1. Introduction
1.1. Transport of Medical Supplies in a Hospital
The performance of the internal distribution of goods at a hospital has a considerable impact on the quality and efficiency of patient care [
1,
2]. However, healthcare logistics differ greatly from any other industry logistics because of clinical, material and information workflows [
1,
2,
3]. For example, hospital policies may impose different paths for certain transport services (e.g., certain corridors may be excluded for moving patients). Moreover, the delivery of specific materials may require precautions that constrain the flow (e.g., infectious substances). Indeed, internal hospital delivery includes a large variety of logistic flows each subject to different rules, procedures and routes. Hence, modern hospitals need to have an efficient infrastructure as well as operational organization supporting intra-hospital logistics of diagnostic samples and supplies [
1].
Currently, the transport of medical goods in a hospital heavily relies on dedicated human resources that carry the materials on foot. This mode of transport may prove inefficient because of inflexibility to a variable and hardly predictable demand, especially at particular times of emergency. Other problems of a human-based transportation system that remain to be addressed are: long walking distances, inefficient routing, elevator problems and communication issues [
2] and risk of nosocomial infections [
4]. Moreover, it was observed that logistics activities are often executed by clinical staff, subtracting time for patient care [
1].
A 2019 literature study identified Key Performance Indicators (KPIs) that are used specifically in internal hospital logistics to drive the strategy for a more efficient supply chain [
2]. The authors identified various clusters of KPIs for inventory management and distribution activities in hospitals (i.e., quality, time, financial and productivity). These KPIs allow for an objective assessment of the performance of distribution activities to address possible inefficiencies. It emerged that novel strategies to improve supply chain management should aim at the integration, standardization and coordination of the processes in order to maintain safety, affordability and availability of supplies.
1.2. Recent Advancements on Transportation of Medical Supplies with Drones
Unmanned aircraft systems (UAS), so-called ‘drones’, are increasingly revealing their potentially groundbreaking role in healthcare [
5,
6,
7,
8]. The majority of tests performed during the last few years regarded the use of UAS to deliver medical supplies (e.g., blood products, automated external defibrillators, drugs, vaccines and diagnostic samples). UAS started to reveal their usefulness for medical delivery from and to remote areas with poor road infrastructure and for emergency response [
6,
9,
10]. The main upsides of drone applications found in these early experiences were the efficiency in time-critical situations, lower CO
2 emissions and saving costs related to road transport [
9]. Experimentations frequently took place in developing countries, in which not particularly stringent regulations facilitated more extensive and economical experimental campaigns [
11].
Conversely, drone routine applications by healthcare organizations above densely populated areas and in congested airspace, such as the Milan metropolitan area, introduce specific criticalities. In fact, technological shortcomings, social acceptance, urban planning and the demonstrability of operations’ safety in densely populated areas constitute major challenges for the integration of UAS technology in the digital and physical infrastructure of the urban environment [
12], as well as for safe coexistence with other air traffic.
1.3. The Concept of Urban Air Mobility
After the signature of the Chicago Convention in 1944, the International Civil Aviation Organization (ICAO) has been the central pillar for safe, secure, economic and efficient air navigation on the global scale. In fact, Art. 44 of that Convention clearly limits the role of the ICAO to standardization only of international flights, among which long-range commercial air transport (CAT) by fixed-wing airplanes is the most prominent service [
13]. Consequently, across the decades, the ICAO has never standardized domestic aviation activities, not relevant on the global scale, such as gliders, aerostats, model aircraft and aerial work through manned aircraft. Therefore, at the current time, the ICAO is standardizing only Remotely Piloted Aircraft Systems (RPAS) when flying along long-range international routes in controlled airspace under Instrument Flight Rules (IFR) and at heights higher than 500 ft (150 m) Above Ground Level (AGL). Nevertheless, ICAO contracting States have the duty of regulating all other domestic drone activities because these flights may, anyway, endanger international commercial aviation, in particular during the departure and arrival phases of flight. However, no specific ICAO standards apply to such domestic drone operations.
