Abstract
Off-target drift from aerial pesticide applications in croplands can be a major source of pesticide exposure to pollinators. Pesticide adjuvants (PAs) are added to pesticides but can be as toxic as pesticides’ active ingredients. Ongoing experiments have identified sodium alginate (SA) as a drift-reducing PA less toxic to honeybees. Hence, SA and fenugreek polymer (FP) have been tested as drift-reducing PAs for aerial applications using the Remotely Piloted Aerial Application System (RPAAS). Two spray experiments were carried out in the field: (i) water only (W) and (ii) water and adjuvant (WA). Droplet spectrum and on-target coverage were collected using a VisiSize P15 image analyzer and kromekote cards, respectively. The drift reduction potentials (DRPs) of the adjuvants were analyzed based on droplet size (diameters of 10%, 50%, and 90% volume) and the proportion of driftable volume with droplets < 200 µm. Compared to the W only, the W-A treatment produced larger droplets, suggesting the presence of DRP. There were 14.5%, 8.3% to 14.4%, and 2.3% to 7.7% driftable fines in the W, WA (SA), and WA (FP) treatments, respectively. The FP treatment improved the on-target coverage (3.0% to 3.1%) compared to water (2.7%). Our results indicate that SA and FP have the potential to mitigate off-target drift and protect pollinator health.
1. Introduction
Profitable commercial crop production often involves the use of pesticides to maintain pest densities below economic thresholds. Most pesticide applications in the lower Mississippi Delta (LMD) are carried out by piloted agricultural aircraft. The drone-based aerial application of pesticides to large agricultural areas, such as LMD, has not been widely practiced due to the newness of the technology. However, to address existing concerns, such as off-target drift [1,2,3], aerial applicator fatalities with conventional manned agricultural aircraft [4,5], and high costs of repair and maintenance, drone-based aerial pesticide applications can be a valuable alternative.
Like piloted aerial applications, the distance between the nozzle and the target remains high in the RPAAS, which favors conditions for off-target drift. In addition, the high concentration of pesticide spray because of low volume applications (because of small tank size and limited battery capacity in most cases) affects the spreading and volatilization of the pesticide. Although most of the pesticide labels have recommendations on doses required to improve spray characteristics, they are tailored for ground-based and piloted large-scale aerial applications only. Therefore, research is needed to develop pesticide adjuvants specific to the RPAAS platform to mitigate drift and improve pesticide efficacy.
Adjuvants have been used in conventional aerial pesticide applications for many years, and in drone-based aerial applications, in recent years [6,7,8]. Several adjuvants added to pesticides to improve the efficacy, adhesion, and incorporation into plant tissues and reduce off-target drift may be equally or more toxic than the active ingredient [9,10,11]. Commercially available adjuvants such as polyacrylamides, polyvinyl chlorides, sodium salts of fatty acids, alkyl phenyl ethoxylates, and other petroleum-based distillates are also not environmentally friendly [12]. The most-used adjuvants in drone-based aerial applications include vegetable oils, organosilicon, and high-molecular polymers, which mostly act to decrease the surface tension and therefore increase the wetting, spreading, and retention of spray droplets on leaves [6,8]. These adjuvants, especially the petroleum-derived ones, are environmentally harmful and could be toxic to pollinators. This calls for the development of pollinator and environmentally friendly adjuvants with better efficacies.
The dependence of crop production on agrochemicals creates a need to improve our understanding of how these practices affect our ecosystem, notably the detrimental effects on pollinating insect populations. The impacts of pesticides on pollinator insects such as honeybees were well documented [13,14,15,16,17,18,19,20]. To protect insect pollinators from exposure to pest control products, there is an urgent need to develop environmentally safe adjuvants. Those adjuvants should have the following characteristics: (i) compatible with commonly used herbicides, fungicides, and insecticides; (ii) not adversely affecting pesticide efficacy against target crop pests; and (iii) not having adverse effects on beneficial insects, such as bees and other insect pollinators. Such adjuvants will have an enormous positive impact on the pesticide market and an increased acceptance among crop producers.
In this study, we tested two plant-derived polymers for their potential as drift-reducing pesticide adjuvants to reduce pesticide exposure and related toxicity to insect pollinators. Most of the plant-based polymers are found widely in nature, including in algae (as alginate), plants (as cellulose, pectin, cyclodextrin, and starch), microorganisms (as dextran), and animals (as chitosan) [21,22]. These polymers can be linear (for example, chitosan) or cyclic (such as cyclodextrin) and differ in their charge, which can be neutral, positive, or negative [23]. The advantages of plant-based polymers include low cost, environmental friendliness, and abundant availability, which can facilitate their large-scale production. Data from our prior and ongoing projects provided the rationale for conducting the study reported here. Our ongoing research with sodium alginate (SA) as an adjuvant to commonly used insecticides in the LMD region resulted in less toxicity to honeybees. Our prior study in ground-based field herbicide application and in laboratory settings revealed the drift reduction potential (DRP) of SA [24]. Prior study results demonstrated that when added to commonly used insecticides as an adjuvant, SA did not interfere with targeted insect kill mechanisms [25]. Preliminary no-choice honeybee visitation field experiments were carried out using sunflower (Helianthus annuus) plants with flowers. We sprayed water (control), fenugreek polymer (FP) suspension (FP is a nontoxic food-grade polysaccharide derived from the seeds of fenugreek (Trigonella foenum-graecum L.), a legume (Fabaceae) grown in North Africa and Asia), insecticides imidacloprid, and bifenthrin as different treatments. Bee visitation results for fenugreek solution were similar to water and significantly higher than insecticide-alone treatments (unpublished).
