Predicting Nitrogen Flavanol Index (NFI) in Mentha arvensis Using UAV Imaging and Machine Learning Techniques for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. UAV Data Acquisition
2.3. UAV Data Processing
2.4. Calculation of Vegetation Indices
2.5. Ground Truth Data Collection
2.6. Machine Learning Model Development
2.6.1. Support Vector Regression
2.6.2. Random Forest Regression
2.6.3. Gradient Boosting Regression
2.6.4. Selection of Predictor Variables
2.6.5. Model Performance
3. Results
3.1. Variation in NFI Content During Different Growth Stage
3.2. Feature Selection
3.3. Model Evaluation
4. Discussion
4.1. Feature Selection and Vegetation Indices’ Contribution Across Growth Stages
4.2. Model Performance and Variability Across Growth Stages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UAV | Unmanned Aerial Vehicle |
| NFI | Nitrogen Flavanol Index |
| DAP | Days After Planting |
| VI | Vegetation Index |
| VIs | Vegetation Indices |
| RGB | Red–Green–Blue |
| DLS | Downwelling Light Sensor |
| SfM | Structure-from-Motion |
| GIS | Geographic Information System |
| RFE | Recursive Feature Elimination |
| SVR | Support Vector Regression |
| RF | Random Forest |
| GBR | Gradient Boosting Regression |
| SR | Simple Ratio |
| NDVI | Normalized Difference Vegetation Index |
| RDVI | Renormalized Difference Vegetation Index |
| ARVI | Atmospherically Resistant Vegetation Index |
| MSR1 | Modified Simple Ratio 1 |
| MSR2 | Modified Simple Ratio 2 |
| DVI | Difference Vegetation Index |
| SAVI | Soil Adjusted Vegetation Index |
| OSAVI | Optimized Soil-Adjusted Vegetation Index |
| MSAVI | Modified Soil-Adjusted Vegetation Index |
| SARVI | Soil and Atmosphere Resistant Vegetation Index |
