Microstructure Development during Low-Current Resistance Spot Welding of Aluminum to Magnesium
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Cold Pressing and Resistance Spot Welding
2.3. Microstructural Characterization
2.4. Microhardness Measurements
3. Results
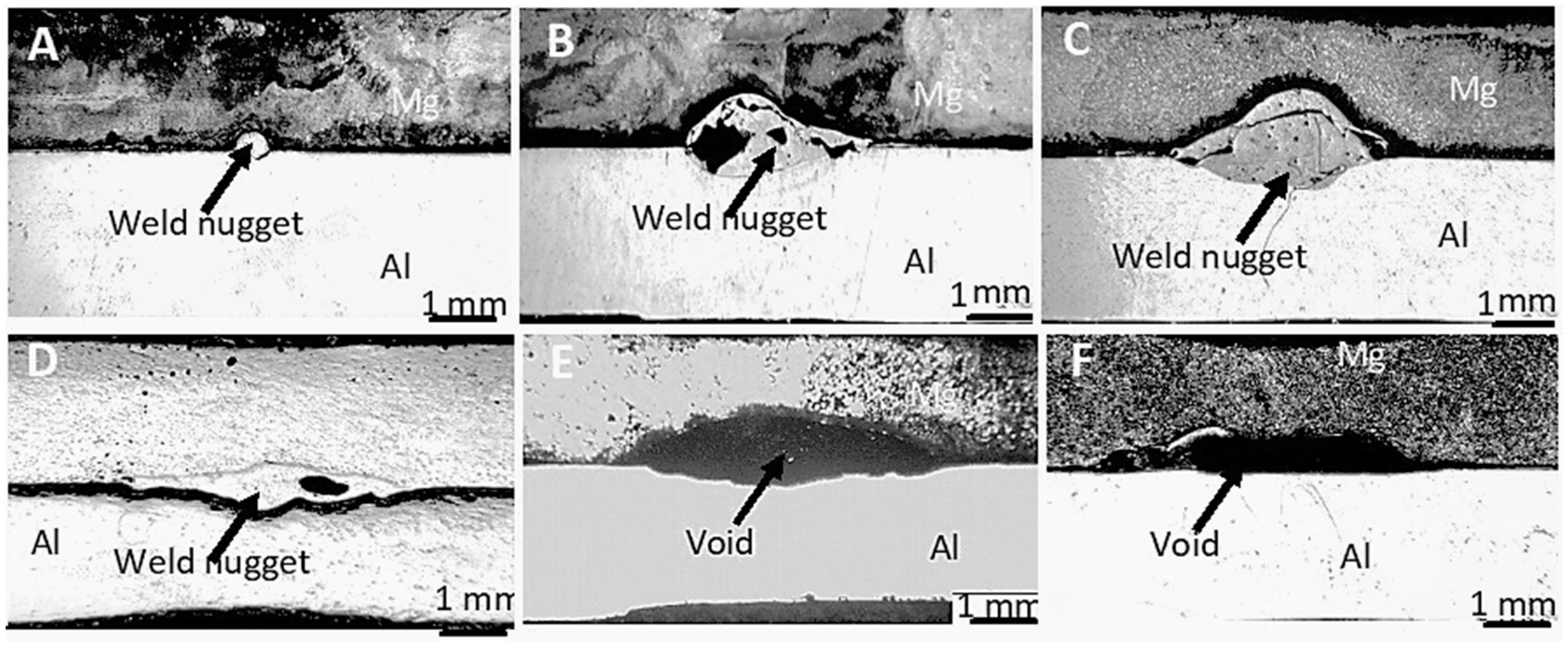
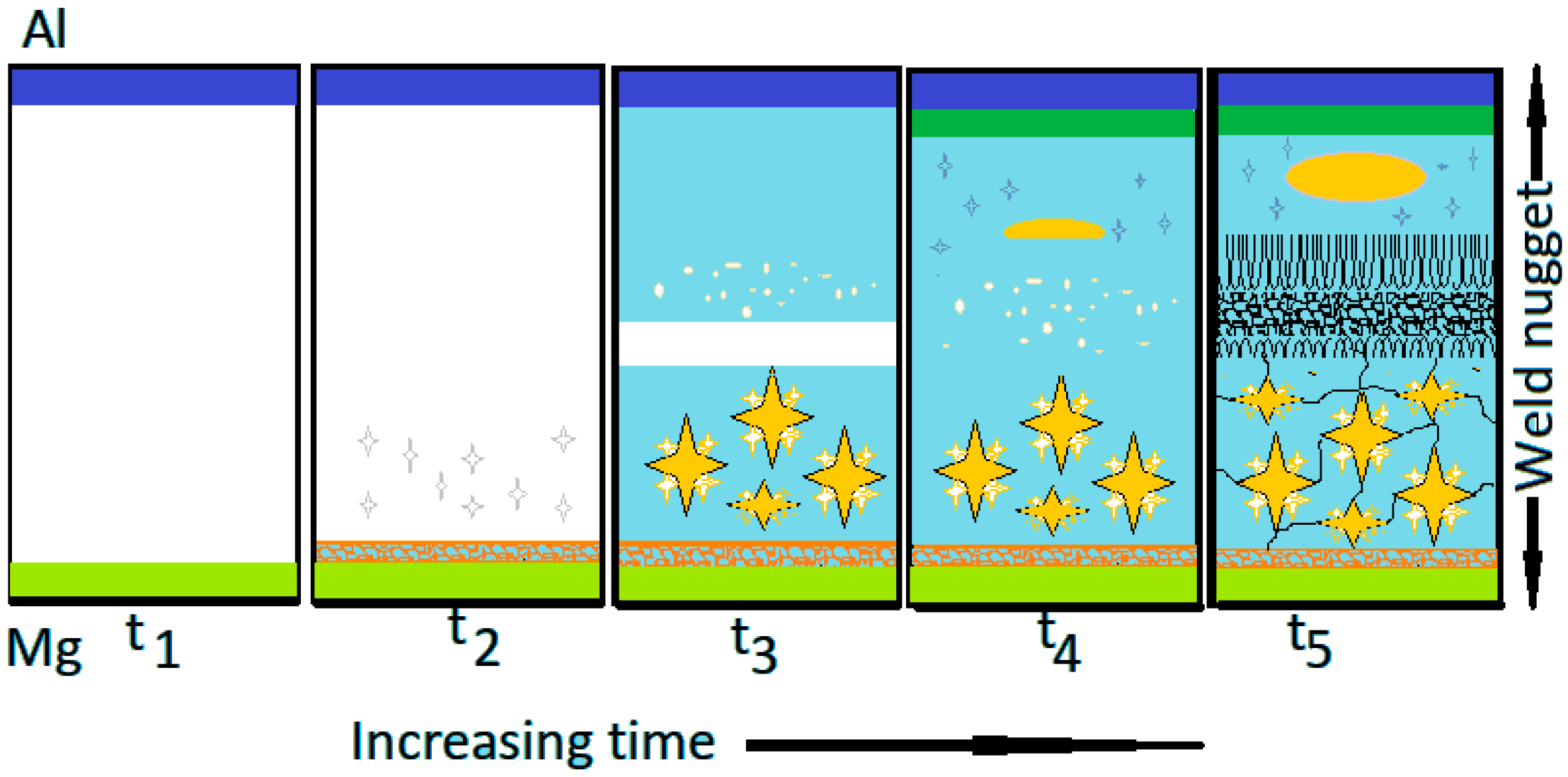
3.1. Formation of the Weld Nugget
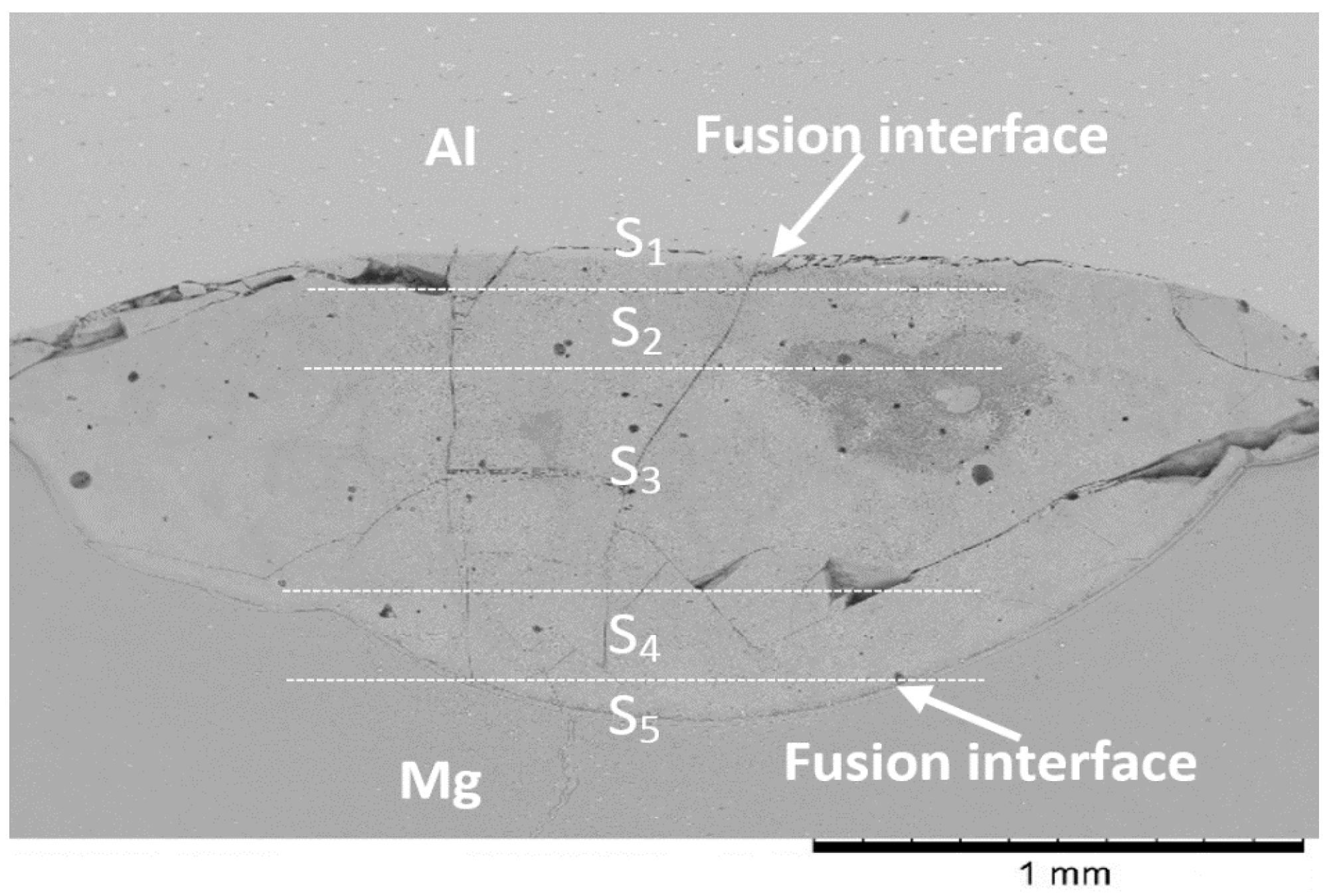
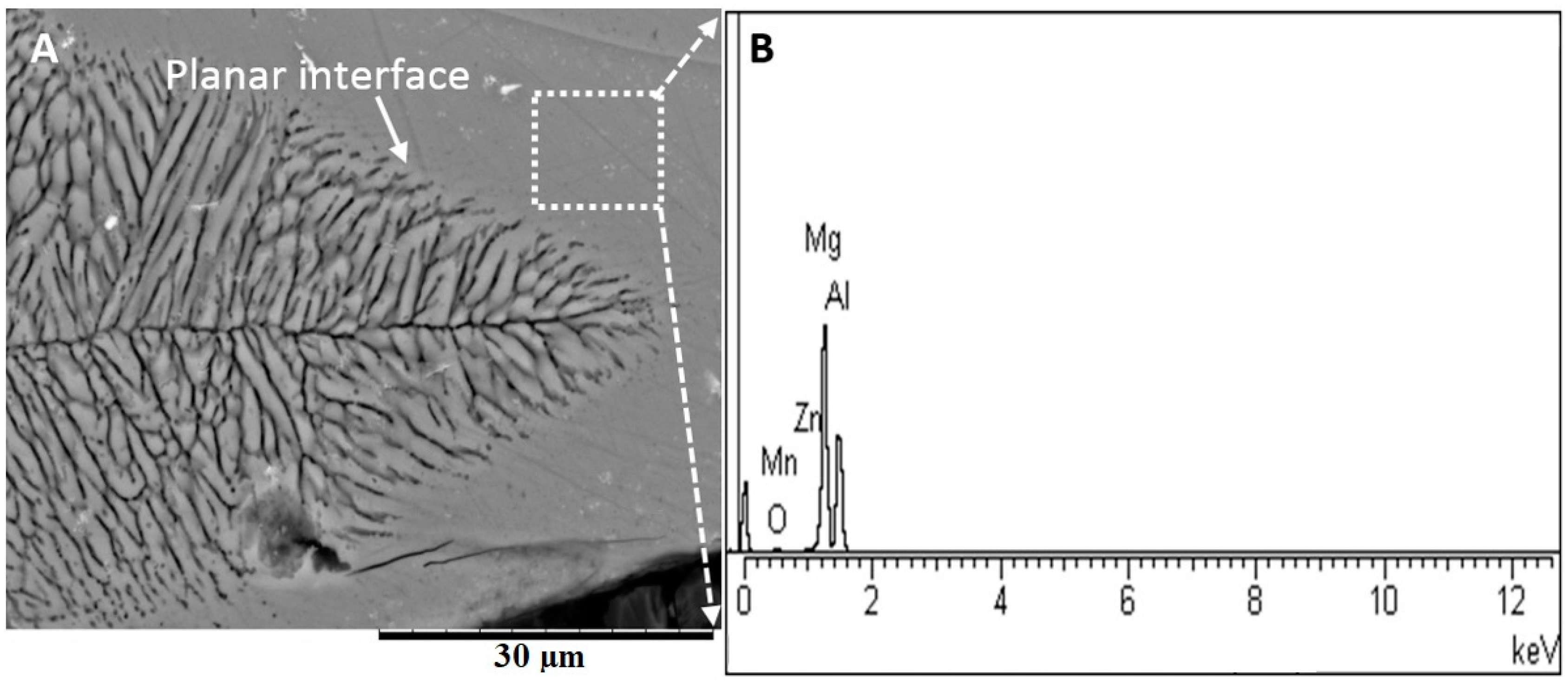
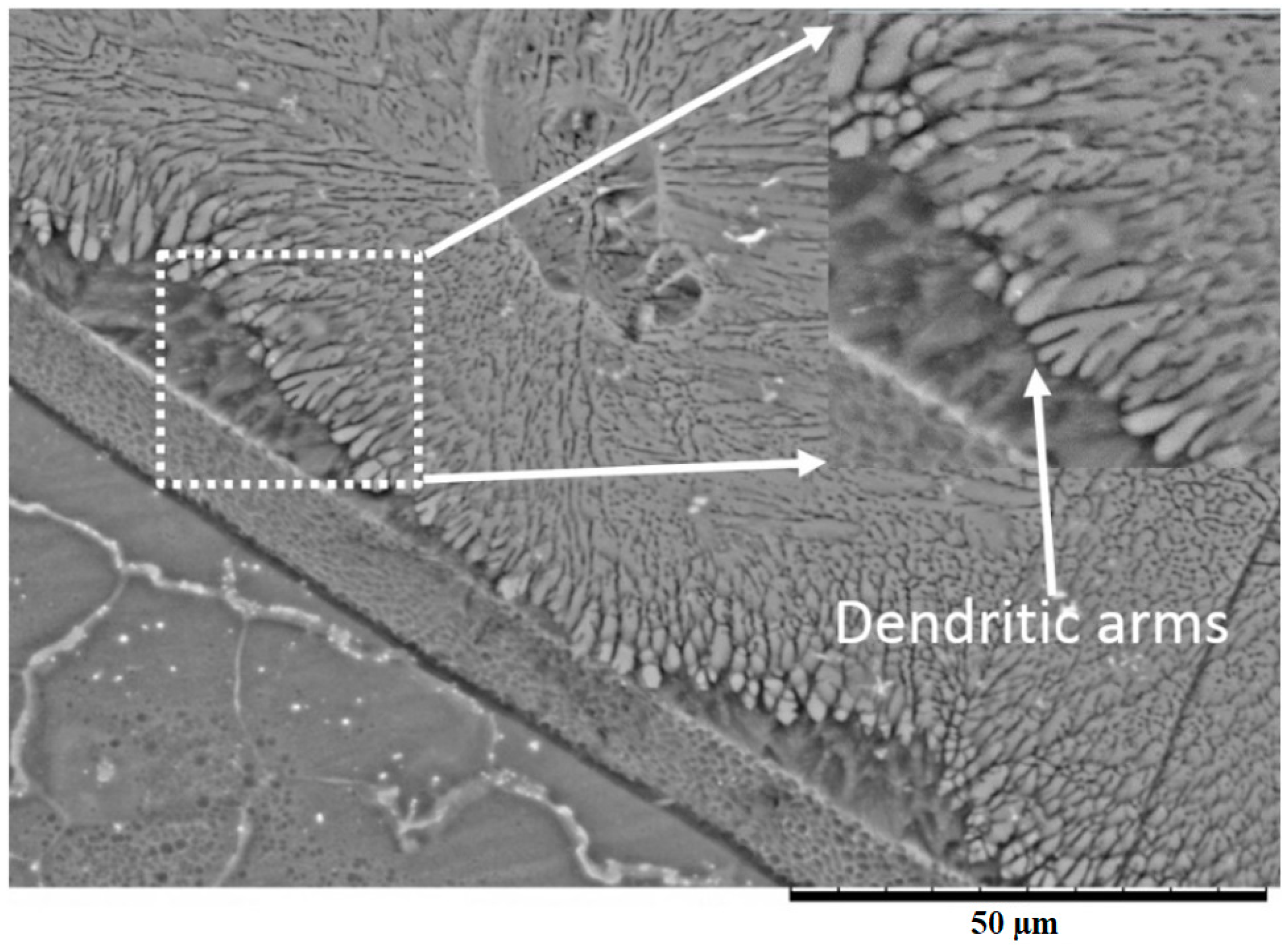
3.2. Microstructure Analysis of the Fusion Interfaces
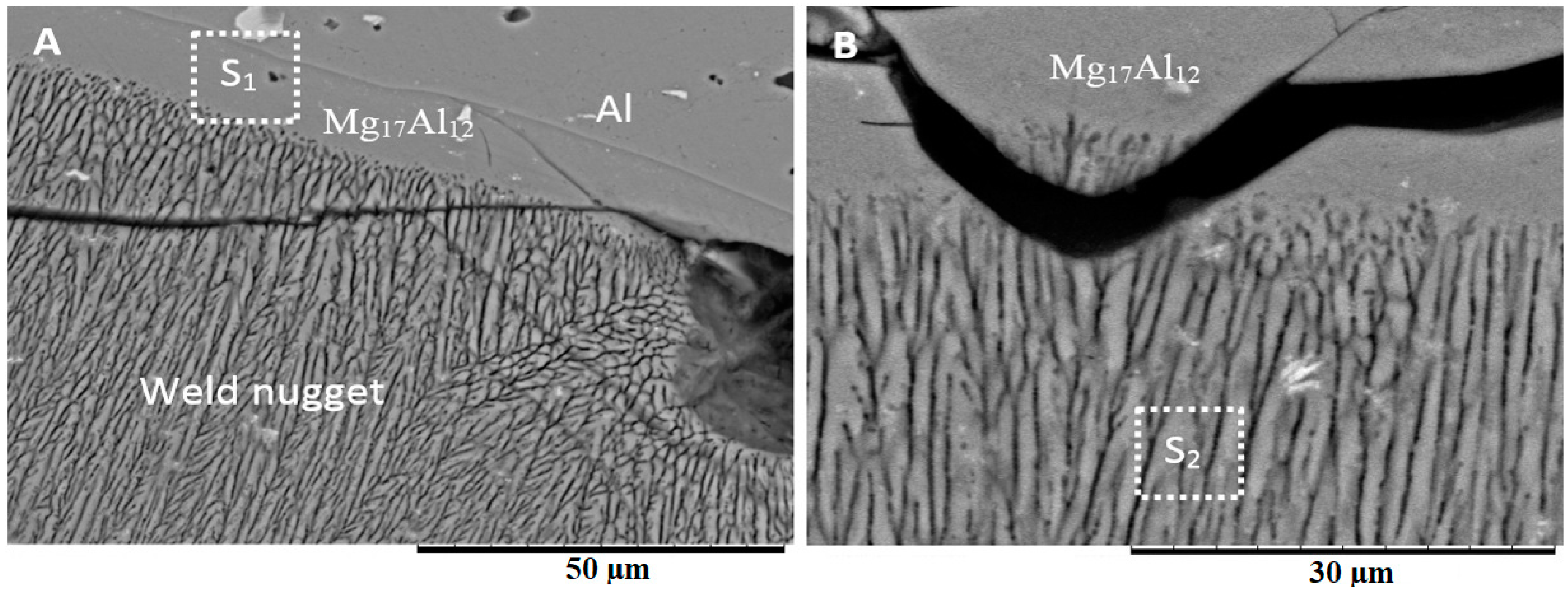
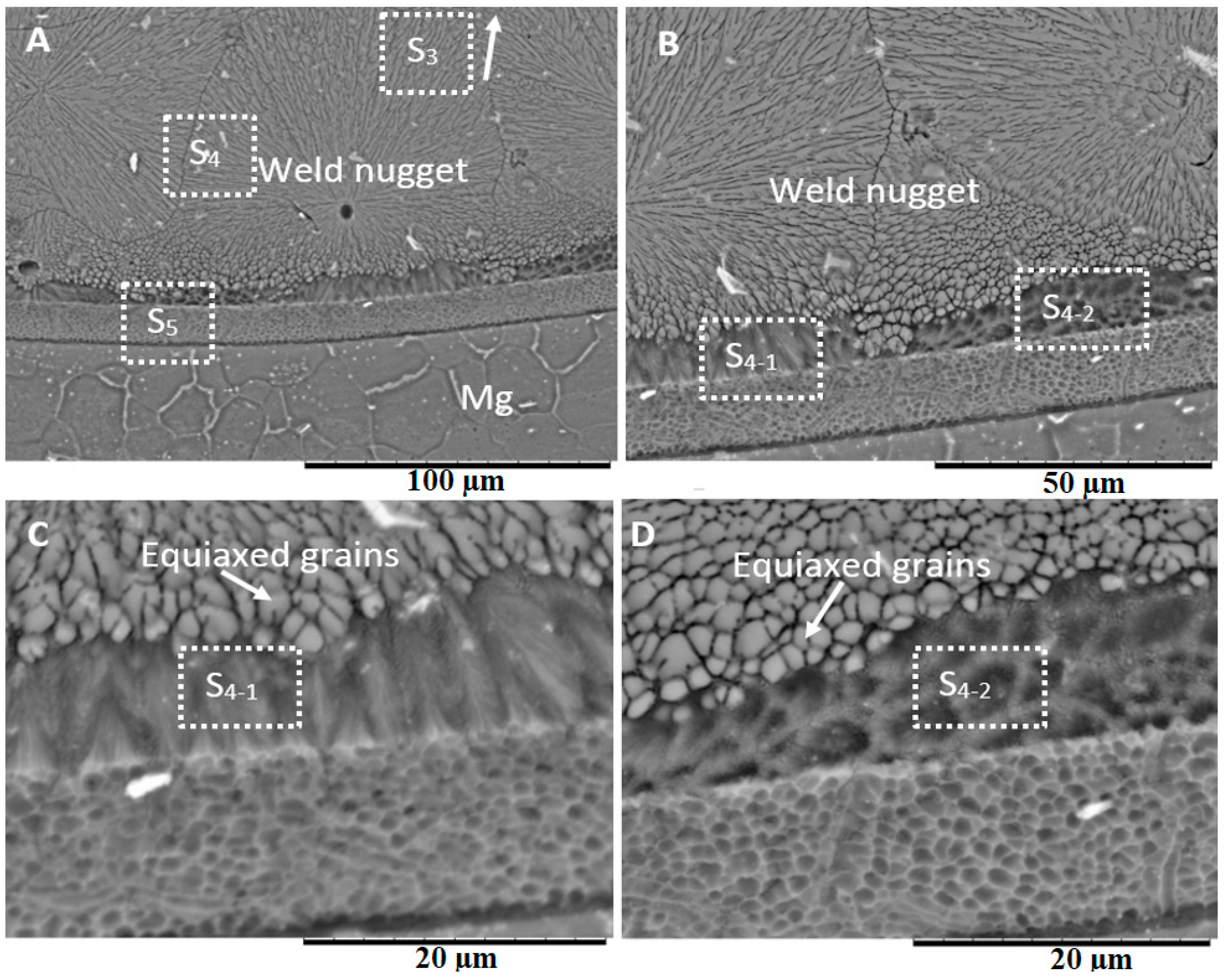
3.3. Microstructure at the Center of the Weld
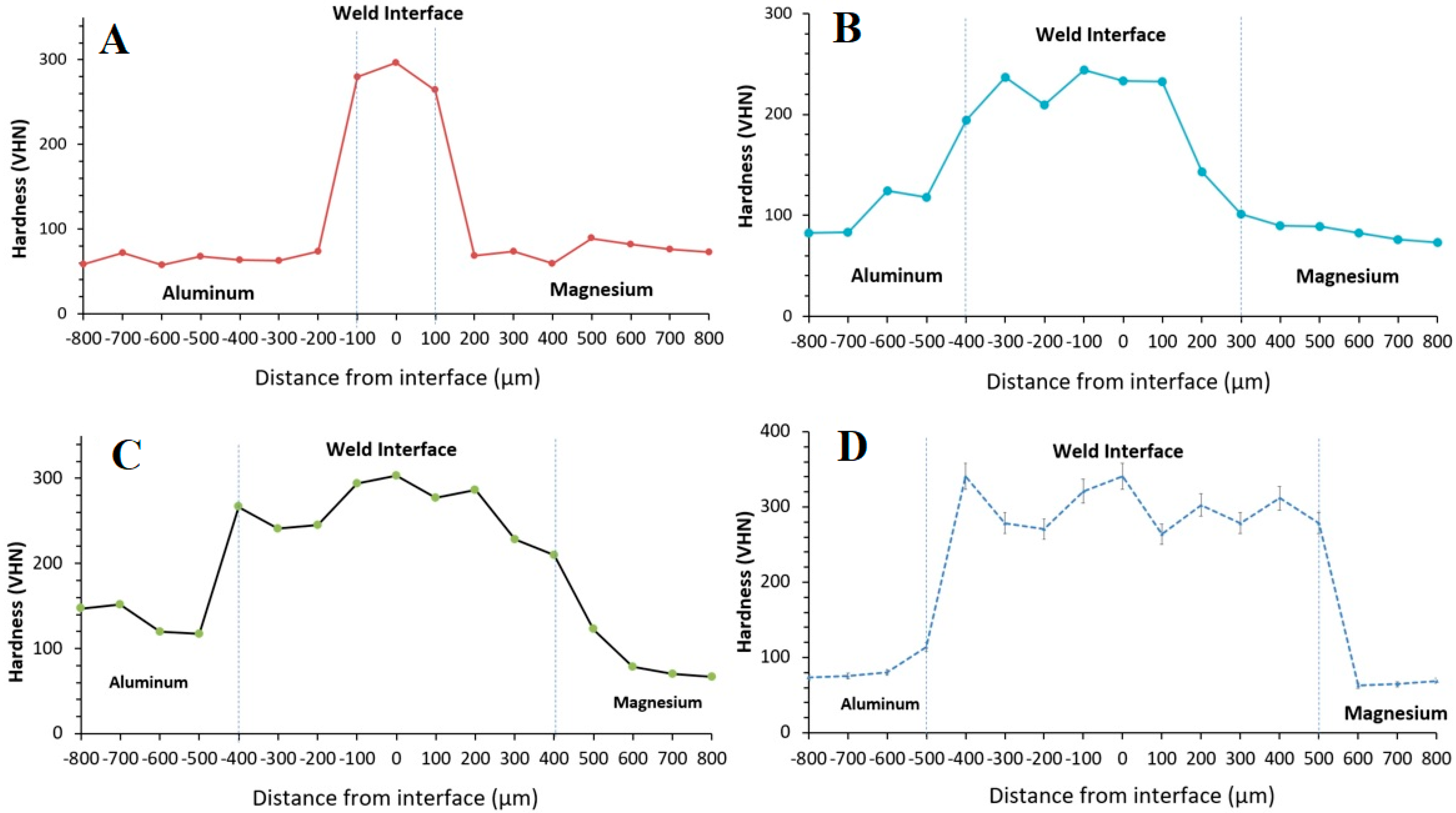
3.4. Microhardness Measurements
4. Discussion
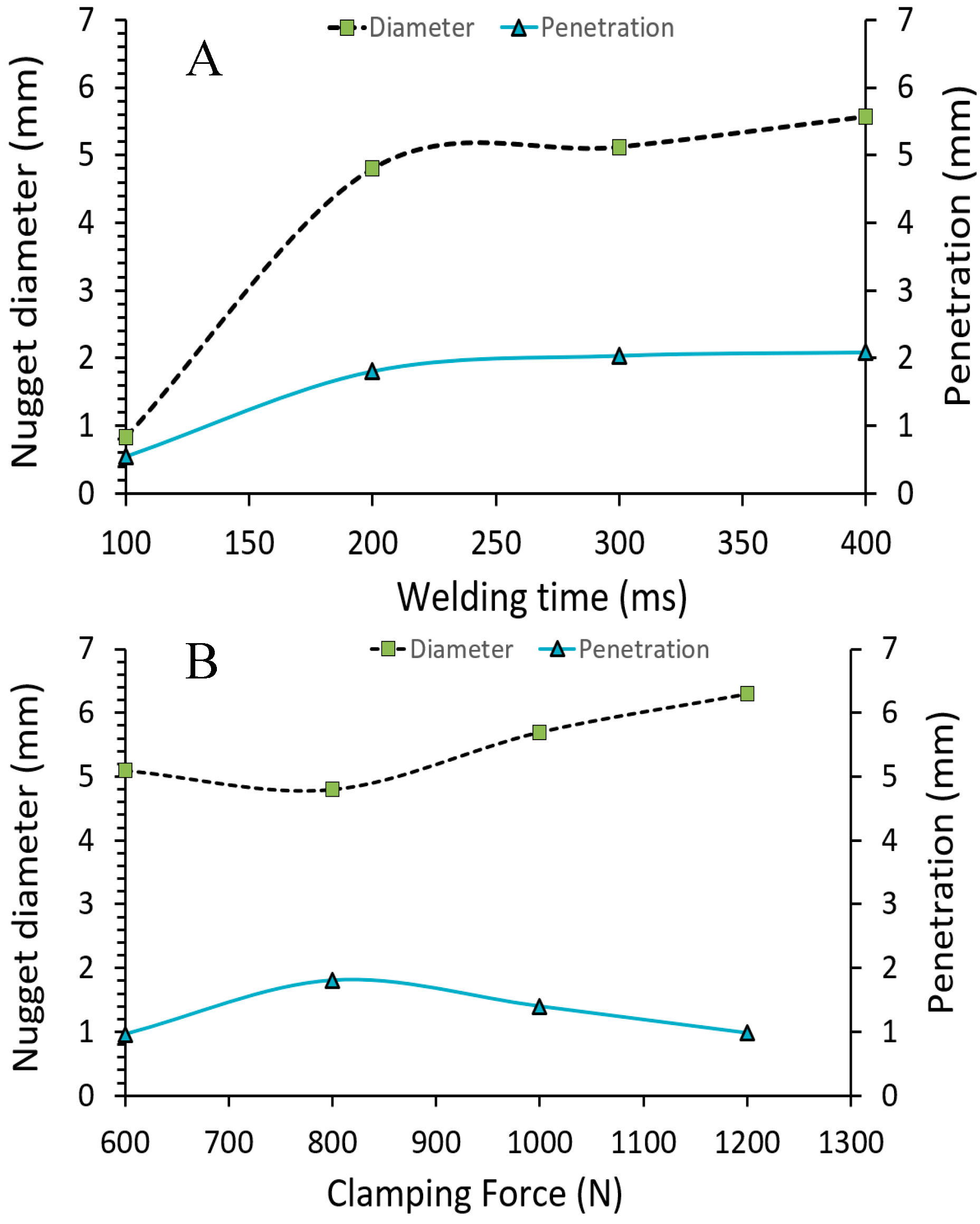
4.1. Effect of Welding Parameters on Weld Nugget Formation
4.2. Weld Nugget Microstructures
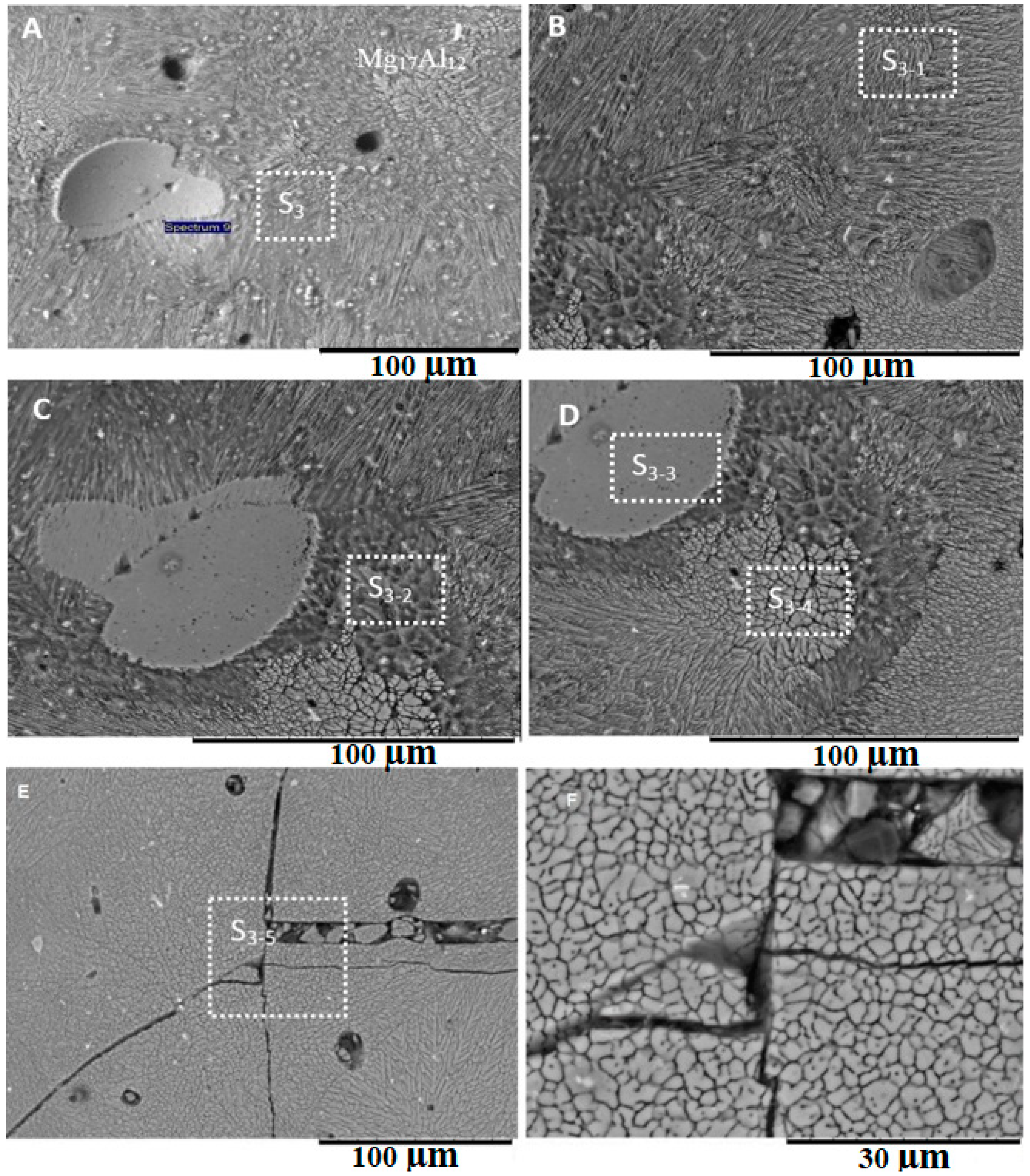
4.3. Formation of Intermetallic Compounds
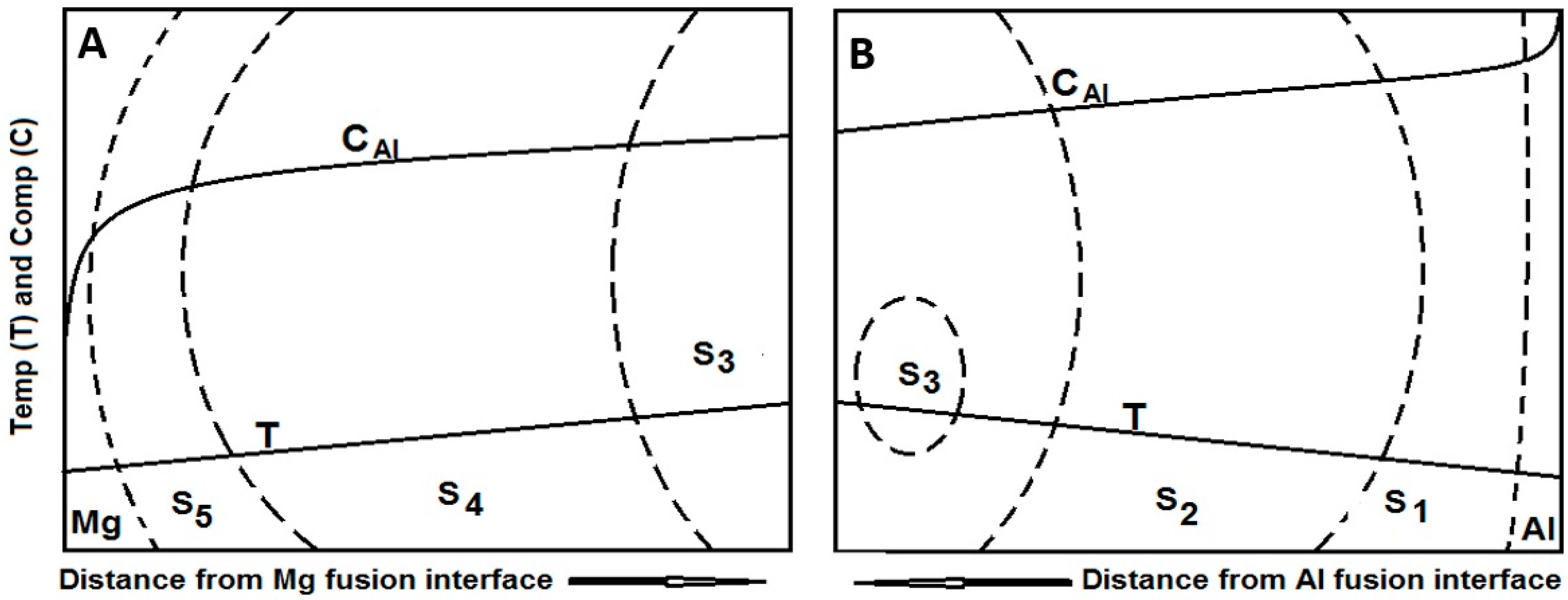
4.4. Macrosegregation in the Weld
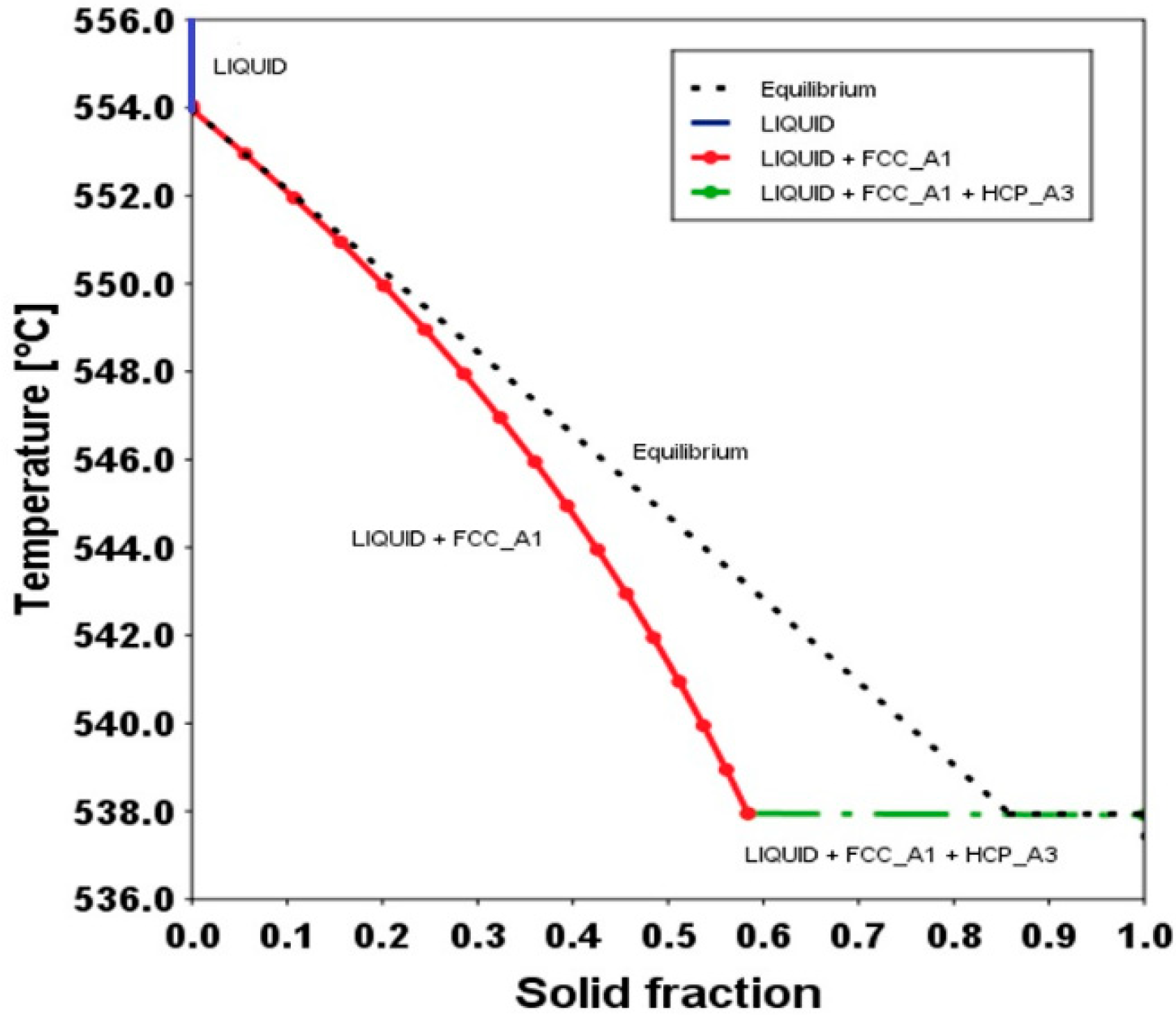
4.5. Solidification Sequence
4.6. Microhardness Measurements
5. Conclusions