The major goal of this study was to evaluate the drift reduction potential of sodium alginate (SA) and fenugreek polymer (FP) for aerial pesticide applications using the Remotely Piloted Aerial Application System (RPAAS). The specific objectives include the analysis of (i) the droplet spectrum of spray volume; (ii) the proportion of driftable droplets (driftable fines) in the spray volume; and (iii) the on-target spray coverage. With better on-target coverage, we can expect less off-target drift. Therefore, on-target coverage was used in combination with droplet spectrum to determine the drift reduction potential. The data required to estimate DRP were obtained by spraying water (control) and water with adjuvant (treatments). To our knowledge, this is the first study in which SA and FP were tested as drift-reducing adjuvants when aerially applied with the RPAAS platform.
2. Materials and Methods
2.1. Study Area
This study was conducted in an unpaved area surfaced with gravel in Burleson County, near College Station, TX (30°40′ N, 96°18′ W) (Figure 1). The location had access to a weather station that recorded local weather data continuously.
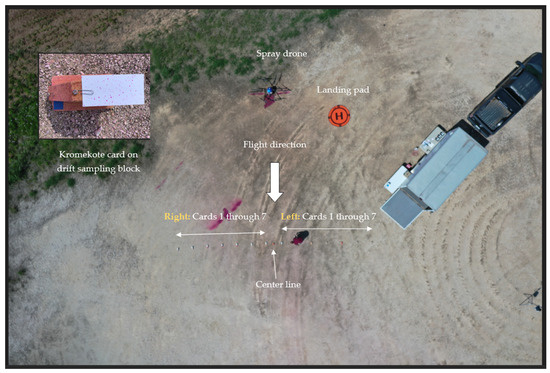
Figure 1.
Experimental setup for drone-based aerial pesticide application and testing spray drift.
2.2. Characteristics of Adjuvants and Their Current Applications
Sodium alginate (SA) (C6H7 O6Na)n, with a molar mass of 1.93 × 105 g/mole, is nontoxic, biocompatible, and biodegradable [26]. It is nearly odorless and tasteless [27,28]. After dissolving in water, a thick colloidal solution tends to form with increasing concentration. SA is listed by the United States Food and Drug Administration (USFDA) [29] as a “generally recognized as safe” (GRAS) material. In the European Union (EU), it is registered as a food-improvement agent [30]. The United States Environmental Protection Agency (USEPA) rated SA as a safer chemical [31]. SA is used in drug delivery systems and as a wound dressing material in the pharmaceutical and medical fields [32,33,34]. In the food industry, SA is used as a texturizer, stabilizer, firming agent, and flavor adjuvant [35]. In water and wastewater treatment, it is used as a flocculant [36]. In other industries, it is used as a surface active agent, processing aid, emulsifier, drilling mud, and formulation aid. Commercially available SA produced by Spectrum Chemical Manufacturing Corporation (Gardena, CA, USA and New Brunswick, NJ, USA) was used in this study.
The fenugreek polymer (FP) is a nontoxic food-grade polysaccharide derived from the seeds of fenugreek (Trigonella foenum-graecum L.), a legume (Fabaceae) grown in North Africa and Asia. FP is partially soluble in water and completely soluble in one molar of sodium hydroxide (NaOH) [37]. The pH of FP aqueous suspension was found to be almost neutral. FP is an amorphous non-ionic polymer made up of D-galactose and D-mannose with a galactose-to-mannose ratio of 1:1.2 [38]. FP has shown promising results as a plant-derived flocculant in water treatment studies [39]. FP was prepared by following the method of Srinivasan et al. [37]. Briefly, fenugreek seeds (purchased from a local grocery store) were soaked overnight in approximately 3 to 5 times the volume of deionized (DI) water. The soaked seeds were finely blended, the dissolved mucilage was filtered with a muslin cloth, and the remnants were discarded. Then, the mucilage was precipitated with 99% isopropyl alcohol at a ratio of one part extracted mucilage solution to three parts isopropyl alcohol. The precipitated FP was separated using vacuum filtration, washed with acetone two to three times to remove impurities, and then dried in a hot-air oven at 70 °C. The dried FP was blended into powder and stored at 4 °C for future use.
The physicochemical characteristics of both adjuvants used in this study are presented in Table 1.

Table 1.
Physicochemical characteristics of water and different adjuvant solutions.
2.3. Experimental Setup
The Remotely Piloted Aerial Application System (RPAAS) used for this study was a six-rotor Precision Vision 35X (Leading Edge Aerial Technologies, New Smyrna Beach, FL, USA) with a payload capacity of 16 L. The system was equipped with four Turbo TeeJet 110-01 nozzles (two on each side of the RPAAS) mounted to spray booms. Prior pattern testing with this setup showed an effective swath width of 6.1 m (20 ft). To achieve the assumed application rate of 28 L/ha (3 gal./acre), the RPAAS was flown at 5.5 km/h (3.3 mph) at an altitude of 3.05 m (10 ft) with an industry-standard spray system pressure of 276 kPa (40 psi). The required speed of the aircraft was back-calculated as follows using the above-stated parameters:
where 167 is a conversion factor to resolve units to L∙ha−1.
Application Rate (L∙ha−1) = System Flowrate (L∙min−1)/[(Ground Speed (m∙s−1) x_Effective Swath (m))/167]
For each treatment, three trials were carried out. Each trial required less than one minute of flight time. A total of 14 Kromekote cards (7 cards each on the left and right side of the centerline) were used to collect spray droplets (Figure 1) in each trial. Each Kromekote card was placed flat on the sampling block, as shown in the inner left panel of Figure 1, with a 1 m distance between cards.
