Using Drones with Thermal Imaging to Estimate Population Counts of European Hare (Lepus europaeus) in Denmark
Abstract
1. Introduction
2. Methods
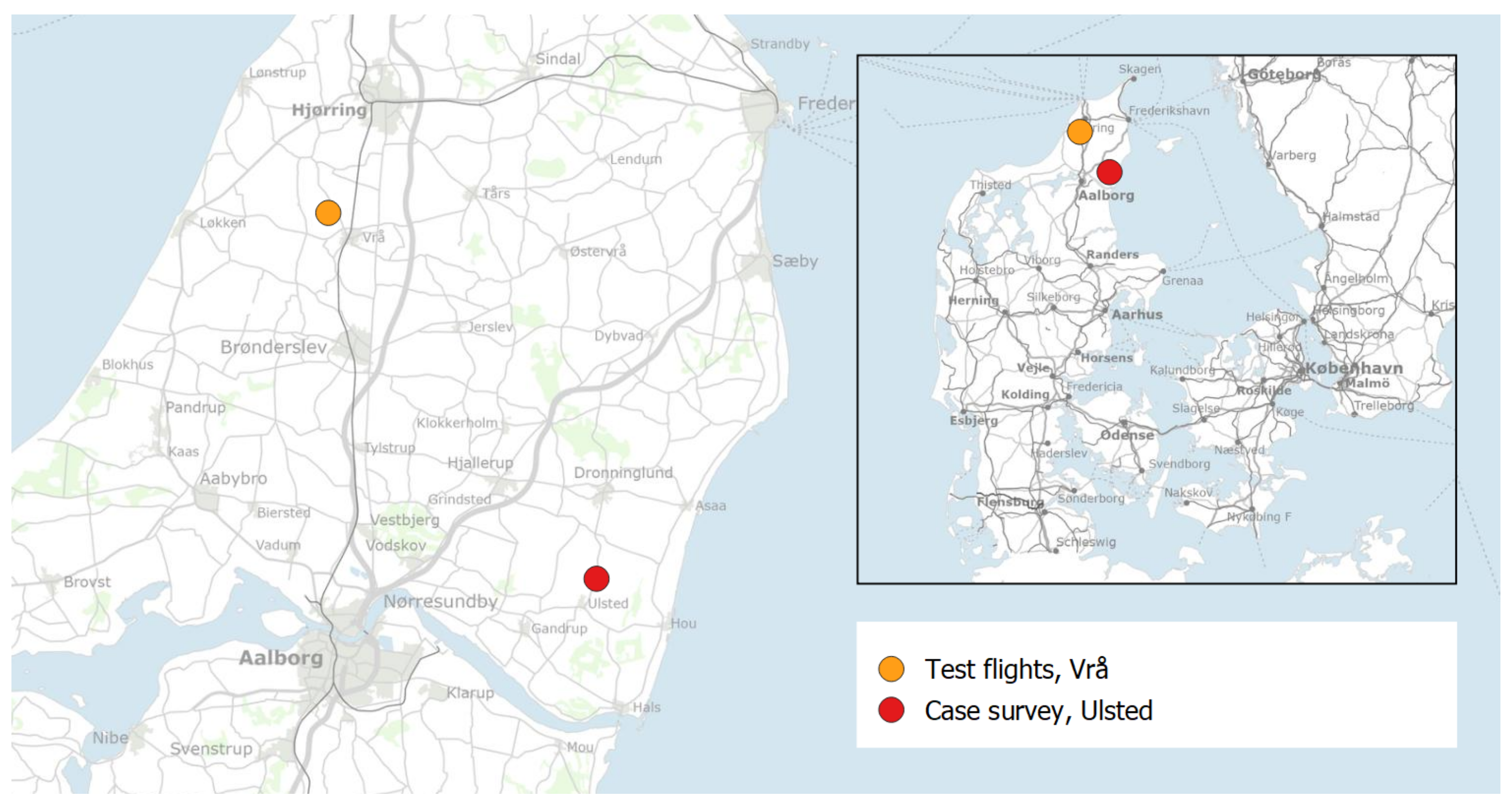
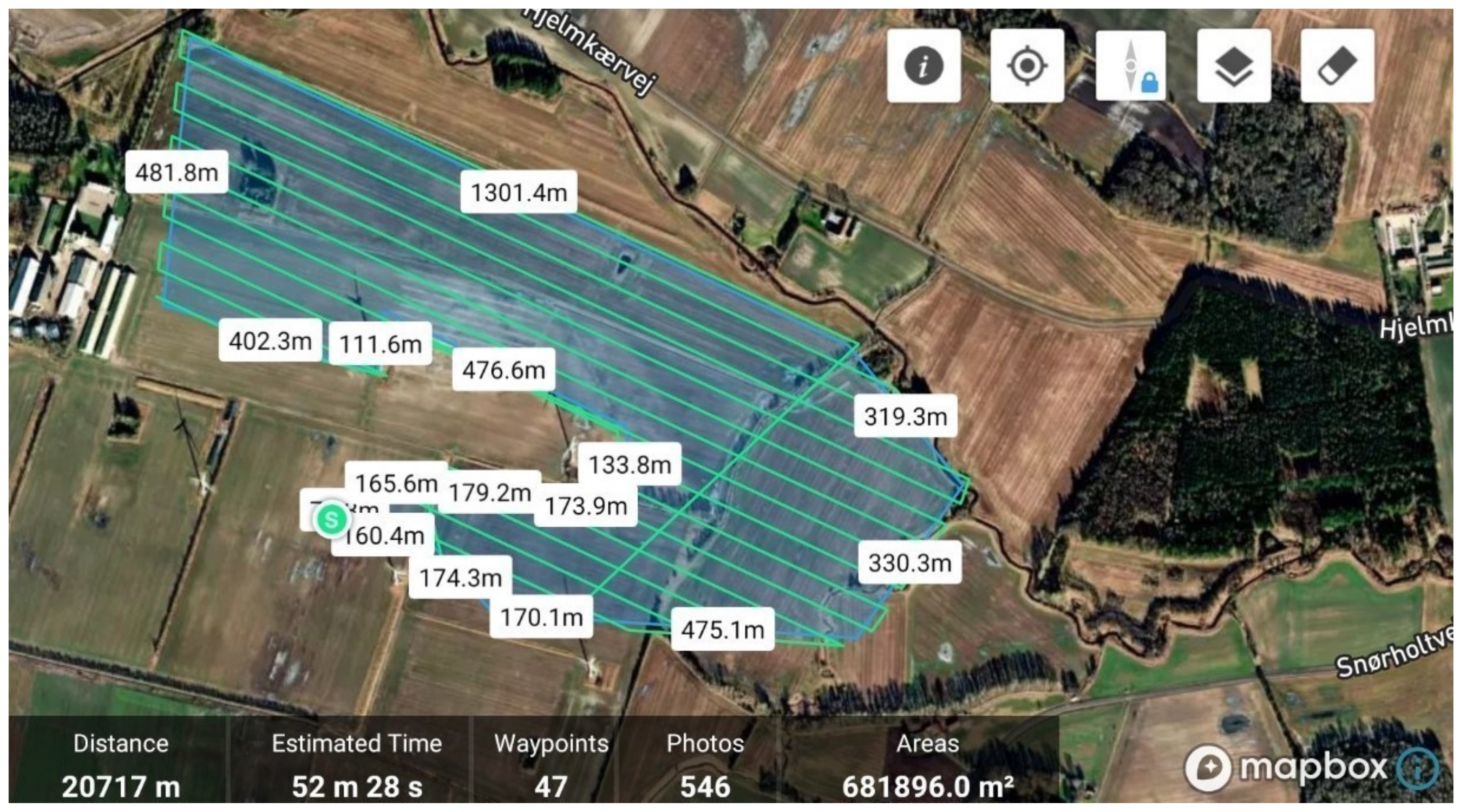
2.1. Flight Parameters
2.2. Case Survey
2.3. Data Analysis
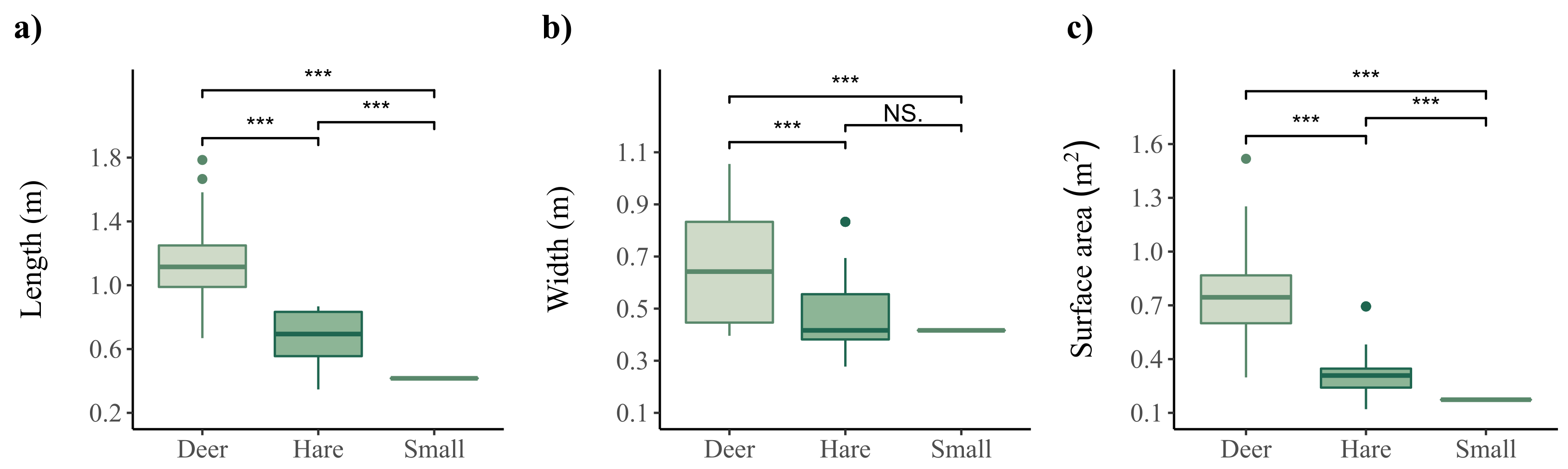
2.3.1. Species Classification
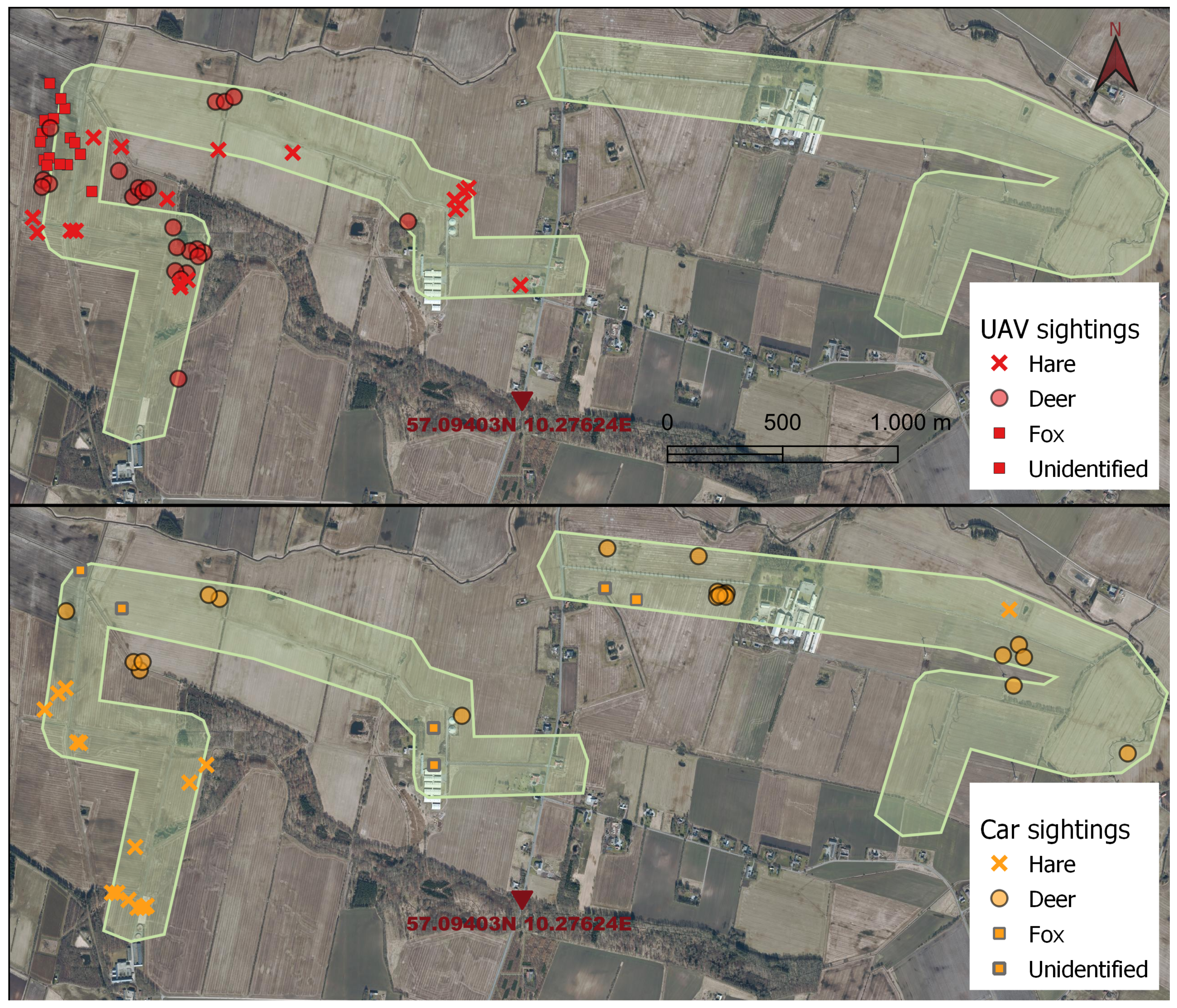
2.3.2. Mapping Observations
3. Results
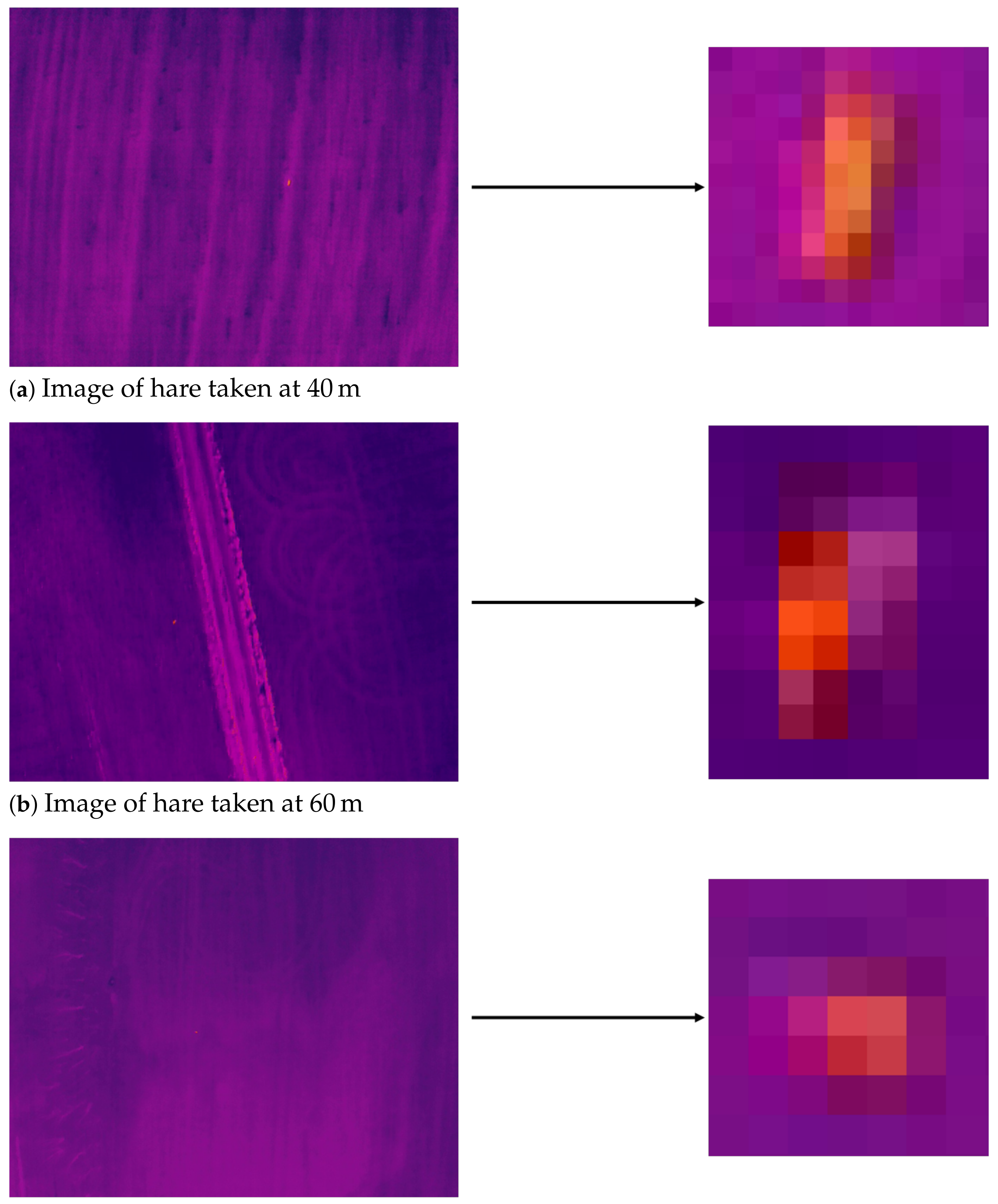
3.1. Flight Altitude
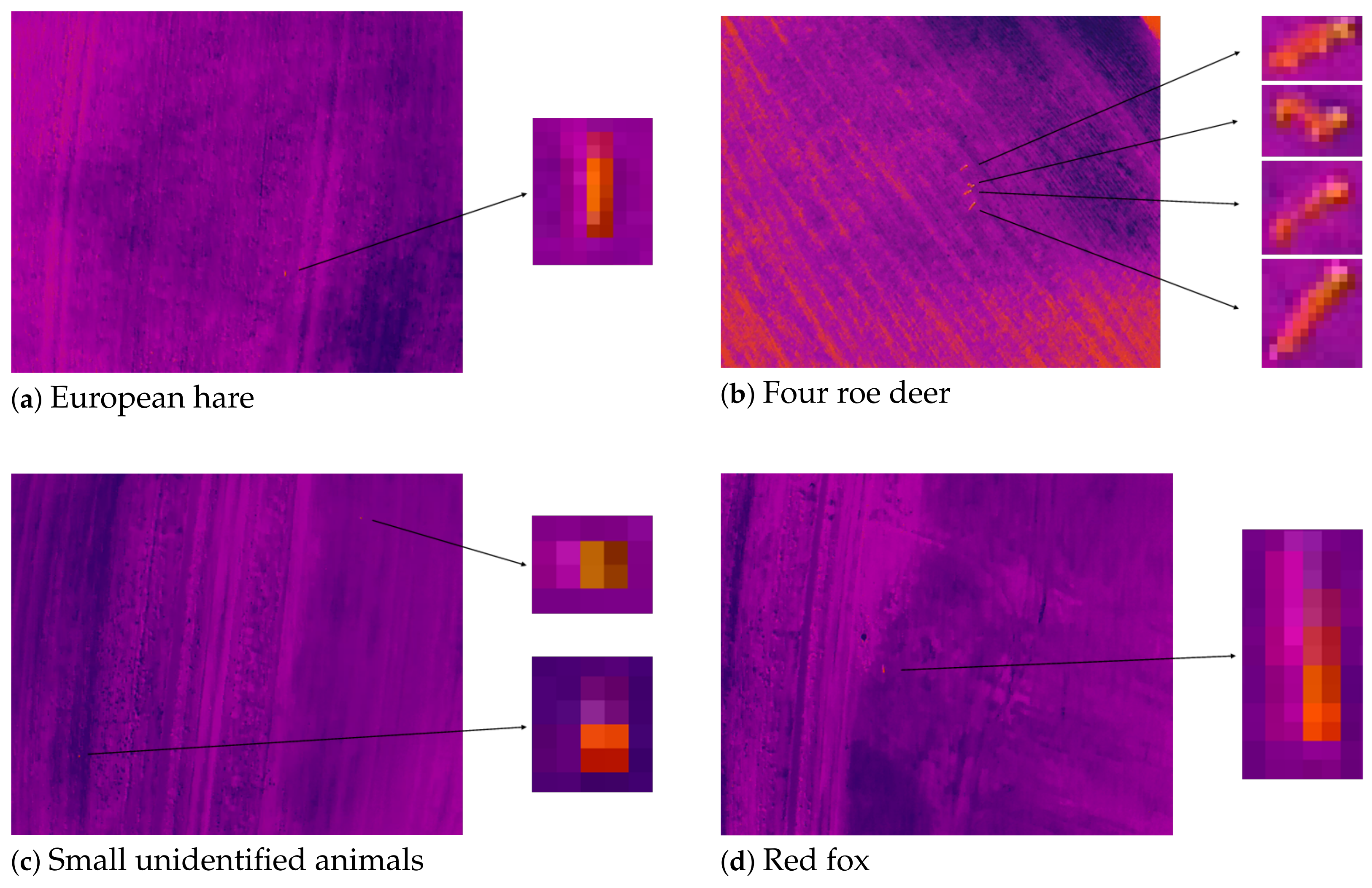
3.2. Species Classification
3.3. Case Survey
4. Discussion
4.1. Flight Parameters
4.2. Species Classification
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webbon, C.C.; Baker, P.J.; Harris, S. Faecal Density Counts for Monitoring Changes in Red Fox Numbers in Rural Britain. J. Appl. Ecol. 2004, 41, 768–779. [Google Scholar] [CrossRef]
- Aebischer, N.J.; Baines, D. Evaluating the Use of Drones Equipped with Thermal Sensors as an Effective Method for Estimating Wildlife. Rev. Catalana d’Ornitol. 2008, 24, 30–43. [Google Scholar]
- Begon, M.; Howarth, R.; Townsend, C. Essentials of Ecology, 4th ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kahlert, J.; Fox, A.D.; Heldbjerg, H.; Asferg, T.; Sunde, P. Functional Responses of Human Hunters to Their Prey—Why Harvest Statistics may not Always Reflect Changes in Prey Population Abundance. Wildl. Biol. 2015, 21, 294–302. [Google Scholar] [CrossRef]