| EVI | Enhanced Vegetation Index |
| NDRE | Normalized Difference Red Edge Index |
| RRI1 | Red Edge Ratio Index 1 |
| RRI2 | Red Edge Ratio Index 2 |
| MCARI | Modified Chlorophyll Absorption Ratio Index |
| MCARI1 | Modified Chlorophyll Absorption Ratio Index 1 |
| MCARI2 | Modified Chlorophyll Absorption Ratio Index 2 |
| MTVI | Modified Triangular Vegetation Index |
| Datt Index | Datt Index |
| aDVI | Adjusted Difference Vegetation Index |
| GNDVI | Green Normalized Difference Vegetation Index |
| PSSRc | Pigment-Specific Simple Ratio for Carotenoids |
| RARSa | Ratio Analysis of Reflectance Spectra for Chlorophyll a |
| SIPI | Structure-Insensitive Pigment Index |
References
- Pinter, P.J., Jr.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.T.; Upchurch, D.R. Remote Sensing for Crop Management. Photogramm. Eng. Remote Sens. 2003, 69, 647–664. [Google Scholar] [CrossRef]
- Novoa, R.; Loomis, R.S. Nitrogen and Plant Production. Plant Soil. 1981, 58, 177–204. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and Nitrogen Relationships in Leaves of C3 Plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Watson, M.E.; Galliher, T.L. Comparison of Dumas and Kjeldahl Methods with Automatic Analyzers on Agricultural Samples under Routine Rapid Analysis Conditions. Commun. Soil. Sci. Plant Anal. 2001, 32, 2007–2019. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf Nitrogen Determination Using Non-Destructive Techniques—A Review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Bista, M.K.; Adhikari, B.; Sankarapillai, L.V.; Pieralisi, B.; Reddy, K.R.; Jenkins, J.; Bheemanahalli, R. Drought and Heat Stress Induce Differential Physiological and Agronomic Trait Responses in Cotton. Ind. Crops Prod. 2024, 222, 119540. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A New Instrument for Field Measurements of Epidermal Ultraviolet Absorbance by Chlorophyll Fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G. Optically Assessed Contents of Leaf Polyphenolics and Chlorophyll as Indicators of Nitrogen Deficiency in Wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A New Optical Leaf-clip Meter for Simultaneous Non-destructive Assessment of Leaf Chlorophyll and Epidermal Flavonoids. Physiol. Plant 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Moigne, M. Le Nondestructive Diagnostic Test for Nitrogen Nutrition of Grapevine (Vitis vinifera L.) Based on Dualex Leaf-Clip Measurements in the Field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Fan, K.; Li, F.; Chen, X.; Li, Z.; Mulla, D.J. Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning. Remote Sens. 2022, 14, 3504. [Google Scholar] [CrossRef]
- Khan, M.S.; Yadav, P.; Semwal, M.; Prasad, N.; Verma, R.K.; Kumar, D. Predicting Canopy Chlorophyll Concentration in Citronella Crop Using Machine Learning Algorithms and Spectral Vegetation Indices Derived from UAV Multispectral Imagery. Ind. Crops Prod. 2024, 219, 119147. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-Based Chlorophyll Content Estimation by Evaluating Vegetation Index Responses under Different Crop Coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance Measurement of Canopy Biomass and Nitrogen Status in Wheat Crops Using Normalized Difference Vegetation Indices and Partial Least Squares Regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Wiegand, C.L.; Richardson, A.J.; Escobar, D.E.; Gerbermann, A.H. Vegetation Indices in Crop Assessments. Remote Sens. Environ. 1991, 35, 105–119. [Google Scholar] [CrossRef]