2.4. Spray Experiments
The spray tank mixture was prepared as follows: For each liter of tank mix, 26.5 mL of diluted rhodamine dye (Keystone Aniline Corp., Chicago, IL, USA, 10% v/v) was used to adequately stain the Kromekote paper that received droplets for subsequent analysis. The spray system pressure was set to 276 kPa (40 psi), and the pressure was measured with an inline pressure gauge (4FLR1, Grainger, Lake Forest, IL, USA) to achieve a total system flow rate of 1.5 L/min (0.4 GPM). Treatments comprised two concentrations of SA, (1.25 and 2.5 g of polymer/L of water) and two concentrations of FP (0.5 and 1 g/L) (Table 2). Water was used as the control (Table 2). For each treatment, three trials were carried out for the spray coverage study. Four separate trials were carried out for the droplet spectrum analysis. For the droplet spectrum analysis, a VisiSize P15 image analyzer (Oxford Lasers, Didcot, UK) was used. It was set at the center of the flight line to capture the size and speed of droplets as the drone was flying. However, because of the limited field of view, the instrument could not capture adequate droplets for any meaningful droplet spectrum analysis. Therefore, the drone was kept stationary above the instrument to collect 500 droplets per trial to facilitate droplet size and speed analysis.

Table 2.
Details of spray experiment.
2.5. Weather Conditions
Instantaneous measurements of wind speed during the spray coverage experiment using Kromekote cards were recorded using a hand-held anemometer (Kestrel 5500 Portable Weather Station, Kestrel Corp., Albuquerque, NM, USA), placed near the test site. Drone flight in each experimental trial involved flying into the wind. However, when testing in an outdoor environment, meteorological parameters such as wind velocity, temperature, and humidity cannot be controlled. Changes in wind speed and direction can impact downwind deposition, with higher wind speeds moving spray particles farther downwind. As temperature rises and humidity falls, evaporation of spray droplets will increase, reducing the overall size of the spray droplet spectrum, especially the smaller, more driftable fraction due to higher surface area-to-volume ratios. These smaller droplets have a lower terminal velocity and are more prone to downwind displacement due to wind. In addition, smaller droplets will result in less coverage. Recognizing these factors, but having no control over them, the studies were conducted in the shortest timeframe possible to provide meteorological consistency between treatments. In addition, to control for varying wind direction, it was constantly monitored, and the RPAAS flights were only conducted when the wind direction was within 30° of the flight line. Continuous observations of wind speed, air temperature, and relative humidity measured during the spray coverage experiment are presented in Figure 2. The instantaneous weather data collected during the spray experiments for collecting droplet spectrum characteristics using an image analyzer are presented in Table 3.
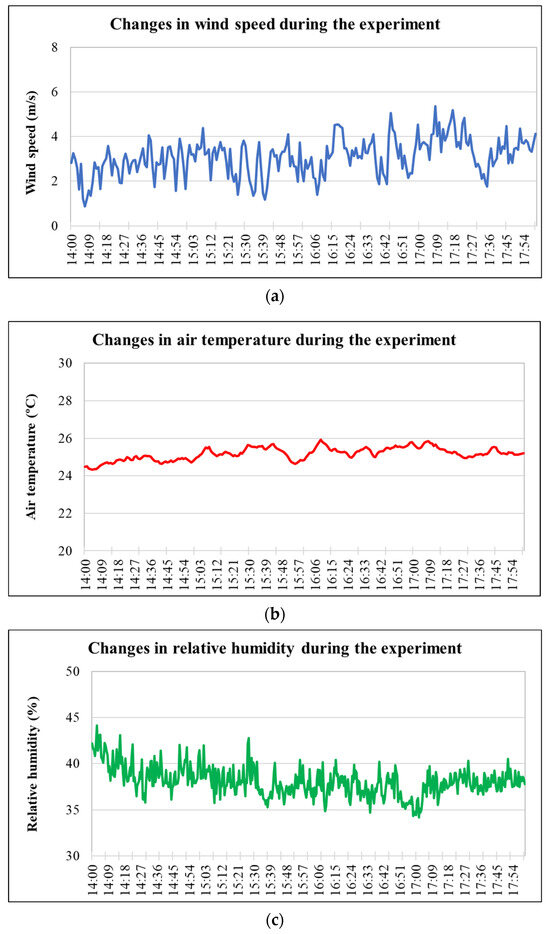
Figure 2.
Changes in weather parameters during the experiment: (a) wind speed, (b) air temperature, and (c) relative humidity. (Data sources: (a,b), Delta T weather station; (c), mesonet). Note: the X axis indicates the time during which the experiments were conducted.

Table 3.
Instantaneous weather record during spray experiments to collect droplet spectrum data using a VisiSize P15 image analyzer.
2.6. Data Collection on Droplet Size and Speed
To analyze the size and speed of spray droplets in the field, a VisiSize P15 (image analysis system was used. This image analysis system uses a short, double-light pulse to illuminate a plane of spray that is photographed such that droplets show up as dark spots against a bright background. A digital camera is used to capture the snapshots of the spray particles. The parameters displayed by the instrument are average diameter (by number, area, and volume); the Sauter mean diameter (DV32) (the diameter of a collection of spherical droplets of different sizes is equal to the diameter of equisized spherical droplets forming a collection [40]; 10%, 50%, and 90% percentile volumes (DV10, DV50, and DV90); standard deviation; relative span; and all the curves related to the parameters describing the droplet spectrum [41,42].
