Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reactive Extrusion of PTT-g-MA
2.3. Characterization of the PTT-g-MA
2.3.1. FTIR Spectroscopy
2.3.2. Rheological Characterization
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Verification of the effectiveness of the PTT-g-MA in the phase formation of the PTT/ABS blend
3. Results and Discussion
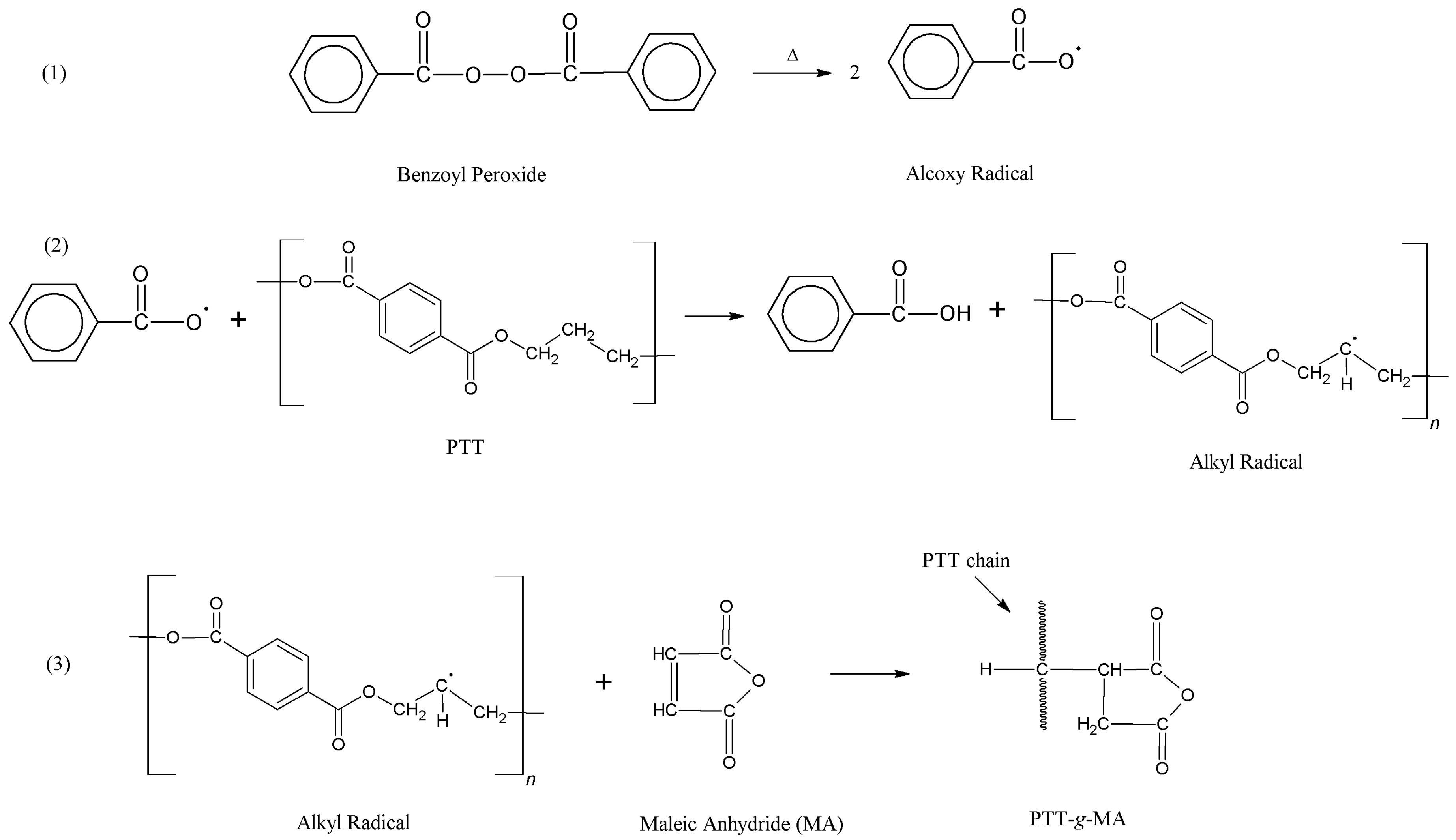
3.1. Grafting Mechanism Between PTT and MA
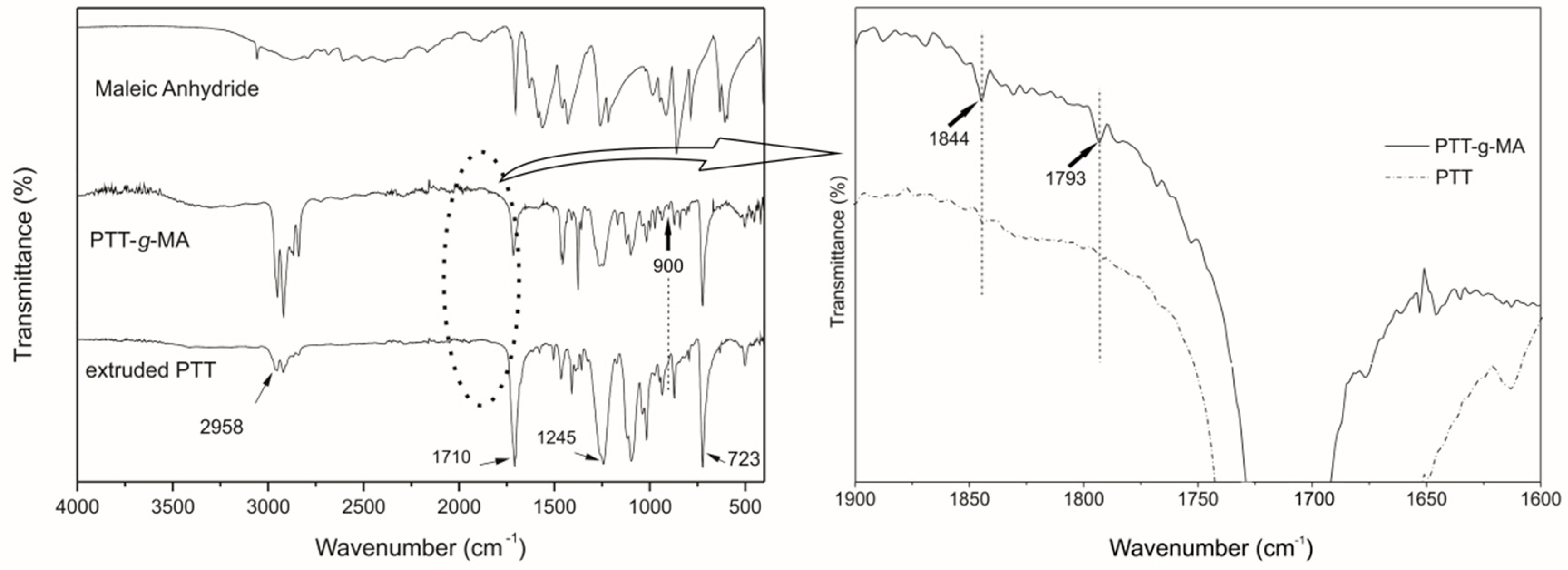
3.2. FTIR Spectroscopy
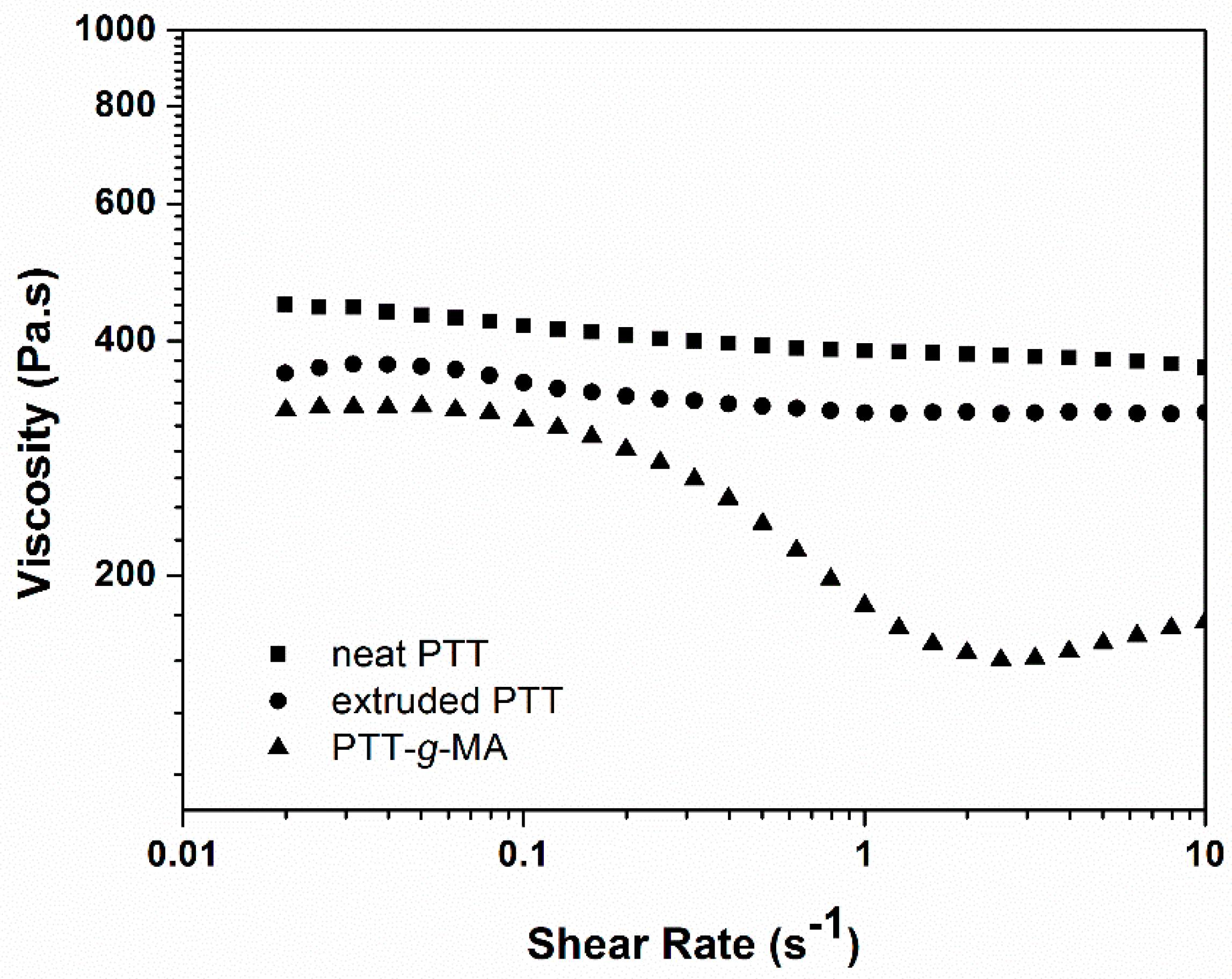
3.3. Rheological analysis
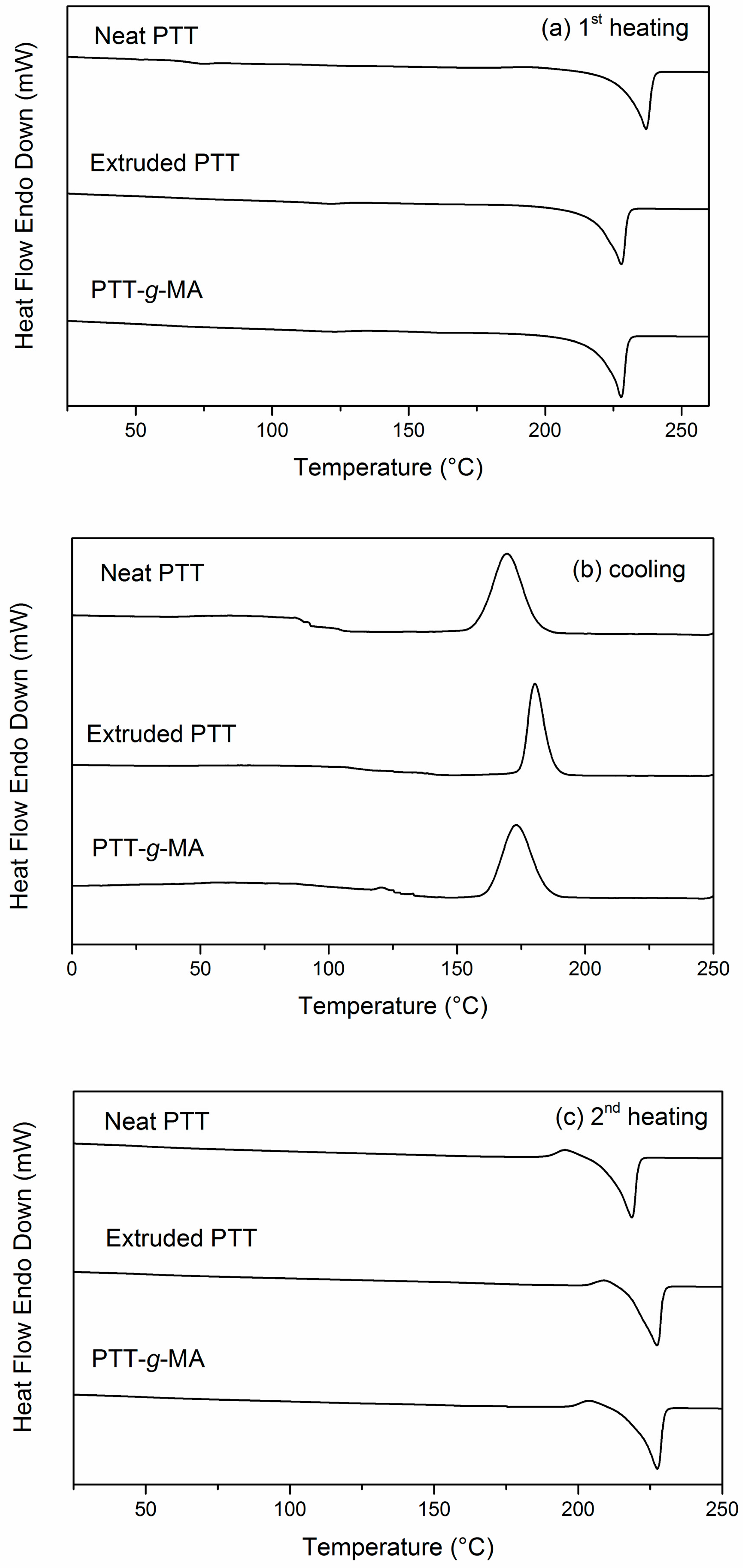
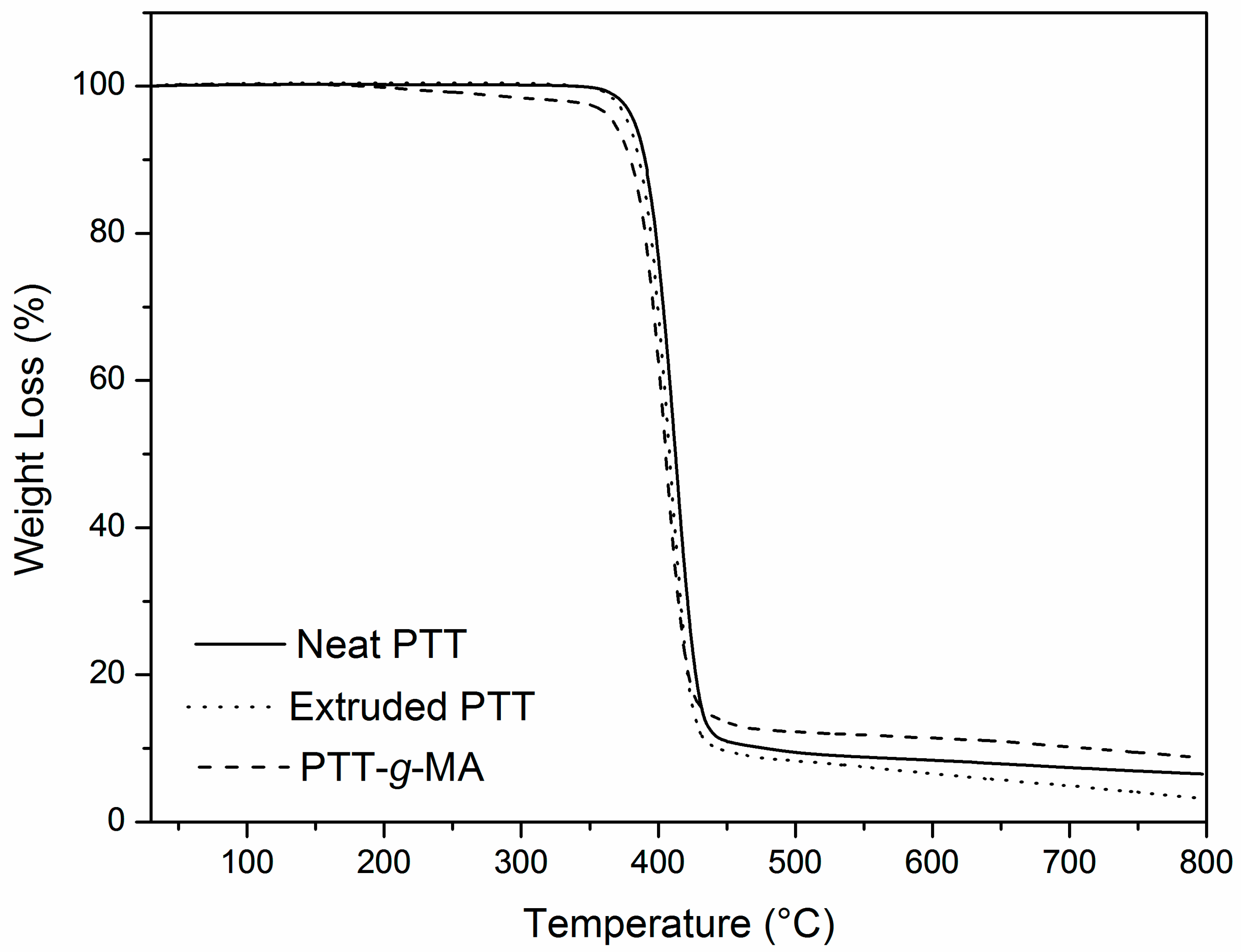
3.4. Thermal Analysis: DSC and TGA
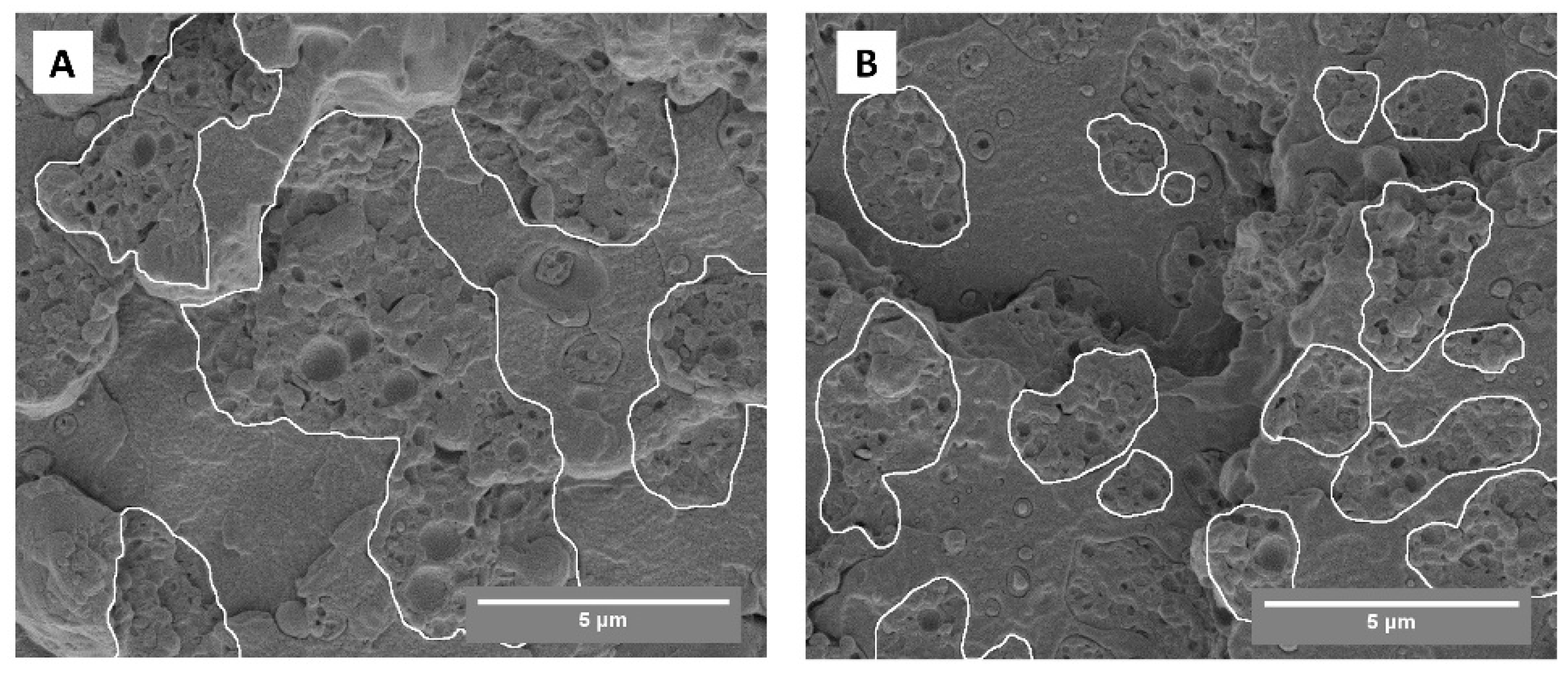
3.5. Effectiveness of PTT-g-MA as a Compatibilizer for PTT/ABS Blend
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montanheiro, T.L.D.A.; Passador, F.R.; Oliveira, M.P.D.; Durán, N.; Lemes, A.P. Preparation and Characterization of Maleic Anhydride Grafted Poly(Hydroxybutirate-co-Hydroxyvalerate) – PHBV-g-MA. Mater. Res. 2016, 19, 229–235. [Google Scholar] [CrossRef]