- Chlingaryan, A.; Sukkarieh, S.; Whelan, B. Machine Learning Approaches for Crop Yield Prediction and Nitrogen Status Estimation in Precision Agriculture: A Review. Comput. Electron. Agric. 2018, 151, 61–69. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.; Dong, R.; Zha, H.; Xia, T.; Chen, Z.; Kusnierek, K.; Mi, G.; Sun, H.; Li, M. Machine Learning-Based in-Season Nitrogen Status Diagnosis and Side-Dress Nitrogen Recommendation for Corn. Eur. J. Agron. 2021, 123, 126193. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L.; Zarco-Tejada, P. Airborne Hyperspectral Images and Ground-Level Optical Sensors as Assessment Tools for Maize Nitrogen Fertilization. Remote Sens. 2014, 6, 2940–2962. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Qian, X.; Zhu, Y.; Shen, G. Quantification of Rice Canopy Nitrogen Balance Index with Digital Imagery from Unmanned Aerial Vehicle. Remote Sens. Lett. 2015, 6, 183–189. [Google Scholar] [CrossRef]
- Kumar, R.; Murthy, P.V.S.; Kumar, A. Unmanned Aerial Vehicle-Based Multispectral Remote Sensing for Commercially Important Aromatic Crops in India for Its Efficient Monitoring and Management. J. Indian Soc. Remote Sens. 2021, 50, 397–407. [Google Scholar]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote Mapping of Standing Crop Biomass for Estimation of the Productivity of the Shortgrass Prairie, Pawnee National Grasslands, Colorado; CABI: Wallingford, UK, 1972. [Google Scholar]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with Erts. In Third Earth Resources Technology Satellite-1 Symposium. Volume I: Technical