- The optimal parameter settings for small-scale resistance welding were found to be 300 ms welding time and 800 N clamping force. Weld nuggets formed with lower welding time and clamping force were undersized and contained extensive porosity, while clamping force above 800 N cause gross deformation of the test samples and the expulsion of the molten metal during the welding process.
- The differences in the thermokinetic properties of aluminum and magnesium alloys led to the formation of an asymmetrical joint with most of the melting taking place on the Mg-side of the weld. Geometric analysis of the weld nugget showed that 67% of the weld nugget occurred on the Mg-side. Verification of the composition of the weld nugget using EDS confirmed the dominance of Mg content within the weld nugget, which led to the formation of the Mg17Al12 and the Mg5Al8 intermetallic compounds.
- Various morphological transitions were observed on the Al-side between planar, cellular, and dendritic, as predicted by the increasing growth rate when moving from the base metal into the center of the weld nugget. Meanwhile, on the Mg-side, a thick MgAl2O4 layer was observed, and beyond this layer, a transition between cellular, equiaxed, and columnar dendritic morphologies was observed.
- The diameter of the weld nugget was found to increase with an increase of both welding time and clamping force. However, at higher clamping force, melt expulsion occurred, leading to the formation of voids within the weld nugget.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muhamed, M.; Omar, M.; Abdullah, S.; Sajuri, Z.; Wan Zamri, W.; Abdullah, M. Brazed Joint Interface Bonding Strength of AR500 Steel and AA7075 Aluminium Alloy. Metals 2018, 8, 668. [Google Scholar] [CrossRef]
- Materials, L. Lighweight Materials 2016 Annual Report; United States Department of Energy: Washington DC, USA, 2017.
- Liu, L.; Ren, D.; Liu, F. A review of dissimilar welding techniques for magnesium alloys to aluminum alloys. Materials 2014, 7, 3735–3757. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, D. A novel weld-bonding hybrid process for joining Mg alloy and Al alloy. Mater. Des. 2011, 32, 3730–3735. [Google Scholar] [CrossRef]
- Kah, P.; Suoranta, R.; Martikainen, J.; Magnus, C. Techniques for joining dissimilar materials: Metals and polymers. Rev. Adv. Mater. Sci. 2014, 16, 229–237. [Google Scholar]
- Urbikain, G.; Perez, J.M.; López de Lacalle, L.N.; Andueza, A. Combination of friction drilling and form tapping processes on dissimilar materials for making nutless joints. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 1007–1020. [Google Scholar] [CrossRef]