- Chen, C.C.; White, J.L. Compatibilizing Agents in Polymer Blends: Interfacial Tension, Phase Morphology, and Mechanical Properties. Polym. Eng. Sci. 1993, 33, 923–930. [Google Scholar] [CrossRef]
- Gaylord, N.G. Compatibilizing Agents: Structure and Function in Polyblends. J. Macromol. Sci. Part A - Chem. 1989, 26, 1211–1229. [Google Scholar] [CrossRef]
- Rzayev, Z.M.O. Graft Copolymers of Maleic Anhydride and Its Isostructural Analogues: High Performance Engineering Materials. Int. Rev. Chem. Eng. 2011, 3, 153–215. [Google Scholar]
- Al-Malaika, S.; Axtell, F.; Rothon, R.; Gilbert, M. Additives for Plastics. In Brydson’s Plastics Materials; Gilbert, M., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2016; pp. 127–168. [Google Scholar]
- Hezavehi, E.; Bigdeli, A.; Zolgharnein, P. Morphological, thermal and mechanical properties of nanostructured materials based on polypropylene/poly (trimethylene terephthalate) blended fibers and organoclay. Mater. Sci. Pol. 2012, 30, 82–91. [Google Scholar] [CrossRef]
- Passador, F.R.; Ruvolo-Filho, A.C.; Pessan, L.A. Effects of different compatibilizers on the rheological, thermomechanical, and morphological properties of HDPE/LLDPE blend-based nanocomposites. J. Appl. Polym. Sci. 2013, 130, 1726–1735. [Google Scholar] [CrossRef]
- Xue, M.L.; Yu, Y.L.; Chuah, H.H. Reactive compatibilization of poly(trimethylene terephthalate)/polypropylene blends by polypropylene-graft-maleic anhydride. Part 2. Crystallization behavior. J. Macromol. Sci. Part B Phys. 2007, 46, 603–615. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Wu, Q.; Song, L.; Wu, G. Thermal analysis of the melting process of poly(trimethylene terephthalate) using FTIR micro-spectroscopy. Eur. Polym. J. 2005, 41, 2216–2223. [Google Scholar] [CrossRef]
- Huang, J. Polymer Blends of Poly (trimethylene terephthalate) and Polystyrene Compatibilized by Styrene-Glycidyl Methacrylate Copolymers. J. Appl. Polym. Sci. 2003, 88, 2247. [Google Scholar] [CrossRef]
- Wang, D.; Sun, G. Novel Polymer Blends from Polyester and Bio-Based Cellulose Ester. J. Appl. Polym. Sci. 2011, 119, 2302–2309. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Characterization and antistatic behavior of SiO2-functionalized multiwalled carbon nanotube/poly(trimethylene terephthalate) composites. J. Polym. Res. 2013, 20, 253. [Google Scholar] [CrossRef]
- Szymczyk, A.; Paszkiewicz, S.; Roslaniec, Z. Influence of intercalated organoclay on the phase structure and physical properties of PTT-PTMO block copolymers. Polym. Bull. 2013, 70, 1575–1590. [Google Scholar] [CrossRef]
- Tolstoy, V.P.; Chernyshova, I.V.; Skryshevsky, V.A. Handbook of Infrared Spectroscopy of Ultrathim Films; John Wiley and Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Yamen, M.; Ozkaya, S.; Vasanthan, N. Structural and Conformational Changes During Thermally-Induced Crystallization of Poly(trimethylene terephthalate) by Infrared Spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1497–1504. [Google Scholar] [CrossRef]
- Farahani, M.F.; Jafari, S.H.; Khonakdar, H.A.; Yavari, A.; Melati, A. FTIR and Thermal Analyses of Intermolecular Interactions in Poly(trimethylene terephthalate)/Phenoxy Blends. Polym. Eng. Sci. 2011, 51, 518–525. [Google Scholar] [CrossRef]
- Al-omairi, L.M. Crystallization, Mechanical, Rheological and Degradation Behavior of Polytrimethylene Terephthalate, Polybutylene Terephthalate and Polycarbonate Blend. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2010. [Google Scholar]
- Bai, Y.; Li, N.; Liu, Y.; Run, M. Morphology and Mechanical Properties of Poly(trimethylene terephthalate)/Maleinized Acrylonitrile-Butadiene-Styrene Blends. Asian J. Chem. 2015, 27, 2548–2554. [Google Scholar] [CrossRef]
- Smith, A.L. Applied Infrared Spectroscopy: Fundamentals, Techniques, and Analytical Problem-Solving; Smith, A.L., Ed.; John Wiley & Sons: Chichester, UK, 1979. [Google Scholar]
- Sarathchandran, C.; Chan, C.H.; Abdul Karim, S.R.; Thomas, S. Poly(trimethylene terephthalate) – The New Generation of Engineering Thermoplastic Polyester. In Physical Chemistry of Macromolecules: Macro to Nanoscales; Chan, C.H., Chia, C.H., Thomas, S., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2014; pp. 573–618. [Google Scholar]