2.7. Data Analysis
Raw data showing the size and speed of each droplet collected from VisiSize P15 under different spray treatments were analyzed, and parameters such as the minimum, maximum, average, DV10, Sauter mean diameter (DV32), DV50, and DV90 were obtained for each spray treatment to gain a full understanding of the droplet spectrum. To visualize the droplet size distributions (Figure 3), kernel density estimates were computed for each set of droplet sizes (from all the trials in a treatment) using the density function in R version 4.3.1. A Gaussian kernel was used, and the nrd0 algorithm was used to choose the smoothing bandwidth. Droplets smaller than 200 µm are more likely to drift away from the target spray area [43,44,45]. To analyze the DRP of the adjuvants, the proportion of droplets smaller than 200 µm was estimated from the raw data on droplet sizes observed using VisiSize P15.
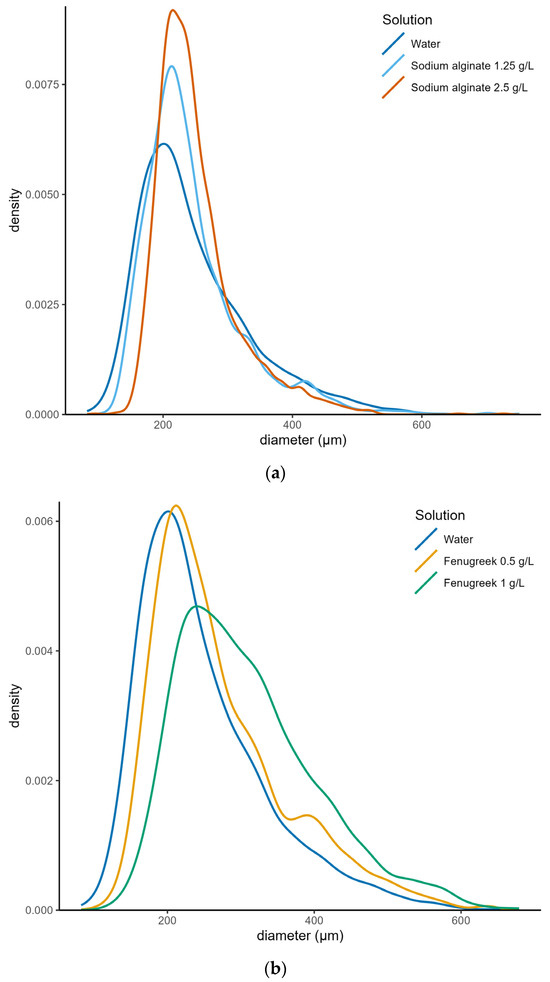
Figure 3.
Droplet spectrum of spray solutions with and without adjuvants: (a) sodium alginate; (b) fenugreek polymer.
AccuStain (Version 0.35), a public domain software (University of Illinois), was used to analyze the spray droplet stains deposited on the Kromekote paper to estimate the on-target coverage. The Kromekote cards were scanned at 600 dots/pixel per inch (DPI/PPI) using an Epson Perfection V39 desktop scanner. An identification was generated for each card in the AccuStain software based on the user-entered information, which in turn was based on the location of the card in the field experiment. The AccuStain software generated a complete swath report including in-field spray pattern uniformity values along with the size of droplets at different locations across the flight line.
3. Results
3.1. Adequacy of the Experimental Setup
The drone was flown into the wind in each spray application. Therefore, changes in the wind direction should not affect the results. It should be noted that the weather data presented in Figure 2 and Table 3 were observed on two different days, and therefore, there may not be a correlation. The weather data presented in Table 3 are applicable for spray droplet size and speed estimation using the VisiSize P15 image analyzer. The temperature variation during the entire experiment was 1.1 °C. The relative humidity (RH) values decreased progressively as the experiment continued. However, the drop in RH was not significant except for one trial that reached 67%. The wind speed during DI water and SA at 1.25 g/L spray treatments ranged between 3.3 and 4.2 m/s. For both FP treatments, the wind speed remained slightly higher (4.2 to 5.6 and 3.3 to 5.1, respectively) than for DI water. However, for SA at 2.5 g/L, the wind speed was higher than those in other treatments. In the spray coverage experiment, the relative humidity varied from 35% to 40% (Figure 2). The air temperature remained stable (variation within 2 degrees). The wind speed varied between 2 and 4 m/s for most of the time during the experiment (Figure 2). The influence of the changes in wind speed on droplet size, speed and coverage could not be ruled out in a field experiment (like this study). The variation in weather parameters was not significant (except for wind direction), suggesting the adequacy of the experimental setup for spray applications to analyze the drift reduction potential of the adjuvants. The infield uniformity results estimated by the coefficient of variation (CV) ranged from 8.7 to 13%, which was well within the accepted industry standard for aerial spray applications [43].
3.2. Results of the Droplet Spectrum
Larger droplets are less likely to drift. The droplet spectra estimated using the VisiSize P15 image analyzer are shown in Figure 3 and Table 4 and Table 5. All the adjuvant treatments produced larger droplets than water alone in all the important size categories, except for DV75 and DV90 droplets of SA treatment (Table 4, Figure 3). Droplets generated in the DV10 and DV25 regions of the droplet spectrum are more likely to drift away. Notably, both SA and FP adjuvants produced larger droplets (than water) in the DV10 and DV25 sections of the droplet spectrum, suggesting the potential of these adjuvants to reduce drift when they are added to water. The average size of droplets could be a misleading parameter for determining the base drift reduction potential for a spray volume with droplets of a wide range of sizes. The median (DV50) or Sauter mean diameter (DV32) could be a better parameter for quickly evaluating the drift reduction potential between different treatments. The DV32 or DV50 (VMD) for water and different adjuvant treatments indicated that all adjuvant treatments produced relatively larger droplets, thus reducing drift potential. Among the adjuvants tested, FPs produced relatively larger droplets (9% to 18% larger droplets than water) than SAs did (7% larger droplets than water), suggesting that FPs have greater potential to reduce drift than SAs in aerial pesticide applications. A comparison of the relative span (RS) of water only and adjuvant treatments revealed that adding the adjuvant to water produced relatively more uniform droplets than water alone (Table 4). Combined with droplet size data, RS analysis substantiates the DRP of both SA and FP.