Nowadays, small drones are proliferating at heights below 500 ft AGL, which are suitable for several applications, including short range local transport of pharmaceuticals and biological samples. Furthermore, industry is developing an entire range of new aircraft, manned or unmanned, among which are gyroplanes, which can also travel along roads in the same way as cars, electrically powered multi-rotor aircraft capable of Vertical Take-Off and Landing (eVTOL), which can be ‘manned’ (i.e., with the pilot on board) or remotely piloted.
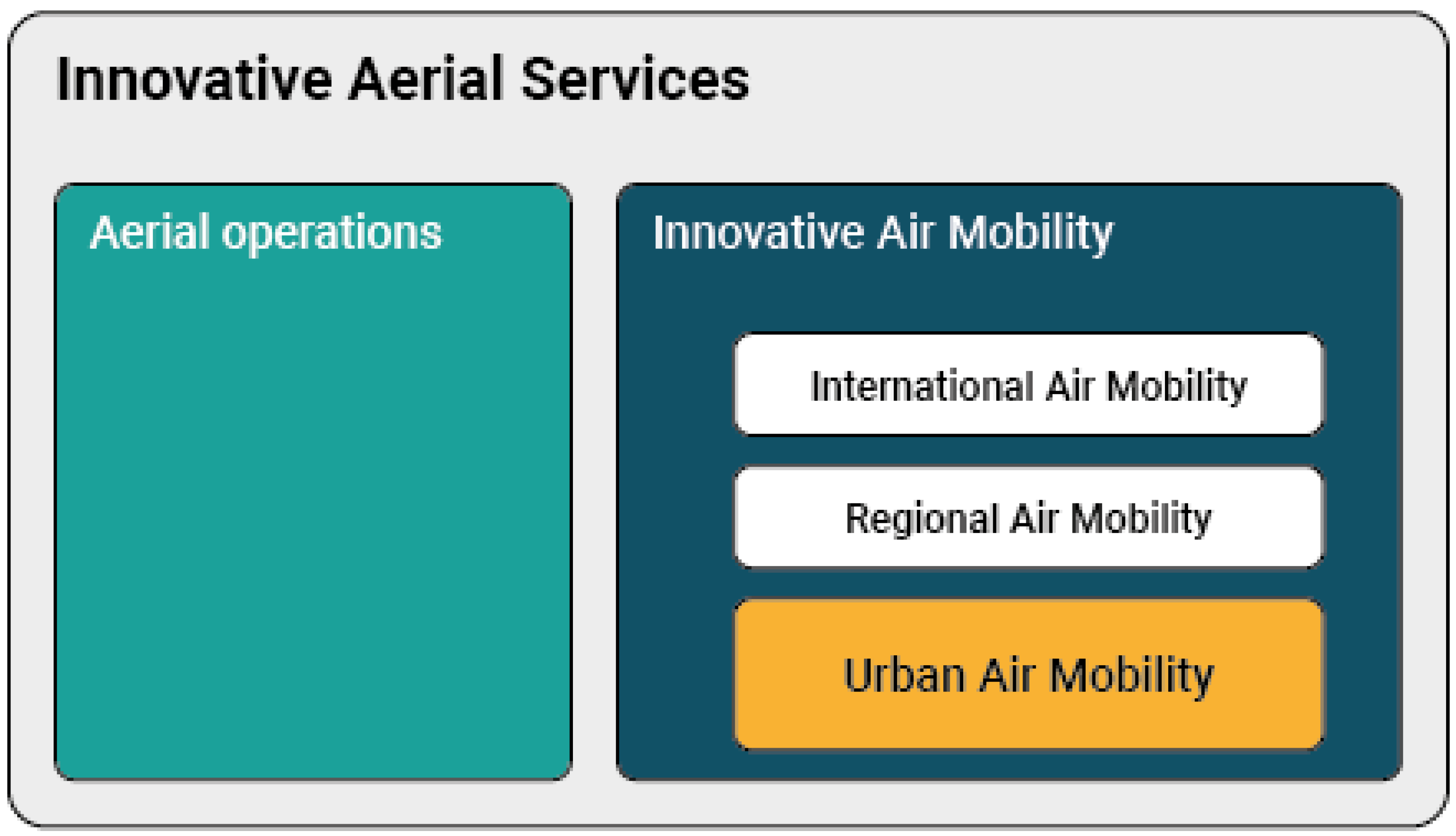
Taking the latest developments into account, the European Union Aviation Safety Agency (EASA) has developed a Concept of Operations (CONOPS) for these Innovative Aerial Services (IAS) based on new airborne technologies, enabling new services beneficial for society. As shown in
Figure 1, these services include new types of air operations (e.g., specialized operations in agriculture), but also Innovative Air Mobility (IAM). The latter includes a new generation of technologies enabling safe, secure and sustainable air mobility of passengers and cargo, likely integrated into a multimodal transportation system, whose backbone would no longer be the vehicle but a digital ‘ecosystem’ in which all vehicles and several other digital actors will be integrated. IAM comprises international air transport (subject to ICAO provisions), but also regional flights on a shorter range, as well as Urban Air Mobility (UAM), which is the subset of IAM operations conducted into, within or out of urban environments, using manned or unmanned aircraft.
UAM is expected to unburden road transport in the future by means of eVTOL, new aircraft types and even small drones for goods transport. All these air operations would rely on a high level of automation and exploitation of digital technologies. As said above, EASA has already acknowledged that these operations should be integrated into a multimodal transportation system and into logistic systems, which are also becoming more and more