- Cooke, K.O. A comparative analysis of techniques used for joining intermetallic MMCs. In Intermetallic Matrix Composites; Woodhead Publishing: Sawston, UK, 2018; pp. 221–241. ISBN 9780857093462. [Google Scholar]
- Shahid, F.; Khan, A.A.; Saqib Hameed, M. Mechanical and Microstuctural Analysis of Dissimilar Metal Welds. IJRRAS 2015, 25, 6. [Google Scholar]
- Rodríguez, A.; Calleja, A.; López de Lacalle, L.; Pereira, O.; González, H.; Urbikain, G.; Laye, J. Burnishing of FSW Aluminum Al–Cu–Li Components. Metals 2019, 9, 260. [Google Scholar] [CrossRef]
- Choi, D.H.; Ahn, B.W.; Lee, C.Y.; Yeon, Y.M.; Song, K.; Jung, S.B. Formation of intermetallic compounds in Al and Mg alloy interface during friction stir spot welding. Intermetallics 2011, 19, 125–130. [Google Scholar] [CrossRef]
- Sato, Y.S.; Shiota, A.; Kokawa, H.; Okamoto, K.; Yang, Q.; Kim, C. Effect of interfacial microstructure on lap shear strength of friction stir spot weld of aluminium alloy to magnesium alloy. Sci. Technol. Weld. Join. 2010, 15, 319–324. [Google Scholar] [CrossRef]
- Kumar, D.S.; Sasanka, C.T.; Ravindra, K.; Suman, K.N.S. Magnesium and Its Alloys in Automotive Applications—A Review. Am. J. Mater. Sci. Technol. 2015, 4, 12–30. [Google Scholar] [CrossRef]