Table 4.
Droplet diameter of spray solutions with and without adjuvants (measured using the VisiSize P15 image analyzer).

Table 5.
Droplet velocity of spray solutions with and without adjuvants (measured using the VisiSize P15 image analyzer).
Faster droplets were more likely to hit the target and less likely to drift. The droplet velocities estimated using the VisiSize P15 image analyzer are shown in Table 5. Droplets generated in the 10th percentile and 25th percentile regions of the droplet spectrum (corresponding to diameters DV10 and DV25) were more likely to drift away. Both SA and FP adjuvants produced faster droplets (than water) in the 10th and 25th percentile sections of the droplet spectrum, suggesting the potential of these adjuvants to reduce drift when they are added to water. The 32nd percentile and 50th percentile (corresponding to DV32 and DV50 (VMD) regions of the droplet size) for water and FP treatment at 0.5 g/L produced relatively faster (on average 10% faster than water) droplets, thus reducing drift potential. However, similar results could not be observed for FP treatment at 1 g/L. The SA treatment at both the concentrations tested did not produce droplets faster than water beyond the 10th and 25th percentiles. Among the adjuvants tested, FP at 0.5 g/L alone produced relatively faster droplets, suggesting that FP has greater potential to reduce drift than SA in aerial pesticide applications.
3.3. Analysis of Drift Reduction in Aerial Application
An analysis of droplet size less than 200 µm will shed additional light on the drift reduction potential of various spray treatments. Water alone produced 14.5% driftable spray volume. The addition of either adjuvant to water produced appreciable reductions in driftable droplets. Compared with water alone, the SA treatment reduced driftable volume by 0.1% to 6.2%. Similarly, the FP treatment reduced the driftable fines by 6.8% to 12.3% during the spray treatment (Table 6). Between these two adjuvants, the drift reduction potential of FP was greater than that of SA.

Table 6.
Driftable proportion of spray volume (volume of droplets less than 200 µm size) in the sodium alginate and fenugreek polymer spray solutions (droplet spectrum was measured using VisiSize P15 image analyzer).
3.4. On-Target Spray Coverage Analysis
Achieving adequate spray coverage of applied pesticides on target is equally important as reducing off-target drift. From the drift reduction standpoint, it is desirable to have fewer larger droplets with more on-target coverage rather than otherwise. Table 7 shows the average on-target coverage of spray solutions with and without adjuvants. Figure 4 shows the average (of three replications in each treatment) on-target coverage for each Kromekote card kept across the flight direction. Compared to water alone, the SA treatment resulted in fewer droplets on Kromekote cards (Table 7), and most of the droplet reductions appear to be on the smaller side (Table 4 and Table 5). However, the SA treatment produced less overall coverage than the water-alone treatment. Additionally, the FP treatment resulted in fewer droplets on Kromekote cards. However, the FP treatment produced better target coverage than the water-alone treatment (Table 7, Figure 4) possibly because of the increased speed of droplets from FP treatment compared to water (Table 5). On the other hand, the SA treatment reduced the droplet speed compared to water droplets (Table 5), probably justifying the reduction in on-target coverage compared to water.

Table 7.
On-target spray coverage results of spray solutions with and without adjuvants.
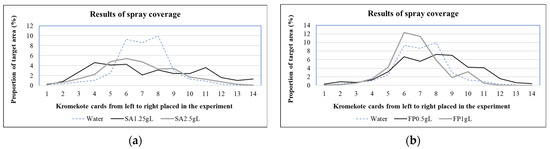
Figure 4.
Results of droplet spray coverage of (a) sodium alginate (SA) and (b) fenugreek polymer (FP).
4. Discussion
The preliminary results from some of the ongoing experiments indicate that when SA was added to a pesticide mixture as an adjuvant, it (i) reduced the toxicity of the pesticide mixture to honeybees (Table 8), (ii) did not interfere with the targeted pest kill mechanisms [25], and (iii) reduced drift in ground-based pesticide applications (Table 9) [24]. In addition, our results showed that SA is compatible with commonly used herbicides and insecticides [24,25]. Therefore, the use of SA and FP as potential pesticide adjuvants and their use in drone-based aerial applications were investigated in this study.

Table 8.
Mortality data of honeybees exposed to the pesticide Bifenthrin with or without adjuvants.

Table 9.
Summary results of ground-based herbicide application with sodium alginate as a pesticide adjuvant.
Three physical properties of spray liquids have been shown to affect the characteristics of spray droplets: viscosity, surface tension, and the presence of inhomogeneities [43]. Adjuvants can act by increasing viscosity and/or decreasing the surface tension of the spray liquid to shift the droplet size distribution to a larger size [43]. An analysis of the physicochemical characteristics of the water and adjuvant solutions highlights their DRPs. With respect to water, neither the FP nor the SA solutions showed noticeable differences in density. Dose-dependent changes in pH and surface tension were not significant either, although FP is slightly more acidic than SA, and when added to water, FP reduced the surface tension of the solution better than SA. However, the dose-dependent increase in viscosity for the SA and FP (Table 1) solutions suggested that they could form droplets larger than water alone, although this behavior was less apparent for FP than for SA. This may be due to the partial solubility of FP in water and the formation of a spray suspension. The observed better drift reduction potential of FP could be due to the combination of the above-mentioned factors. Conventional adjuvants generally work to influence one of these parameters [6,8].