- Nakashima, Y.; Fukasawa, K.; Samejima, H. Estimating Animal Density Without Individual Recognition Using Information Derivable Exclusively from Camera Traps. J. Appl. Ecol. 2018, 55, 735–744. [Google Scholar] [CrossRef]
- Hyun, C.U.; Park, M.; Lee, W.Y. Remotely Piloted Aircraft System (RPAS)-Based Wildlife Detection: A Review and Case Studies in Maritime Antarctica. Animals 2020, 10, 2387. [Google Scholar] [CrossRef]
- Ramos, P.L.; Sousa, I.; Santana, R.; Morgan, W.H.; Gordon, K.; Crewe, J.; Rocha-Sousa, A.; Macedo, A.F. A Review of Capture-recapture Methods and Its Possibilities in Ophthalmology and Vision Sciences. Ophthalmic Epidemiol. 2020, 27, 310–324. [Google Scholar] [CrossRef]
- Delisle, Z.J.; Flaherty, E.A.; Nobbe, M.R.; Wzientek, C.M.; Swihart, R.K. Next-Generation Camera Trapping: Systematic Review of Historic Trends Suggests Keys to Expanded Research Applications in Ecology and Conservation. Front. Ecol. Evol. 2021, 9, 617996. [Google Scholar] [CrossRef]
- Sweeney, K.L.; Helker, V.T.; Perryman, W.L.; LeRoi, D.J.; Fritz, L.W.; Gelatt, T.S.; Angliss, R.P. Flying Beneath the Clouds at the Edge of the World: Using a Hexacopter to Supplement Abundance Surveys of Steller Sea Lions (Eumetopias Jubatus) in Alaska. J. Unmanned Veh. Syst. 2016, 4, 70–81. [Google Scholar] [CrossRef]