- Pyda, M.; Boller, A.; Grebowicz, J.; Chuah, H.; Lebedev, B.V.; Wunderlich, B. Heat Capacity of Poly (trimethylene terephthalate). J. Polym. Sci. Part B Polym. Phys. 1998, 36, 2499–2511. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Cristóvan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Boztuǧ, A.; Basan, S. The modification and characterization of maleic anhydride-styrene-methyl metacrylate terpolymer by poly(ethylene adipate). J. Mol. Struct. 2007, 830, 126–130. [Google Scholar] [CrossRef]
- Zhang, J. Study of Poly (trimethylene terephthalate) as an Engineering Thermoplastics Material. J. Appl. Polym. Sci. 2004, 91, 1657–1666. [Google Scholar] [CrossRef]
| First Heating | ||||
| Sample | Tg (°C) | Tm1 (°C) | ΔHm1 (J/g) | Xc1 (%) |
| Neat PTT | 67.2 | 227 | 64.3 | 44.1 |
| Extruded PTT | - | 227 | 55.4 | 37.9 |
| PTT-g-MA | - | 227 | 59.5 | 42.4 |
| Cooling | ||||
| Sample | Tc (°C) | |||
| Neat PTT | 170 | |||
| Extruded PTT | 180 | |||
| PTT-g-MA | 173 | |||
| Second Heating | ||||
| Sample | Tm2 (°C) | ΔHm2 (J/g) | Xc2 (%) | |
| Neat PTT | 228 | 64.0 | 43.8 | |
| Extruded PTT | 227 | 60.6 | 41.5 | |
| PTT-g-MA | 227 | 64.2 | 45.8 | |
| Sample | Tonset (°C) | T10% (°C) | T50% (°C) | Tmax (°C) |
|---|---|---|---|---|
| Extruded PTT | 387 | 385 | 409 | 407 |
| Neat PTT | 388 | 389 | 411 | 408 |
| PTT-g-MA | 378 | 379 | 405 | 405 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, N.F.; Zaggo, H.M.; Montanheiro, T.L.A.; Passador, F.R. Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing. J. Manuf. Mater. Process. 2019, 3, 37. https://doi.org/10.3390/jmmp3020037
Braga NF, Zaggo HM, Montanheiro TLA, Passador FR. Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing. Journal of Manufacturing and Materials Processing. 2019; 3(2):37. https://doi.org/10.3390/jmmp3020037
Chicago/Turabian StyleBraga, Natália F., Henrique M. Zaggo, Thaís L. A. Montanheiro, and Fabio R. Passador. 2019. "Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing" Journal of Manufacturing and Materials Processing 3, no. 2: 37. https://doi.org/10.3390/jmmp3020037
APA StyleBraga, N. F., Zaggo, H. M., Montanheiro, T. L. A., & Passador, F. R. (2019). Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing. Journal of Manufacturing and Materials Processing, 3(2), 37. https://doi.org/10.3390/jmmp3020037