- Thakur, A.; Nandedkar, V. Optimization of the Resistance Spot Welding Process of Galvanized Steel Sheet Using the Taguchi Method. Arab. J. Sci. Eng. 2014, 39, 1171–1176. [Google Scholar] [CrossRef]
- Webber, T.; Lieb, T.; Mazumder, J. Welding Fundamentals and Processes. In ASM Handbook; ASM International: Materials Park, OH, USA, 2011; ISBN 9781615031337. [Google Scholar]
- Manladan, S.M.; Yusof, F.; Ramesh, S.; Fadzil, M.; Luo, Z.; Ao, S. A review on resistance spot welding of aluminum alloys. Int. J. Adv. Manuf. Technol. 2017, 90, 605–634. [Google Scholar] [CrossRef]
- Gullino, A.; Matteis, P.; D’Aiuto, F. Review of Aluminum-To-Steel Welding Technologies for Car-Body Applications. Metals 2019, 9, 315. [Google Scholar] [CrossRef]
- Sun, M.; Niknejad, S.T.; Gao, H.; Wu, L.; Zhou, Y. Mechanical properties of dissimilar resistance spot welds of aluminum to magnesium with Sn-coated steel interlayer. Mater. Des. 2016, 91, 331–339. [Google Scholar] [CrossRef]
- Cooke, K.O.; Khan, T.I. Resistance spot welding aluminium to magnesium using nanoparticle reinforced eutectic forming interlayers. Sci. Technol. Weld. Join. 2017, 23, 271–278. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Mostafa, A.O.; Medraj, M. Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data. J. Mater. 2014, 2014, 704283. [Google Scholar] [CrossRef]