Presentations; Freden, S.C., Mercanti, E.P., Becker, M.A., Eds.; NASA SP-351; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 1973; pp. 309–317. [Google Scholar]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanre, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus Hippocastanum L. and Acer Platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Ehammer, A.; Fritsch, S.; Conrad, C.; Lamers, J.; Dech, S. Statistical Derivation of FPAR and LAI for Irrigated Cotton and Rice in Arid Uzbekistan by Combining Multi-Temporal RapidEye Data and Ground Measurements. In Proceedings of the SPIE Remote Sensing, Toulouse, France, 20–23 September 2010; Volume 7824, p. 782409. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral Vegetation Indices and Novel Algorithms for Predicting Green LAI of Crop Canopies: Modeling and Validation in the Context of Precision Agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Datt, B. Visible/near Infrared Reflectance and Chlorophyll Content in Eucalyptus Leaves. Int. J. Remote Sens. 1999, 20, 2741–2759. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote Estimation of Chlorophyll Content in Higher Plant Leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying Chlorophylls and Caroteniods at Leaf and Canopy Scales: An Evaluation of Some Hyperspectral Approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio Analysis of Reflectance Spectra (RARS): An Algorithm for the Remote Estimation of the Concentrations of Chlorophyll A, Chlorophyll B, and Carotenoids in Soybean Leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Penuelas, J.; Frederic, B.; Filella, I. Semi-Empirical Indices to Assess Carotenoids/Chlorophyll-a Ratio from Leaf Spectral Reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Rehman, T.H.; Borja Reis, A.F.; Akbar, N.; Linquist, B.A. Use of Normalized Difference Vegetation Index to Assess N Status and Predict Grain Yield in Rice. Agron. J. 2019, 111, 2889–2898. [Google Scholar] [CrossRef]
- Boiarskii, B.; Hasegawa, H. Comparison of NDVI and NDRE Indices to Detect Differences in Vegetation and Chlorophyll Content. J. Mech. Contin. Math. Sci. 2019, 4, 20–29. [Google Scholar] [CrossRef]
- Gianquinto, G.; Goffart, J.P.; Olivier, M.; Guarda, G.; Colauzzi, M.; Dalla Costa, L.; Delle Vedove, G.; Vos, J.; Mackerron, D.K.L. The Use of Hand-Held Chlorophyll Meters as a Tool to Assess the Nitrogen Status and to Guide Nitrogen Fertilization of Potato Crop. Potato Res. 2004, 47, 35–80. [Google Scholar] [CrossRef]
- Chiu, M.S.; Wang, J. Evaluation of Machine Learning Regression Techniques for Estimating Winter Wheat Biomass Using Biophysical, Biochemical, and UAV Multispectral Data. Drones 2024, 8, 287. [Google Scholar] [CrossRef]
- Lu, J.; Dai, E.; Miao, Y.; Kusnierek, K. Developing a New Active Canopy Sensor- and Machine Learning-Based in-Season Rice Nitrogen Status Diagnosis and Recommendation Strategy. Field Crops Res. 2024, 317, 109540. [Google Scholar] [CrossRef]
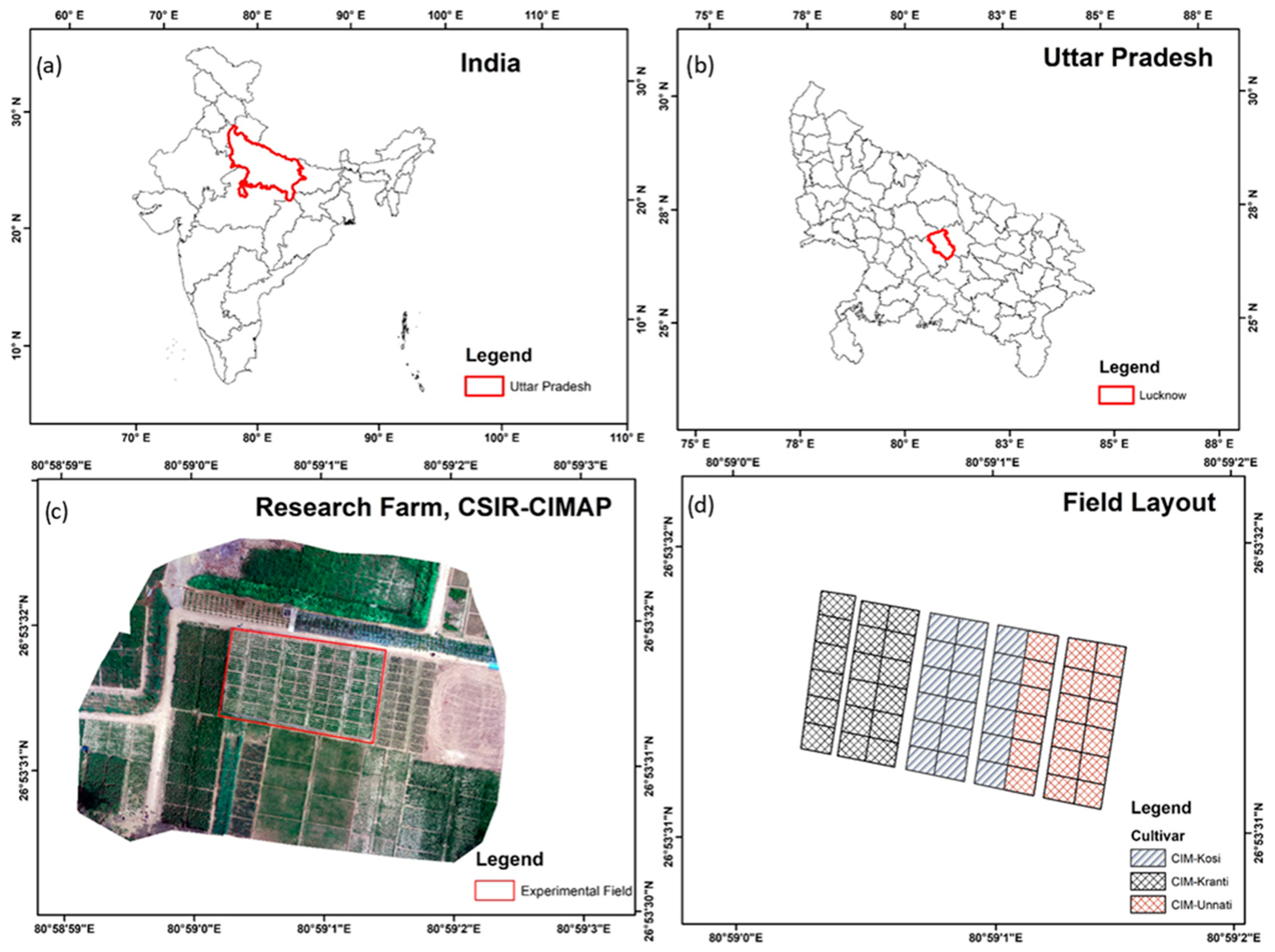
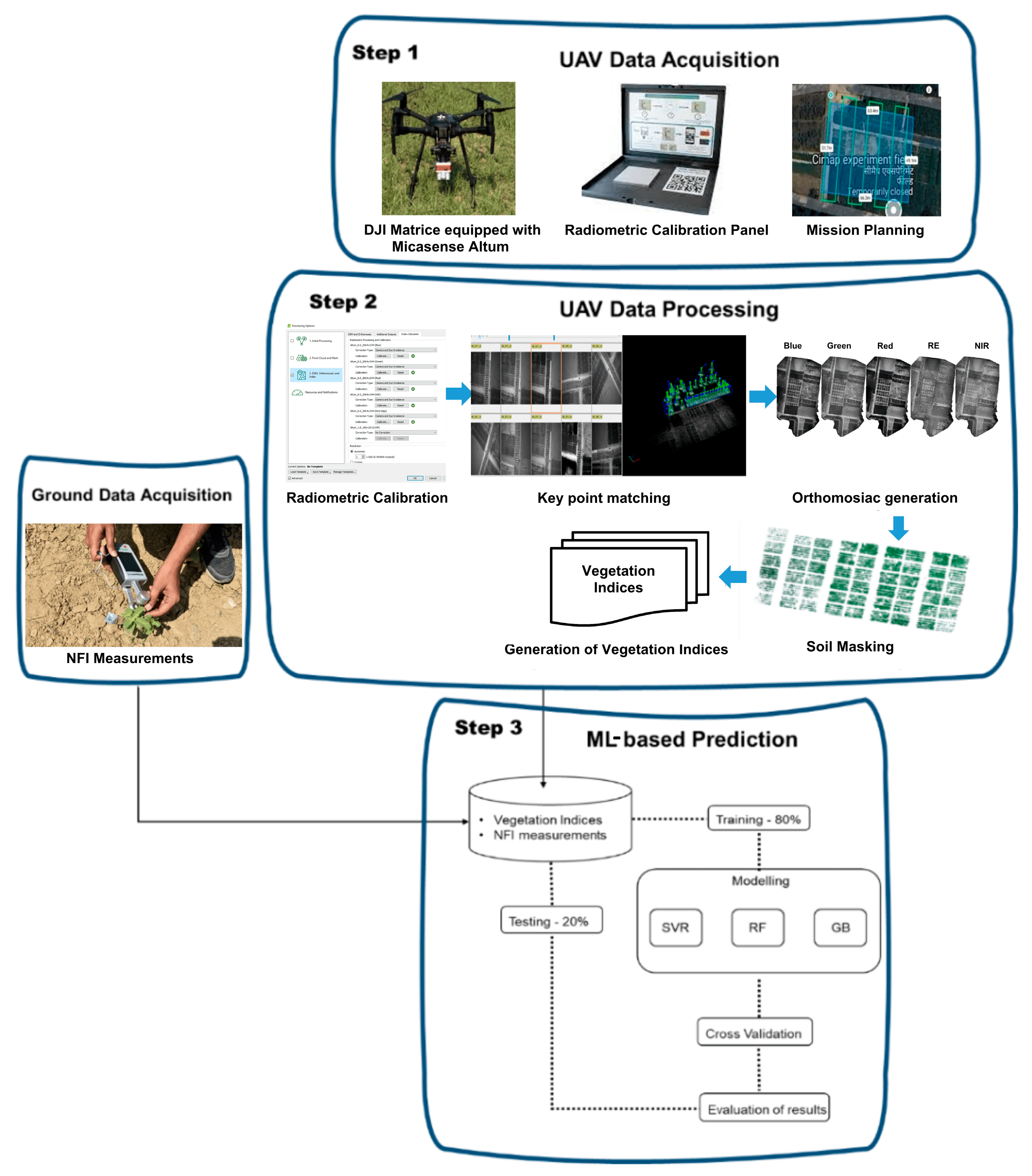
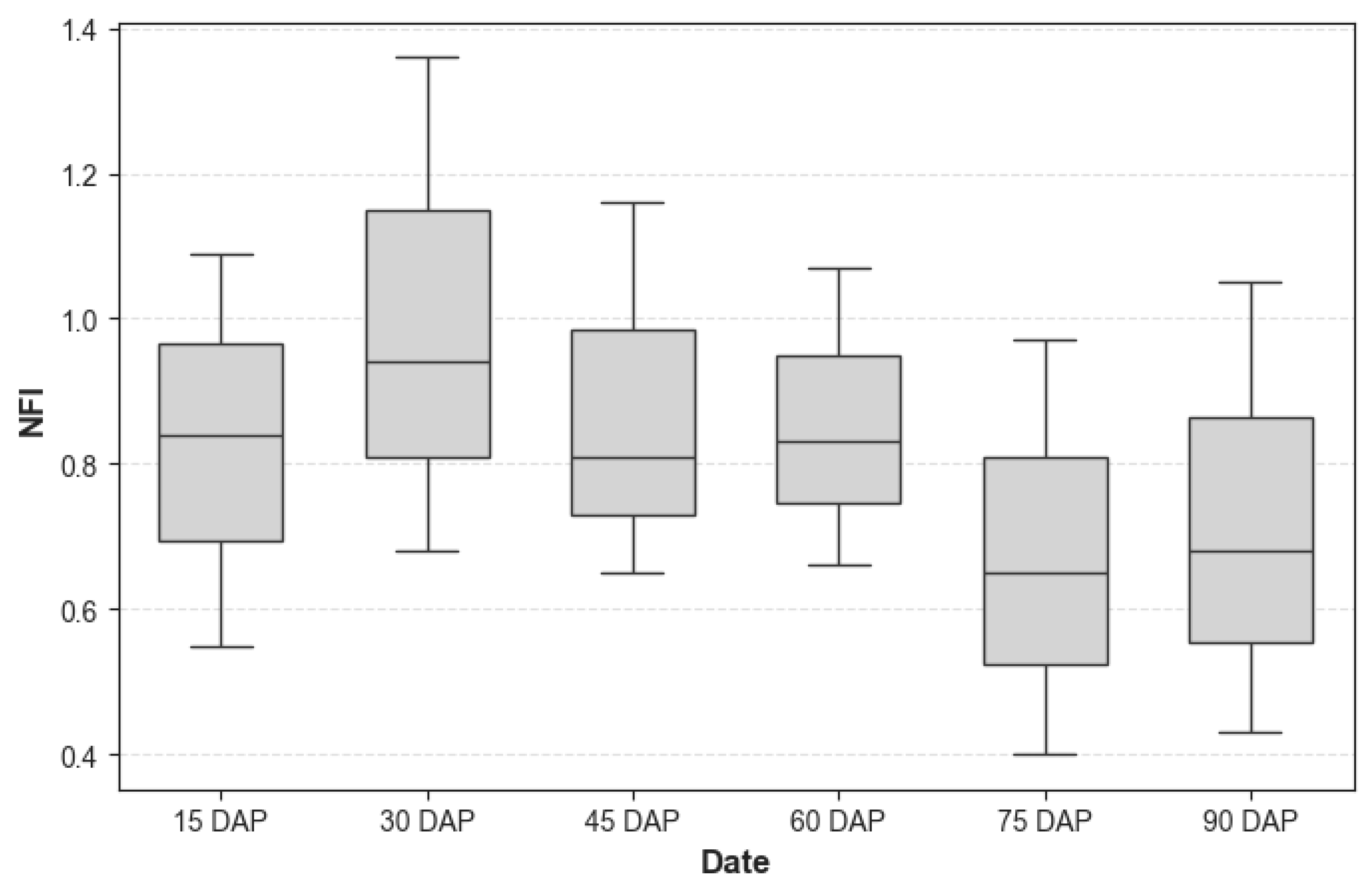
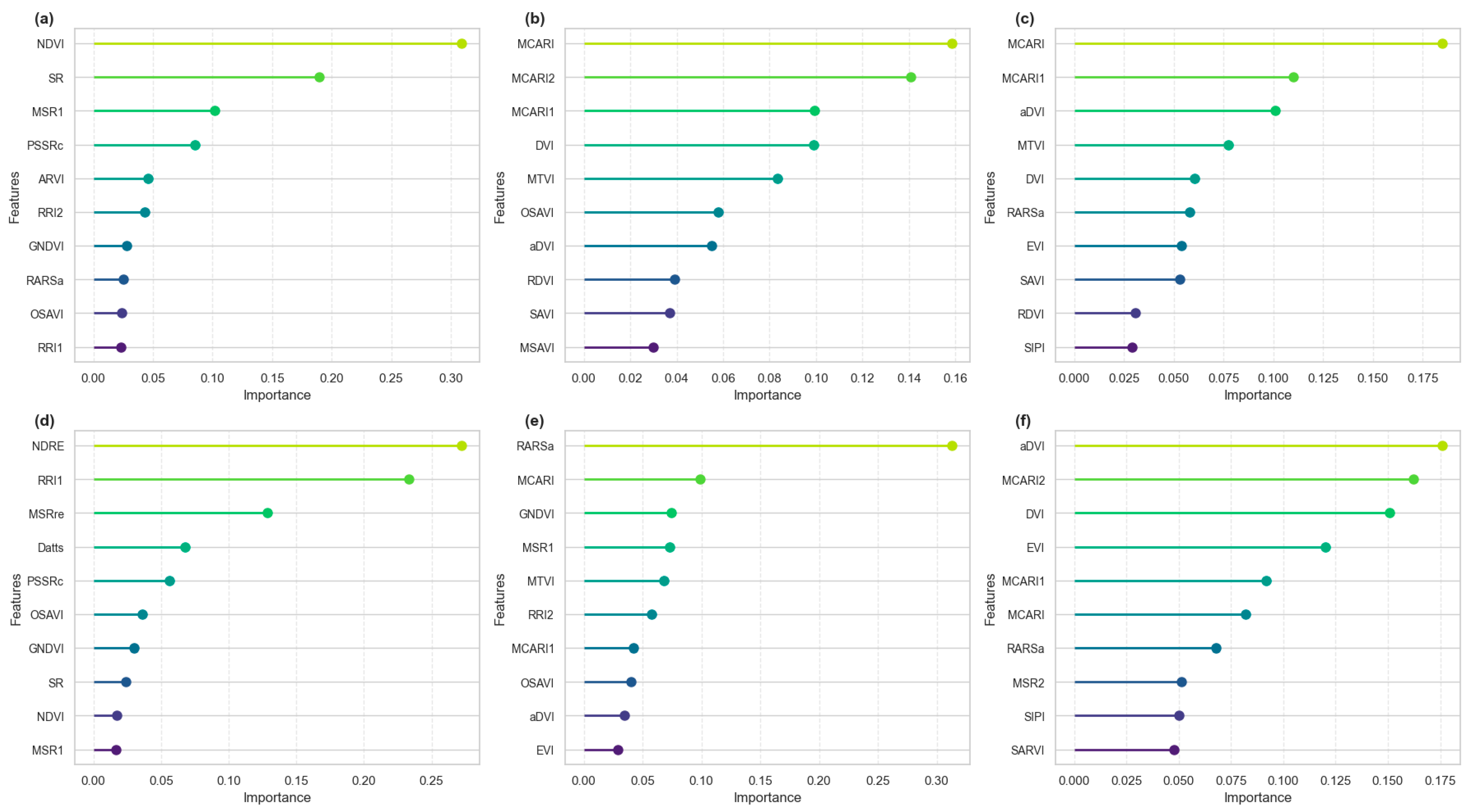

| Date | Day After Planting |
|---|---|
| 2 April 2022 | 15 |
| 17 April 2022 | 30 |
| 2 May 2022 | 45 |
| 17 May 2022 | 60 |
| 1 June 2022 | 75 |
| 16 June 2022 | 90 |
| Index Name | Formula | Reference |
|---|---|---|
| SR | NIR/RED | [21,22] |
| NDVI | (NIR − RED)/(NIR + RED) | [23] |