- Bech-Hansen, M.; Kallehauge, R.M.; Bruhn, D.; Castenschiold, J.H.F.; Gehrlein, J.B.; Laubek, B.; Jensen, L.F.; Pertoldi, C. Effect of Landscape Elements on the Symmetry and Variance of the Spatial Distribution of Individual Birds within Foraging Flocks of Geese. Symmetry 2019, 11, 1103. [Google Scholar] [CrossRef]
- Rahman, D.A.; Sitorus, A.B.Y.; Condro, A.A. From Coastal to Montane Forest Ecosystems, Using Drones for Multi-Species Research in the Tropics. Drones 2021, 6, 6. [Google Scholar] [CrossRef]
- Wen, D.; Su, L.; Hu, Y.; Xiong, Z.; Liu, M.; Long, Y. Surveys of Large Waterfowl and Their Habitats Using an Unmanned Aerial Vehicle: A Case Study on the Siberian Crane. Drones 2021, 5, 102. [Google Scholar] [CrossRef]
- Castenschiold, J.H.F.; Bregnballe, T.; Bruhn, D.; Pertoldi, C. Unmanned Aircraft Systems as a Powerful Tool to Detect Fine-Scale Spatial Positioning and Interactions between Waterbirds at High-Tide Roosts. Animals 2022, 12, 947. [Google Scholar] [CrossRef]
- Fettermann, T.; Fiori, L.; Gillman, L.; Stockin, K.A.; Bollard, B. Drone Surveys Are More Accurate Than Boat-Based Surveys of Bottlenose Dolphins (Tursiops Truncatus). Drones 2022, 6, 82. [Google Scholar] [CrossRef]
