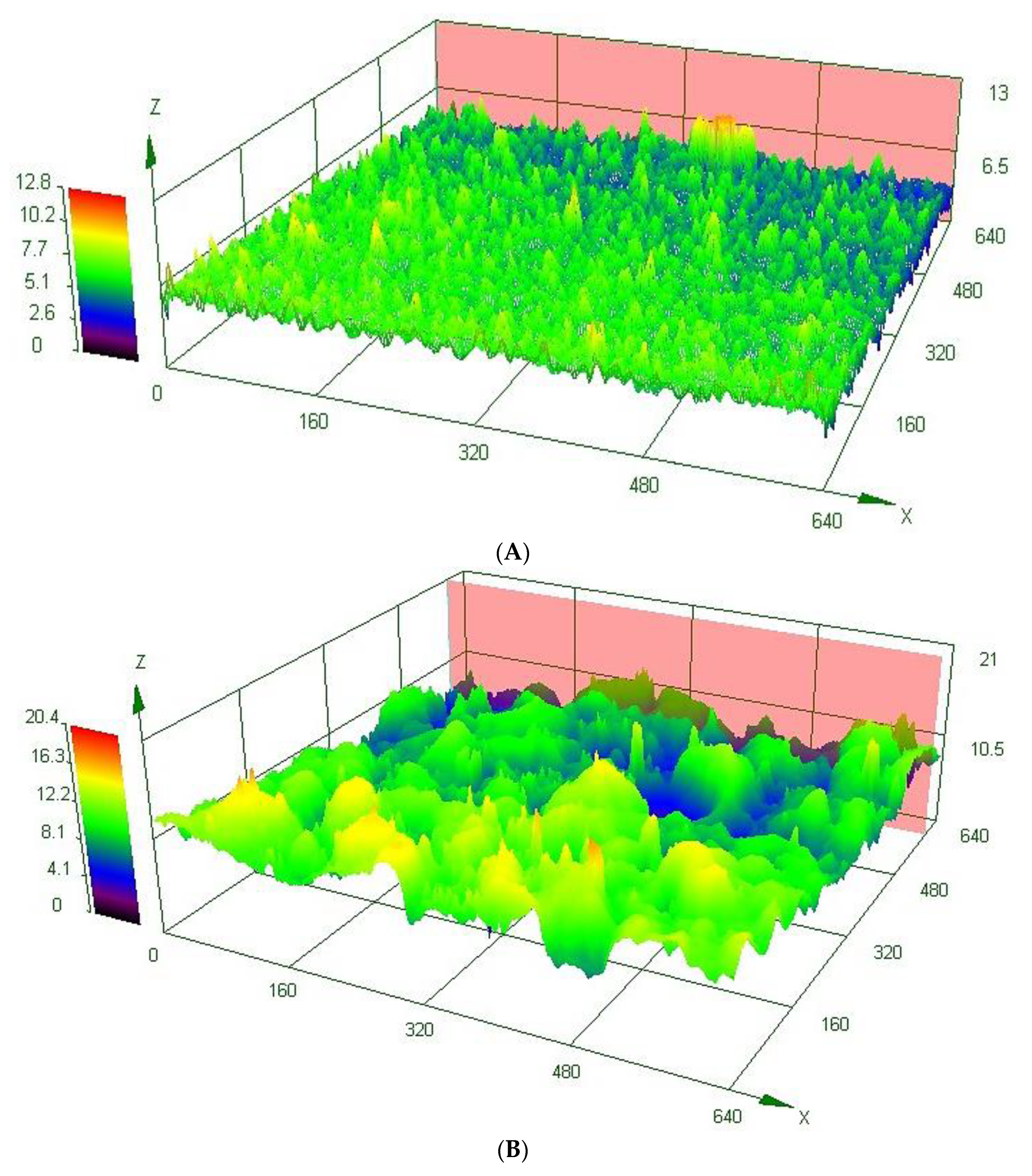
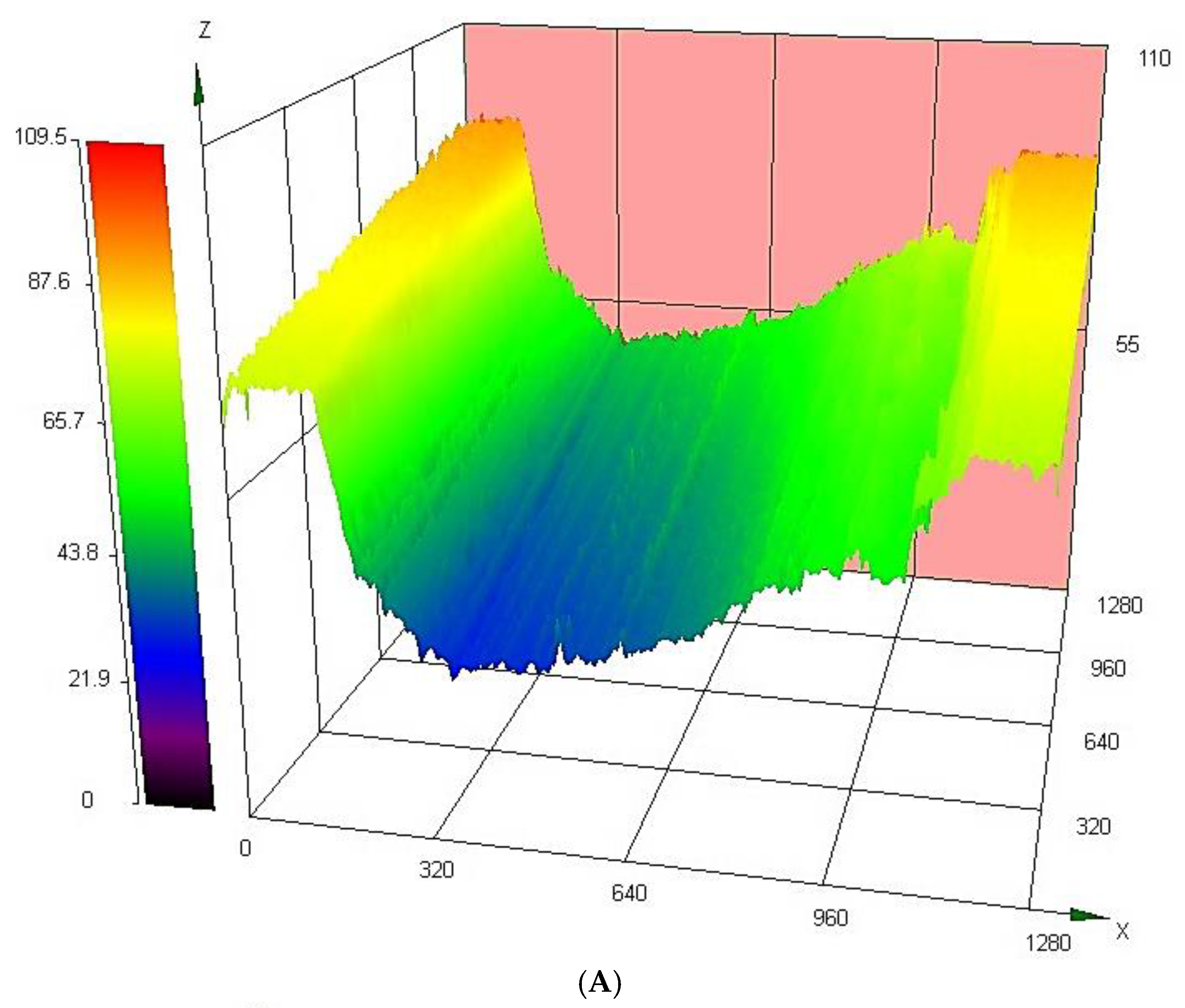

| Heat Treatment Temperature | Roughness (µm) | |
|---|---|---|
| Ni | Ni/Al2O3 | |
| As-deposited | 0.157 | 0.547 |
| 473 K | 0.162 | 0.624 |
| 673 K | 0.234 | 0.659 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooke, K.O.; Khan, T.I.; Ali Shar, M. Effect of Heat-Treatment on the Thermal and Mechanical Stability of Ni/Al2O3 Nanocrystalline Coatings. J. Manuf. Mater. Process. 2020, 4, 17. https://doi.org/10.3390/jmmp4010017
Cooke KO, Khan TI, Ali Shar M. Effect of Heat-Treatment on the Thermal and Mechanical Stability of Ni/Al2O3 Nanocrystalline Coatings. Journal of Manufacturing and Materials Processing. 2020; 4(1):17. https://doi.org/10.3390/jmmp4010017
Chicago/Turabian StyleCooke, Kavian O., Tahir I. Khan, and Muhammad Ali Shar. 2020. "Effect of Heat-Treatment on the Thermal and Mechanical Stability of Ni/Al2O3 Nanocrystalline Coatings" Journal of Manufacturing and Materials Processing 4, no. 1: 17. https://doi.org/10.3390/jmmp4010017
APA StyleCooke, K. O., Khan, T. I., & Ali Shar, M. (2020). Effect of Heat-Treatment on the Thermal and Mechanical Stability of Ni/Al2O3 Nanocrystalline Coatings. Journal of Manufacturing and Materials Processing, 4(1), 17. https://doi.org/10.3390/jmmp4010017