In general, large and faster droplets are less likely to drift away. Key statistics on the droplet spectra indicate that both FP and SA produced larger droplets and a smaller number of driftable fine fractions than water alone. Important parameters to note are DV10 and DV25, which could contain a large proportion of driftable droplets. Both the FP and SA treatments increased DV10 and DV25 and droplet velocities, corresponding to these regions of the droplet spectrum compared with those in the water alone, with FP producing slightly larger droplets than SA (Table 4 and Table 5). FP at 0.5 g/L treatment produced faster droplets than water alone and all other treatments tested. Droplets < 200 µm are more likely to drift [43,44,45]. The analysis of the proportion of droplets < 200 µm for different adjuvant treatments indicated that the DRP activity of both SA and FP was greater than that of water alone. The DRP of FP was better than SA, especially with the treatment at 0.5 g/L. The results of this study showed better performance of FP in DRP at a lower concentration (0.5 g/L) than higher concentration (1 g/L). A similar pattern was exhibited by FP when used as a flocculating agent in water treatment [37], which aligns well with the results of this study. Also, FP has the added advantage of slightly more on-target coverage, which is desirable for more efficient pesticide application.
When comparing important droplet spectrum parameters such as DV10, DV50, and DV90, only moderate correlations were observed between what was observed with the VisiSize P15 image analyzer and what was estimated from the droplet stains deposited on the Kromekote cards (Figure A1). The correlation coefficients were 0.65, 0.62, and 0.58 for DV10, DV50, and DV90, respectively. Given the only moderate correlations between droplet data observed with an image analyzer and those estimated from Kromekote cards, it is highly recommended to use an image analyzer to obtain spray droplet spectrum data. However, given the limited field of view, effort needed, and time taken to obtain a meaningful number of trials for droplet spectrum analysis, it is not possible to obtain droplet spectrum data using an image analyzer for many aerial pesticide application field experiments. A reasonable alternative could be using existing droplet spectrum data or obtaining new data from wind tunnel studies for various pesticide–adjuvant–wind speed data combinations.
A comprehensive review of several manuscripts [46] on drift reduction outlines the importance of using spray adjuvants for drift reduction. The study by Hewitt et al. [46] highlights the use of DV10 of the droplet spectrum as the basis for labels in the aerial application of pesticides in Australia. The study describes the successful application of several adjuvants, including plant-based guar gum. Many other studies have successfully used different types of adjuvants for RPAAS-based aerial pesticide applications. For example, the requirement of spray adjuvants for RPAAS-based insecticide applications to control wheat aphids was highlighted in Wang et al. [6]. In this study, the authors used a wind tunnel to analyze the drift reduction potential of some adjuvants. Their results highlighted (a) the droplet size as an effective indicator of spray drift potential and (b) the importance of wetting and spreading to improve the efficacy of pest control. The adjuvants Breakthrough (mixture of fatty acid ester) and Momentive (made up of polymeric components, organosilicon) showed better drift reduction potential when compared to others. In another study by Hu et al. [47], they tested plant oil, mineral oil, and a mixture of alcohol and ester to improve pesticide spray characteristics and enhance pest control efficiency. The pesticide solution mixed with alcohol and ester adjuvant Quanrun showed excellent wetting and reduced pesticide evaporation, improved droplet deposition, and achieved the highest control efficiency against cotton aphids when applied using the RPAAS at 2.5 m above the canopy. Additionally, the plant oil adjuvant (Beidatong) showed moderate improvement in wetting and the penetration of pesticide into the target and reduced droplet drift.
Adjuvants that do not have adverse effects on beneficial insects such as bees and do not interfere with targeted pest-killing mechanisms could have an enormous positive impact on the pesticide adjuvant market. Research similar to these is currently needed to protect the beekeeping industry and other pollinator species. Additionally, regulatory controls on pesticide adjuvants are needed to protect beneficial insects, such as honeybees.
In this study, only limited field experiments were carried out in noncropped bare fields to estimate the drift reduction potential of SA and FP in drone-based aerial applications. Although our ongoing study showed that, compared to commercial adjuvants such as polyacrylamide, when added to pesticide, SA caused a lower mortality rate for honeybees, additional experiments are needed to test the effect of this adjuvant on other physiological parameters of honey bees. For FP, similar honey bee toxicology studies are ongoing. The laboratory and ground application experiments were not yet carried out. To gain a better understanding of the toxicity of these adjuvants for honey bees, we think that, in addition to caged bee studies, studies in which beehives are placed near the study field need to be performed to determine the infield exposure to individual bees. Honey bees collected from these hives can be analyzed for pesticide and adjuvant residue to determine the exposure level. Another way to analyze pesticide drift is by keeping a series of potted plants along the downwind direction during pesticide spray, collecting samples (e.g., leaves) and analyzing pesticide residue levels (plant bioassay experiment), or making a visual observation of all the plants periodically for likely changes due to pesticide drift. Similarly, experiments could be carried out using plants with flowers to estimate the potential of pesticide exposure to pollinators.
5. Conclusions
Ongoing laboratory and field experiments suggest that some plant-based polymers could be developed as drift-reducing pesticide adjuvants (PAs) to reduce pesticide exposure to insect pollinators inhabiting/foraging areas adjacent to croplands. In this study, sodium alginate (SA) and fenugreek polymer (FP) were tested as drift-reducing PAs when aerially applied with the RPAAS platform. Two spray experiments were carried out in the field: (i) water only as control (W) and (ii) water and adjuvant (WA). The drift reduction potential (DRP) of the adjuvants was determined based on the droplet size, on-target coverage, and proportion of droplets less than 200 µm (driftable fines).