- Setyawan, E.; Stevenson, B.C.; Izuan, M.; Constantine, R.; Erdmann, M.V. How Big Is That Manta Ray? A Novel and Non-Invasive Method for Measuring Reef Manta Rays Using Small Drones. Drones 2022, 6, 63. [Google Scholar] [CrossRef]
- Dronova, I.; Kislik, C.; Dinh, Z.; Kelly, M. A Review of Unoccupied Aerial Vehicle Use in Wetland Applications: Emerging Opportunities in Approach, Technology, and Data. Drones 2021, 5, 45. [Google Scholar] [CrossRef]
- Wich, S.; Dellatore, D.; Houghton, M.; Ardi, R.; Koh, L.P. A Preliminary Assessment of Using Conservation Drones for Sumatran Orangutan (Pongo abelii) Distribution and Density. J. Unmanned Veh. Syst. 2016, 4, 45–52. [Google Scholar] [CrossRef]
- Witczuk, J.; Pagacz, S.; Zmarz, A.; Cypel, M. Exploring the Feasibility of Unmanned Aerial Vehicles and Thermal Imaging for Ungulate Surveys in Forests—Preliminary Results. Int. J. Remote Sens. 2018, 39, 5504–5521. [Google Scholar] [CrossRef]
- Lee, S.; Song, Y.; Kil, S.H. Feasibility Analyses of Real-Time Detection of Wildlife Using UAV-Derived Thermal and RGB Images. Remote Sens. 2021, 13, 2169. [Google Scholar] [CrossRef]
- Brunton, E.A.; Leon, J.X.; Burnett, S.E. Evaluating the Efficacy and Optimal Deployment of Thermal Infrared and True-Colour Imaging When Using Drones for Monitoring Kangaroos. Drones 2020, 4, 20. [Google Scholar] [CrossRef]