- Zhou, Y.; Gorman, P.; Tan, T.; Ely, E.J. Weldability of thin sheet metals during small-scale resistance spot welding using an alternating-current power supply. J. Electron. Mater. 2000, 29, 1090–1099. [Google Scholar] [CrossRef]
- Chen, F.; Tong, G.Q.; Ma, Z.; Yue, X.K. The effects of welding parameters on the small scale resistance spot weldability of Ti-1Al-1Mn thin foils. Mater. Des. 2016, 102, 174–185. [Google Scholar] [CrossRef]
- Senkara, J.; Zhang, S.J.H. Expulsion Prediction in Resistance SW. Weld. J. 2004, 83, 123–132. [Google Scholar]
- McFadden, S.; Browne, D.J. A generalised version of an Ivantsov-based dendrite growth model incorporating a facility for solute measurement ahead of the tip. Comput. Mater. Sci. 2012, 55, 245–254. [Google Scholar] [CrossRef]
- Liang, P.; Su, H.-L.; Donnadieu, P.; Harmelin, M.G.; Quivy, A.; Ochin, P.; Effenberg, G.; Seifert, H.J.; Lukas, H.L.; Aldinger, F. Experimental investigation and thermodynamic calculation of the central part of the Mg-Al phase diagram. Zeitschrift Met. Res. Adv. Tech. 1998, 89, 536–540. [Google Scholar]