Compared to the control, the WA treatments produced larger droplets (9% to 18% larger for FP and 7% larger for SA), suggesting the occurrence of DRP. Notably, 14.5 percent of the total spray volume comprised driftable size droplets for W, whereas only 8.3 to 14.4% and 2.3 to 7.8% of the spray volume were driftable for the SA and FP treatments, respectively. Compared with the W only (2.7%), the FP treatment improved on-target coverage (3.0% to 3.1% average coverage over 14 equally spaced Kromekote cards placed across the flight line). The FP treatment had faster droplet velocities than water alone and SA, especially at 0.5 g/L concentration (10% faster than water alone). In summary, the results of this study indicate that SA and FP have the potential to mitigate off-target drift from drone-based aerial pesticide applications. Compared to SA, FP has the added advantage of providing slightly more on-target coverage, which is desirable for improving pesticide application efficiency. The observed better drift reduction potential of FP could be attributed to, at least in part, a combination of factors, including its ability to increase viscosity; the presence of inhomogeneities (its partial solubility in water); and, to a lesser extent, a decrease in the surface tension. Our study set the foundation for further testing these plant-based polymers in row crops (such as corn, cotton, and soybean) with different densities and varied topographies.
Author Contributions
N.K. conceptualized the study, analyzed the results, and wrote a significant portion of the manuscript. D.M. carried out the drone experiment. R.S. prepared the FP polymer from scratch, identified the appropriate concentration of the adjuvants, and prepared the adjuvant mixture for the experiment. W.Z. analyzed the physicochemical properties of the adjuvants. All the authors contributed to the writing and development of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by internal funding from USDA-ARS under project number # 6066-30500-001-000D.
Data Availability Statement
The data are available upon request.
Acknowledgments
The authors thank Quentin Read, USDA-ARS Southeast Area Statistician for his help in visualizing the droplet size distribution data. The authors also thank Mohamed A. Latheef for his help to collect the droplet spectrum data in the field.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
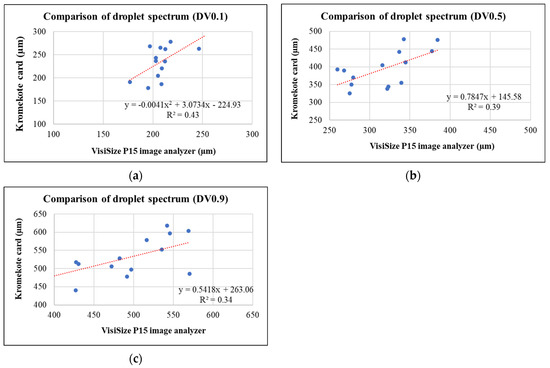
Figure A1.
Relationship between droplet spectrum parameters measured by VisiSize P15 and estimated by droplet stains deposited on Kromekote cards (a) DV0.1 (b) DV0.5 and (c) DV0.9.
References
- Kirk, I.W. Aerial spray drift from different formulations of glyphosate. Trans. ASAE 2000, 43, 555. [Google Scholar] [CrossRef]
- Oliveira, R.B.D.; Antuniassi, U.R.; Mota, A.A.; Chechetto, R.G. Potential of adjuvants to reduce drift in agricultural spraying. Eng. Agric. 2013, 33, 986–992. [Google Scholar] [CrossRef]
- Butts, T.R.; Fritz, B.K.; Kouame, K.B.; Norsworthy, J.K.; Barber, L.T.; Ross, J.; Lorenz, G.M.; Thrash, B.C.; Bateman, N.R.; Adamczyk, J.J. Herbicide spray drift from ground and aerial applications: Implications for potential pollinator foraging sources. Sci. Rep. 2022, 12, 18017. [Google Scholar] [CrossRef] [PubMed]
- Reich, G.A.; Berner, W.H. Aerial Application Accidents 1968 to 1966 An Analysis of the Principal Factors. Arch. Environ. Health 1968, 17, 776–784. [Google Scholar] [CrossRef]
- Cantor, K.P.; Silberman, W. Mortality among aerial pesticide applicators and flight instructors: Follow-up from 1965–1988. Am. J. Ind. Med. 1999, 36, 239–247. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zeng, A.; Song, J.; Xu, T.; Lv, X.; He, X. Effects of Adjuvants on Spraying Characteristics and Control Efficacy in Unmanned Aerial Application. Agriculture 2022, 12, 138. [Google Scholar] [CrossRef]
- Xiao, Q.; Xin, F.; Lou, Z.; Zhou, T.; Wang, G.; Han, X.; Lan, Y.; Fu, W. Effect of Aviation Spray Adjuvants on Defoliant Droplet Deposition and Cotton Defoliation Efficacy Sprayed by Unmanned Aerial Vehicles. Agronomy 2019, 9, 217. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, R.; Yu, M.; Liu, Y.; Ma, Y.; Guo, X.; Gu, Y.; Formstone, C.; Xu, Y.; Wu, X. Enhanced dosage delivery of pesticide under unmanned aerial vehicle condition for peanut plant protection: Tank-mix adjuvants and formulation improvement. Pest Manag. Sci. 2023, 80, 1632–1644. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four Common Pesticides, Their Mixtures and a Formulation Solvent in the Hive Environment Have High Oral Toxicity to Honeybee Larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef]
- Van de Merwe, J.P.; Neale, P.A.; Melvin, S.D.; Leusch, F.D.L. In vitro bioassays reveal that additives are significant contributors to the toxicity of commercial household pesticides. Aquat. Toxicol. 2018, 199, 263–268. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wu, J.; Yu, R.; Zhang, Y.; Sun, L.; Gao, Y. Acute Toxicity and Ecotoxicological Risk Assessment of Three Volatile Pesticide Additives on the Earthworm—Eisenia fetida. Int. J. Environ. Res. Public Health 2021, 18, 11232. [Google Scholar] [CrossRef] [PubMed]
- Straw, E.A.; Thompson, L.J.; Leadbeater, E.; Brown, M.J.F. Inert ingredients are understudied, potentially dangerous to bees and deserve more research attention. Proc. R. Soc. Biol. Sci. 2022, 289, 20212353. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effect of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Pesticide residue and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.-L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef]
- Ellis, J. The honey bee crises. Outlooks Pest Manag. 2012, 23, 35–40. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yañez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef]
- Tison, L.; Hahn, M.-L.; Holtz, S.; Rößner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef]
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 2016, 6, 32108. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharide-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Polysaccharide-based nucleic acid nanofor-mulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef]
- Hassani, L.N.; Hendra, F.; Bouchemal, K. Auto-associative amphiphilic polysaccharides as drug delivery systems. Drug Discov. Today 2012, 17, 608–614. [Google Scholar] [CrossRef]
- Kannan, N.; Read, Q.; Zhang, W. An algae-based polymer material as a pesticide adjuvant for mitigating off-target drift. Heliyon 2024, 10, e35510. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Scheibener, S.; George, J.; Kannan, N.; Portilla, M. Assessing the Efficacy of Sodium Alginate and Polyacrylamide as Spray Adjuvants Combined with Bifenthrin and Imidacloprid against Lygus lineolaris and Piezodorus guildinii. Agriculture 2024, 14, 535. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Chapter 3: Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Elsevier Publications: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-817055-7. [Google Scholar] [CrossRef]
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Compound Summary for Sodium Alginate. 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-Alginate (accessed on 21 December 2023).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- US Food & Drug Administration. CFR—Code of Federal Regulations Title 21. 2017, Volume 3. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1724 (accessed on 15 October 2024).