- Lethbridge, M.; Stead, M.; Wells, C. Estimating Kangaroo Density by Aerial Survey: A Comparison of Thermal Cameras with Human Observers. Wildl. Res. 2019, 46, 639. [Google Scholar] [CrossRef]
- Rahman, D.A.; Setiawan, Y.; Wijayanto, A.K.; Rahman, A.A.A.F.; Martiyani, T.R. An Experimental Approach to Exploring the Feasibility of Unmanned Aerial Vehicle and Thermal Imaging in Terrestrial and Arboreal Mammals Research. E3S Web Conf. 2020, 211, 02010. [Google Scholar] [CrossRef]
- Obermoller, T.R.; Norton, A.S.; Michel, E.S.; Haroldson, B.S. Use of Drones with Thermal Infrared to Locate White-tailed Deer Neonates for Capture. Wildl. Soc. Bull. 2021, 45, 682–689. [Google Scholar] [CrossRef]
- Howell, L.G.; Clulow, J.; Jordan, N.R.; Beranek, C.T.; Ryan, S.A.; Roff, A.; Witt, R.R. Drone Thermal Imaging Technology Provides a Cost-Effective Tool for Landscape-Scale Monitoring of a Cryptic Forest-Dwelling Species across All Population Densities. Wildl. Res. 2021, 49, 66–78. [Google Scholar] [CrossRef]
- Shewring, M.P.; Vafidis, J.O. Using UAV-mounted Thermal Cameras to Detect the Presence of Nesting Nightjar in Upland Clear-fell: A Case Study in South Wales, UK. Ecol. Solut. Evid. 2021, 2, e12052. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Asferg, T.; Forchhammer, M.C. Long-term Patterns in European Brown Hare Population Dynamics in Denmark: Effects of Agriculture, Predation, and Climate. BMC Ecol. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Misiorowska, M.; Wasilewski, M. Survival and Causes of Death Among Released Brown Hares (Lepus europaeus Pallas, 1778) in Central Poland. Acta Theriol. 2012, 57, 305–312. [Google Scholar] [CrossRef]
- Miljøministeriet Naturstyrelsen. Forvaltningsplan for Hare; Nationalt Center for Miljø og Energi, Aarhus Universitet: Roskilde, Denmark, 2013. [Google Scholar]
- Sliwinski, K.; Strauß, E.; Jung, K.; Siebert, U. Comparison of Spotlighting Monitoring Data of European Brown Hare (Lepus europaeus) Relative Population Densities with Infrared Thermography in Agricultural Landscapes in Northern Germany. PLoS ONE 2021, 16, e0254084. [Google Scholar] [CrossRef]
- Hacklander, K.; Schai-Braun, S. Lepus europaeus. In The IUCN Red List of Threatened Species 2019; IUCN Red List: Cambridge, UK, 2019; Available online: https://doi.org/10.2305/IUCN.UK.2019-1.RLTS.T41280A45187424.en (accessed on 15 May 2022).
- Pépin, D.; Angibault, J.M. Selection of Resting Sites by the European Hare as Related to Habitat Characteristics During Agricultural Changes. Eur. J. Wildl. Res. 2007, 53, 183–189. [Google Scholar] [CrossRef]