based on the digital exchange of data and information. In other words, while certain domains (e.g., airworthiness of the aircraft to avoid crashes on the ground or prevention of airborne collisions) will remain in the exclusive realm of aviation, Information Technology (IT) entities will interact among them well beyond aviation, in the so-called digital ‘ecosystem’.
The project Flying Forward 2020 (FF2020), funded by the Innovation and Networks Executive Agency (INEA), is one of those involved in the development of IAM, in fact with particular attention on the digital ecosystem. The architecture of such an ecosystem is based on ISO DIS 23629-5 [
14], which encompasses:
- (a)
An indefinite number of ‘actors’ (i.e., IT entities) digitally interacting in the ecosystem;
- (b)
The 30 possible UAS Traffic Management (UTM, alias U-space) services listed in ISO DIS 23629-12 [
15];
- (c)
An indefinite number of users of the services, several of which are not involved in the execution of drone flights (e.g., the Police for law enforcement, municipal authorities and ‘geozone’ managers, final customers such as hospitals and pharmacies, aviation authorities, etc.);
- (d)
The most prominent users are however the IT entities under the managerial control of the UAS operator, which mainly include:
The work position of the Fleet Manager, which is potentially active 24/7 and which interacts with all other IT entities necessary to plan the flight (e.g., SAMWISE tool for Risk Analysis Assistance) as well as with the final customer (e.g., the pharmacy requesting the delivery of a medicine);
The Command Unit (CU) of the Remote Pilot (RP), which is active only from pre-flight to post-flight;
The UAS itself, which during the flight and only during the flight, exchanges digital data and information with other IT entities.
One may observe that in this perspective the drone becomes not only part of aviation, being an aircraft, but also an entity in the Internet of Things (IoT) able to exchange data without human intervention (e.g., with the vertiport).