| RDVI | (NIR − RED)/√(NIR + RED) | [24] |
| ARVI | (NIR − [RED − γ (BLUE − RED)])/(NIR + [RED − γ (BLUE − RED)]), γ = 1 | [25] |
| MSR1 | ((NIR/RED) − 1)/√((NIR/RED) + 1) | [26] |
| MSR2 | (NIR − BLUE)/(RED − BLUE) | [27] |
| DVI | NIR − RED | [21] |
| SAVI | ((1 + L)(NIR − RED))/(NIR + RED + L), L = 0.5 | [28] |
| OSAVI | (1 + 0.16)(NIR − RED)/(NIR + RED + 0.16) | [29] |
| MSAVI | 0.5 × [2 × NIR + 1 − √((2 × NIR + 1)2 − 8 × (NIR − RED))] | [30] |
| SARVI | (1 + L) × (NIR − (RED − (BLUE − RED)))/(NIR + (RED − (BLUE − RED)) + L), L = 0.5 | [25] |
| EVI | 2.5 × (NIR − RED)/(NIR + 6 × RED − 7.5 × BLUE + 1) | [28] |
| NDRE | (NIR − RE)/(NIR + RE) | [31] |
| RRI1 | NIR/RE | [32] |
| RRI2 | RE/RED | [32] |
| MCARI | [(RE − RED) − 0.2 × (RE − GREEN)] × (RE/RED) | [33] |
| MCARI1 | 1.2 × [2.5 × (NIR − RED) − 1.3 × (NIR − GREEN)] | [34] |
| MCARI2 | [1.5 × (2.5 × (NIR − RED) − 1.3 × (NIR − GREEN))]/√((2 × NIR + 1)2 − (6 × NIR − 5 × √RED) − 0.5) | [34] |
| MTVI | 1.2 × [1.2 × (NIR − GREEN) − 2.5 × (RED − GREEN)] | [34] |
| Datt Index | (NIR − RE)/(NIR − RED) | [35] |
| aDVI | NIR − ((GREEN + RED)/2) | [36] |
| GNDVI | (NIR − GREEN)/(NIR + GREEN) | [37] |
| PSSRc | NIR/BLUE | [38] |
| RARSa | RED/RE | [38,39] |
| SIPI | (NIR − BLUE)/(NIR − RED) | [40] |
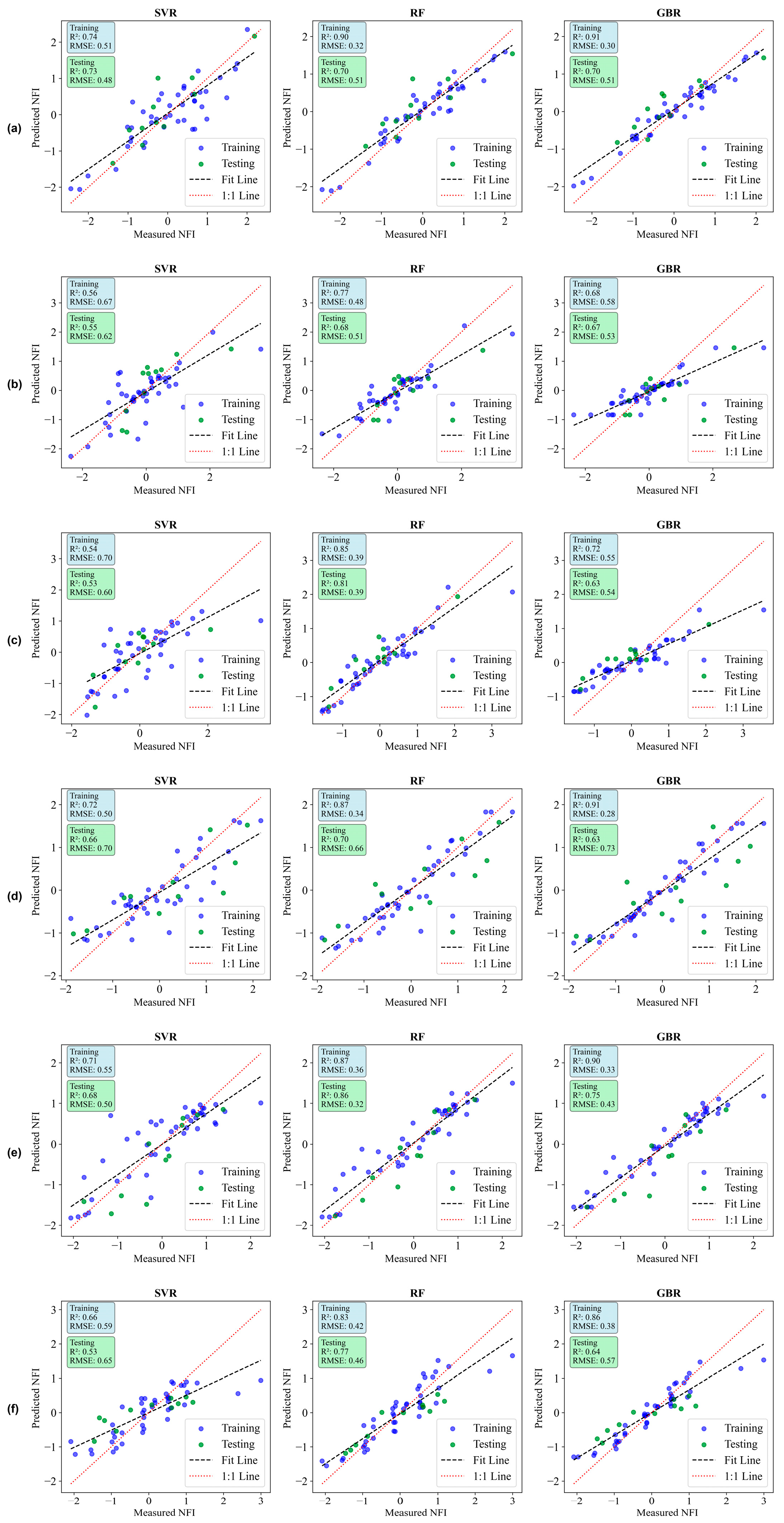
| Growth Stage | Model | Train R2 | Test R2 | Train RMSE | Test RMSE |
|---|---|---|---|---|---|
| 15 DAP | SVR | 0.74 | 0.73 | 0.51 | 0.48 |
| RF | 0.90 | 0.70 | 0.32 | 0.51 | |
| GB | 0.91 | 0.70 | 0.30 | 0.51 | |
| 30 DAP | SVR | 0.56 | 0.55 | 0.67 | 0.62 |
| RF | 0.77 | 0.68 | 0.48 | 0.51 | |
| GB | 0.68 | 0.67 | 0.58 | 0.53 | |
| 45 DAP | SVR | 0.54 | 0.53 | 0.70 | 0.60 |
| RF | 0.85 | 0.81 | 0.39 | 0.39 | |