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; Puente, S.L.; Marcon, F.; et al. Safety efficacy of a feed additive consisting of sodiumalginate for all animal species (ALGAIA). Eur. Food Saf. Auth. J. 2022, 20, 7164. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA); Substance Registry Services (SRS). Sodium Alginate, CAS Number: 9005-38-3. 2023. Available online: https://cdxapps.epa.gov/oms-substance-registry-services/substance-details/162305 (accessed on 21 December 2023).
- Qin, Y. Alginate fibres: An overview of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef]
- Timmons, J. ActivHeal AquaFiber: A new soft, conformable highly absorbent dressing for use with chronic wounds. Wounds UK 2008, 4, 88–91. [Google Scholar]
- United States Food and Drug Administration (USFDA). Guidance for Industry Acrylamide in Foods; (Docket Number: FDA-2013-D-0715); Office of Food Safety, HFS-300, Center for Food Safety and Applied Nutrition, Food and Drug Administration: College Park, MD, USA, 2016. [Google Scholar]
- Srinivasan, R.; Vankar, P.S.; Mishra, A. Treatment of textile effluent using sodium alginate as flocculant. Colourage 2001, 48, 29–32. [Google Scholar]
- Srinivasan, R.; Mishra, A. Okra (Hibiscus esculentus) and Fenugreek (Trigonella foenum graceum) mucilage: Characterization and application as flocculants for textile effluent treatment. Chin. J. Polym. Sci. 2008, 26, 679–687. [Google Scholar] [CrossRef]
- El-Molla, M.M.; Abdel Rehman, A.A.; Abd El-Talouth, I. Chemical modification of fenugreek gum. I. Carbamoylethylation. Am. Dyest. Report. 1998, 87, 56. [Google Scholar]
- Srinivasan, R.; Mishra, A.; McKinney, J. Polysaccharide Agents and Methods of Their Use for Removing Solids from Water. Patent USP No. 10442710, 15 October 2019. [Google Scholar]
- Kowalczuck and Dryzmala (details missing) Przemyslaw B. Kowalczuk and Jan Drzymala, Physical meaning of the Sauter mean diameter of spherical particulate matter. Part. Sci. Technol. 2016, 34, 645–647. [Google Scholar] [CrossRef]
- Oxford Lasers Visisize P15 Research and Development. Available online: http://aams-salvarani.com/en/products/oxford-laser-p15 (accessed on 3 May 2024).
- VisiSize P15 Portable Droplet Sizing and Characterization System. Available online: https://www.oxfordlasers.com/laser-imaging/visisize-p15 (accessed on 3 May 2024).
- Hilz, E.; Vermeer, A.W.P. Spray drift review: The extent to which a formulation can contribute to spray drift reduction. Crop Prot. 2013, 44, 75–83. [Google Scholar] [CrossRef]
- Hong, S.-w.; Park, J.; Jeong, H.; Lee, S.; Choi, L.; Zhao, L.; Zhu, H. Fluid Dynamic Approaches for Prediction of Spray Drift from Ground Pesticide Applications: A Review. Agronomy 2021, 11, 1182. [Google Scholar] [CrossRef]
- Zhu, H.; Reichard, D.L.; Fox, R.D.; Brazee, R.D.; Ozkan, H.E. Simulation of discrete sizes of water droplets from field sprayers. Trans. ASAE 1994, 37, 1401–1407. [Google Scholar] [CrossRef]
- Hewitt, A. Adjuvant use for the management of pesticide drift, leaching and runoff. Pest Manag. Sci. 2024, 80, 4819–4827. [Google Scholar] [CrossRef]
- Hu, H.; Ma, Y.; Song, X.; Wang, D.; Ren, X.; Wu, C.; Liu, C.; Ma, X.; Shan, Y.; Meng, Y.; et al. Tank-Mix Adjuvants Enhance Pesticide Efficacy by Improving Physicochemical Properties and Spraying Characteristics for Application to Cotton with Unmanned Aerial Vehicles. ACS Omega 2024, 9, 31011–31025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).