- Levi, C.G.; Abbaschian, G.J.; Mehrabian, R. Interface interactions during fabrication of aluminum alloy-alumina fiber composites. Metall. Trans. A 1978, 9, 697–711. [Google Scholar] [CrossRef]
- Kim, K.H. Formation of endogenous MgO and MgAl2O4 particles and their possibility of acting as substrate for heterogeneous nucleation of aluminum grains. Surf. Interface Anal. 2015, 47, 429–438. [Google Scholar] [CrossRef]
- Chatterjee, S.; Abinandanan, T.A.; Chattopadhyay, K. Microstructure development during dissimilar welding: Case of laser welding of Ti with Ni involving intermetallic phase formation. J. Mater. Sci. 2006, 41, 643–652. [Google Scholar] [CrossRef]
| Parameters | Welding Time (ms) | Clamping Force (N) | Welding Current (kVA) | |
|---|---|---|---|---|
| Experiment | ||||
| 1 | 100 | 800 | 6.3 | |
| 2 | 200 | 800 | 6.3 | |
| 3 | 300 | 800 | 6.3 | |
| 4 | 400 | 800 | 6.3 | |
| 5 | 300 | 600 | 6.3 | |
| 6 | 300 | 1000 | 6.3 | |
| 7 | 300 | 1200 | 6.3 | |
| Spectra | Al | Si | Mg | Zn | C | O |
|---|---|---|---|---|---|---|
| S1 | 42.11 | 0 | 57.89 | 0 | 0 | 0 |
| S2 | 28.09 | 0 | 47.65 | 0.29 | 13.53 | 10.44 |
| S3 | 23.84 | 0 | 50.15 | 0.22 | 11.05 | 14.73 |
| S3-1 | 17.60 | 0.34 | 26.98 | 0.28 | 22.43 | 29.43 |
| S3-2 | 24.96 | 0 | 41.97 | 0.28 | 9.33 | 19.30 |
| S3-3 | 23.58 | 0 | 48.85 | 0.32 | 11.82 | 13.98 |