| GB | 0.72 | 0.63 | 0.55 | 0.54 | |
| 60 DAP | SVR | 0.72 | 0.66 | 0.50 | 0.70 |
| RF | 0.87 | 0.70 | 0.34 | 0.66 | |
| GB | 0.91 | 0.63 | 0.28 | 0.73 | |
| 75 DAP | SVR | 0.71 | 0.68 | 0.55 | 0.50 |
| RF | 0.87 | 0.86 | 0.36 | 0.32 | |
| GB | 0.90 | 0.75 | 0.33 | 0.43 | |
| 90 DAP | SVR | 0.66 | 0.53 | 0.59 | 0.65 |
| RF | 0.83 | 0.77 | 0.42 | 0.46 | |
| GB | 0.86 | 0.64 | 0.38 | 0.57 | |
| Whole growing period | SVR | 0.71 | 0.61 | 0.08 | 0.11 |
| RF | 0.91 | 0.70 | 0.04 | 0.09 | |
| GB | 0.89 | 0.67 | 0.05 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulati, B.; Zubair, Z.; Sinha, A.; Sinha, N.; Prasad, N.; Semwal, M. Predicting Nitrogen Flavanol Index (NFI) in Mentha arvensis Using UAV Imaging and Machine Learning Techniques for Sustainable Agriculture. Drones 2025, 9, 483. https://doi.org/10.3390/drones9070483
Gulati B, Zubair Z, Sinha A, Sinha N, Prasad N, Semwal M. Predicting Nitrogen Flavanol Index (NFI) in Mentha arvensis Using UAV Imaging and Machine Learning Techniques for Sustainable Agriculture. Drones. 2025; 9(7):483. https://doi.org/10.3390/drones9070483
Chicago/Turabian StyleGulati, Bhavneet, Zainab Zubair, Ankita Sinha, Nikita Sinha, Nupoor Prasad, and Manoj Semwal. 2025. "Predicting Nitrogen Flavanol Index (NFI) in Mentha arvensis Using UAV Imaging and Machine Learning Techniques for Sustainable Agriculture" Drones 9, no. 7: 483. https://doi.org/10.3390/drones9070483
APA StyleGulati, B., Zubair, Z., Sinha, A., Sinha, N., Prasad, N., & Semwal, M. (2025). Predicting Nitrogen Flavanol Index (NFI) in Mentha arvensis Using UAV Imaging and Machine Learning Techniques for Sustainable Agriculture. Drones, 9(7), 483. https://doi.org/10.3390/drones9070483