Project REALITY, funded by the European Union Agency for the Space Programme (EUSPA), has, in addition, demonstrated that small fixed-wing airplanes at low level may reach a Required Navigation Performance (RNP) equal to 0.02 Nautical Miles (NM), 37 m, in the horizontal plane, with sufficient integrity ensured through EGNOS, as necessary to design narrow ‘corridors’ for such drones in urban environments, which are of course obstacle rich. The same project has demonstrated that small multicopters may reach RNP 0.01 (18.5 m), which allows designing even narrower ‘corridors’.
Project BUBBLES, funded by Single European Sky ATM Research Joint Undertaking (SESAR JU), is using the findings of other EU funded projects, to define the separation minima along such corridors or laterally between two adjacent corridors.
Among several other projects underway to develop IAM/UAM, ICARUS, equally funded by SESAR JU, is developing the UTM services listed in said ISO DIS 23629-12, enabling both legacy manned aircraft (flying using barometric altimeter) and drones (using satellite geodetic height) to base vertical separation on a Common Altitude Reference System (CARS), implementable also in a portable Electronic Flight Bag (EFB = no retrofit of any equipment on legacy aircraft).
1.4. Regulations concerning UAM in Europe and in Italy
The European Union (EU) is today the region of the world having the most comprehensive, although not 100% complete, set of regulations for UAS, UTM and UAM.
In fact, the EU common rules are ‘performance-based’ which, inter alia means widely relying on consensus-based standards developed by industry, keeping the legally binding rules, as much as possible, ‘technology-agnostic’. This approach, hence, allows industry to propose new solutions, which, following the established regulatory processes, might be implemented without requiring amendment of the rules. The common EU rules on the matter are also ‘risk based’, meaning that the approval processes are simpler, or even non existing, for operations entailing a lower risk for society.
Drones are subject to all applicable EU legislation, e.g., on liability and insurance or on privacy and data protection. However, from the perspective of aviation safety, the main acts are:
- (a)
Commission Delegated Regulation 2019/945 allowing ‘Notified Bodies’ and manufacturers to verify the airworthiness of a small drone, without involvement of either EASA or the national aviation authority [
16];
- (b)
Commission Implementing Regulation 2019/947, which allows UAS operations in the open category (i.e., no approval by the aviation authority), but subject to strict limitations established in legally binding rules and, conversely, more complex operations in the specific category, without prescribed limitations, but subject to safety risk assessment, which allows industry to progressively implement more ambitious solutions [
17];
- (c)
Commission Implementing Regulation 2021/664 mandating certification of six safety-critical UTM services by aviation authorities [
18], while for the remaining 24 safety-related or operation support services, listed in DIS 23629-12, ISO certification could suffice;
- (d)
Commission Implementing Regulation 2021/666, which requires manned aircraft, when flying into U-space airspace, to also be ‘electronically conspicuous’ [
19].
However, the mentioned set of common EU rules neither covers authorities beyond aviation, nor State flights, or use of the airspace. For the latter, in fact, Art. 15 of 2019/947 tasks EU Member States (MS) to establish their own rules and airspace structures. In these domains, therefore, EU MS have issued several national regulations, among which, in the opinion of the authors, the most relevant include:
- (a)
The Decree of the Belgian Minister for Mobility of 21 December 2020, which not only established ‘geozones’ for the flight of drones, but also institutionalized the role of the ‘geozone manager’, not belonging to aviation, but still a key actor for UAM;
- (b)
The 1st edition of the Regulation UAS-IT of 4 January 2021, adopted by the Italian aviation authority (ENAC), which regulates the vast majority of UAS non-military State flights, through rules modelled on the EU Regulation 2019/947;
- (c)
ENAC Circular ATM 09A, based on Art. 15 of said Regulation 2019/947, which establishes conditions to operate small drones in the close vicinity of airports (e.g., Linate).
All the mentioned regulations, however, do not yet cover the case of carrying passengers on board eVTOL for the purpose of IAM. This gap should soon be filled since EASA is planning to issue a couple of Notices of Proposed Amendment (NPA) in 2022 for UAS operations in the certified category, the only one for which a formal Remote Pilot License issued by the authority and a certificate for the organization of the operator are mandatory. Operators may possibly take credit from ISO certification based on standard 21384-3 [
20].