| S3-4 | 23.84 | 0 | 50.15 | 0.22 | 11.05 | 14.73 |
| S3-5 | 23.16 | 0 | 41.09 | 0.24 | 12.59 | 20.53 |
| S4 | 29.68 | 0 | 45.75 | 0.24 | 11.79 | 11.56 |
| S4-1 | 19.23 | 0.21 | 36.18 | 0.32 | 18.96 | 22.23 |
| S4-2 | 9.39 | 0.16 | 20.64 | 0.17 | 48.46 | 19.39 |
| S5 | 18.23 | 0.27 | 33.24 | 0.20 | 17.48 | 28.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooke, K.O.; Khan, T.I. Microstructure Development during Low-Current Resistance Spot Welding of Aluminum to Magnesium. J. Manuf. Mater. Process. 2019, 3, 46. https://doi.org/10.3390/jmmp3020046
Cooke KO, Khan TI. Microstructure Development during Low-Current Resistance Spot Welding of Aluminum to Magnesium. Journal of Manufacturing and Materials Processing. 2019; 3(2):46. https://doi.org/10.3390/jmmp3020046
Chicago/Turabian StyleCooke, Kavian O., and Tahir I. Khan. 2019. "Microstructure Development during Low-Current Resistance Spot Welding of Aluminum to Magnesium" Journal of Manufacturing and Materials Processing 3, no. 2: 46. https://doi.org/10.3390/jmmp3020046
APA StyleCooke, K. O., & Khan, T. I. (2019). Microstructure Development during Low-Current Resistance Spot Welding of Aluminum to Magnesium. Journal of Manufacturing and Materials Processing, 3(2), 46. https://doi.org/10.3390/jmmp3020046