- Jensen, T.W. Identifying Causes for Population Decline of the Brown Hare (Lepus europaeus) in Agricultural Landscapes in Denmark. Ph.D. Thesis, National Environmental Research Institute, Aarhus University, Aarhus, Denmark, 2009. [Google Scholar]
- Moeslund, J.; Nygaard, B.; Ejrnæs, R.; Bell, N.; Bruun, L.; Bygebjerg, R.; Carl, H.; Damgaard, J.; Dylmer, E.; Elmeros, M.; et al. Den Danske Rødliste. 2019. Available online: https://ecos.au.dk/forskningraadgivning/temasider/redlistframe/roedliste-2019 (accessed on 20 May 2022).
- Asferg, T.; Clausen, P.; Christensen, T.K.; Bregnballe, R.; Clausen, K.K.; Elmeros, M.; Fox, A.D.; Haugaard, L.; Holm, T.E.; Laursen, K.; et al. Vildtbestand og Jagttider i Danmark: Det Biologiske Grundlag for Jagttidsrevisionen 2018. 2016. Available online: http://dce2.au.dk/pub/SR195.pdf (accessed on 15 May 2022).
- Sørensen, I.H.; Midtgaard, L. Notat vedr. Markvildtindsatsens Resultater 2013–2020; Danmarks Jægerforbund: Rønde, Denmark, 2021; Available online: https://www.jaegerforbundet.dk/media/16606/210108-ihs-lmi-notat-markvildt.pdf (accessed on 15 May 2022).
- Jægerforbund, D. Vejledning: Pattedyrstællinger. 2022. Available online: https://www.jaegerforbundet.dk/media/19412/t%C3%A6llevejledning_pattedyr_rev_2022.pdf (accessed on 15 May 2022).
- Sunde, P.; Jessen, L. It Counts Who Counts: An Experimental Evaluation of the Importance of Observer Effects on Spotlight Count Estimates. Eur. J. Wildl. Res. 2013, 59, 645–653. [Google Scholar] [CrossRef]
- Smith, R.K.; Jennings, N.V.; Harris, S. A Quantitative Analysis of the Abundance and Demography of European Hares Lepus europaeus in Relation to Habitat Type, Intensity of Agriculture and Climate. Mammal Rev. 2005, 35, 1–24. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UAS s) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Avola, D.; Cinque, L.; Fagioli, A.; Foresti, G.L.; Pannone, D.; Piciarelli, C. Automatic estimation of optimal UAV flight parameters for real-time wide areas monitoring. Multimed. Tools Appl. 2021, 80, 25009–25031. [Google Scholar] [CrossRef]
- Mesas-Carrascosa, F.J.; Torres-Sánchez, J.; Clavero-Rumbao, I.; García-Ferrer, A.; Peña, J.M.; Borra-Serrano, I.; López-Granados, F. Assessing optimal flight parameters for generating accurate multispectral orthomosaicks by UAV to support site-specific crop management. Remote Sens. 2015, 7, 12793–12814. [Google Scholar] [CrossRef]
- Chrétien, L.P.; Théau, J.; Ménard, P. Visible and Thermal Infrared Remote Sensing for the Detection of White-tailed Deer Using an Unmanned Aerial System. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Rahman, D.A.; Setiawan, Y. Possibility of Applying Unmanned Aerial Vehicle and Thermal Imaging in Several Canopy Cover Class for Wildlife Monitoring – Preliminary Results. E3S Web Conf. 2020, 211, 04007. [Google Scholar] [CrossRef]
- Psiroukis, V.; Malounas, I.; Mylonas, N.; Grivakis, K.E.; Fountas, S.; Hadjigeorgiou, I. Monitoring of Free-Range Rabbits Using Aerial Thermal Imaging. Smart Agric. Technol. 2021, 1, 100002. [Google Scholar] [CrossRef]
- DJI. DJI Pilot Android v2.5.1.3. 2021. Available online: https://www.dji.com/downloads/djiapp/dji-pilot (accessed on 20 March 2022).
- Burke, C.; Rashman, M.; Wich, S.; Symons, A.; Theron, C.; Longmore, S. Optimizing Observing Strategies for Monitoring Animals Using Drone-Mounted Thermal Infrared Cameras. Int. J. Remote Sens. 2019, 40, 439–467. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing, Version 4.0.3; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 May 2022).
- Esri. ArcGIS Pro 2.9.2. 2021. Available online: http://www.esri.com/ (accessed on 25 April 2022).
- Esri. Using the ArcGIS Full Motion Video 1.4 Add-In. 2020. Available online: http://www.esri.com/fmv (accessed on 25 April 2022).
- Python Software Foundation. Python 3.9.12. 2022. Available online: https://www.python.org/ (accessed on 25 April 2022).
- Steen, K.A.; Villa-Henriksen, A.; Therkildsen, O.R.; Green, O. Automatic Detection of Animals in Mowing Operations Using Thermal Cameras. Sensors 2012, 12, 7587–7597. [Google Scholar] [CrossRef]
- Seymour, A.C.; Dale, J.; Hammill, M.; Halpin, P.N.; Johnston, D.W. Automated Detection and Enumeration of Marine Wildlife Using Unmanned Aircraft Systems (UAS) and Thermal Imagery. Sci. Rep. 2017, 7, 45127. [Google Scholar] [CrossRef]
- Corcoran, E.; Winsen, M.; Sudholz, A.; Hamilton, G. Automated Detection of Wildlife Using Drones: Synthesis, Opportunities and Constraints. Methods Ecol. Evol. 2021, 12, 1103–1114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povlsen, P.; Linder, A.C.; Larsen, H.L.; Durdevic, P.; Arroyo, D.O.; Bruhn, D.; Pertoldi, C.; Pagh, S. Using Drones with Thermal Imaging to Estimate Population Counts of European Hare (Lepus europaeus) in Denmark. Drones 2023, 7, 5. https://doi.org/10.3390/drones7010005
Povlsen P, Linder AC, Larsen HL, Durdevic P, Arroyo DO, Bruhn D, Pertoldi C, Pagh S. Using Drones with Thermal Imaging to Estimate Population Counts of European Hare (Lepus europaeus) in Denmark. Drones. 2023; 7(1):5. https://doi.org/10.3390/drones7010005
Chicago/Turabian StylePovlsen, Peter, Anne Cathrine Linder, Hanne Lyngholm Larsen, Petar Durdevic, Daniel Ortiz Arroyo, Dan Bruhn, Cino Pertoldi, and Sussie Pagh. 2023. "Using Drones with Thermal Imaging to Estimate Population Counts of European Hare (Lepus europaeus) in Denmark" Drones 7, no. 1: 5. https://doi.org/10.3390/drones7010005
APA StylePovlsen, P., Linder, A. C., Larsen, H. L., Durdevic, P., Arroyo, D. O., Bruhn, D., Pertoldi, C., & Pagh, S. (2023). Using Drones with Thermal Imaging to Estimate Population Counts of European Hare (Lepus europaeus) in Denmark. Drones, 7(1), 5. https://doi.org/10.3390/drones7010005