1.5. Objectives
Based on the context presented in the previous sections, the objective of this study is to design a state of the art drone service for a hospital urgent pharmaceutical distribution that satisfies (i) users’ needs, (ii) safety requirements and (iii) compliance with rules, thus taking into account the principles of UAM under development by international and European organizations. The scope of the current study focuses on the urgent transport of pharmaceuticals from the pharmacy deposit to the Operative Unit (OU) of San Raffaele Hospital, a large size Scientific Institute for Research, Hospitalization and Healthcare based in Milan, Italy.
2. Materials and Methods
For the design of the drone service for hospital internal delivery of urgent demand of pharmaceuticals, a co-creation methodology was adopted, which can be described as a mix of participatory activities from multiple stakeholders and users.
Such a methodology follows the five principles of service design thinking [
21] to organize resources and planning the project activities. The authors recommended the process to design a new service being (i) user-centered and (ii) co-creative, implying the necessity of the knowledge about how consumers or users feel about the service and the proposed experience by involving them and all the relevant stakeholders in the design process. Hence, the authors suggested (iii) sequencing the service by dividing it in key moments and sequences, and (iv) evidencing it by using visual tools allowed for a better understanding of the service and encouraging feedbacks about the most important matters to the consumers and the reasons behind them. Finally, it was recommended that the process to design new services was (v) holistic, i.e., taking into careful consideration multiple aspects and perspectives of the context hosting the new service to obtain a deeper comprehension of it.
Before applying the co-creation methodology, a ground layer of information was needed. Therefore, desk research was conducted with the aim of collecting necessary information to understand the context and the stakeholders involved in the drug internal distribution process in a hospital and with the use of drones.
In the following phase, the analysis of the collected information led (i) to the definition of a healthcare logistics blueprint that accurately outlined the current service flow in detail (called As Is) and (ii) to the design of a service that employs UAS and the recent concept of UAM (called Aspirational).
Thereafter, two interviews were conducted with prospective users of San Raffaele Hospital: one with the pharmacy manager and one with the nursing staff of one of the Hospital Operative Units (OU). The main objective of this phase was the validation of the service designed in the preceding analysis.
Table 1 shows a scheme of the research design of the current study. The following sections describe each phase in more detail.
2.1. Research
The research phase regarded three subjects: (i) the current hospital OU drugs distribution process, (ii) the study of UAS technologies used for commercial logistic services and, more specifically, for medical logistic services, and (iii) concepts recently developed in the field of UAM to perform safe and compliant routine UAS operations.
San Raffaele Hospital’s internal documentation was consulted to study the hospital OU drug supply process and to identify the workflow phases, the touchpoints and the involved stakeholders.
The second phase of the research was focused on unmanned aircraft technology. In order to better understand the UAS technology used for commercial logistic services in healthcare use cases, market research was conducted on a variety of UAS delivery services that were in use or tested around the globe.
Finally, applicable and upcoming European Regulations and international, European and industry standards were considered to produce a functional scheme of the interfaces among the UAM actors revolving around the designed service to support its safety and scalability. Among the applied standards developed by industry, one may mention ISO 21384-3:2019 for the organization of the operator, DIS 23629-5 for the digital architecture and DIS 23629-12 to identify which UTM services are already available (e.g., electronic registration through d-flight and Risk Analysis Assistance through EuroUSC Italia).
2.2. Analysis
The desk research on hospital documentation regarded each step of the process, the relationships among the users, the employed tools, estimates of the time to fulfil each task and the matrix of data necessary to understand the requirements of the service (e.g., the size of the requested packages). This work enabled the outline of a service blueprint that explained the current drug internal distribution process in detail, i.e., the As Is scenario.
By designing the blueprint, the stakeholders involved in the process were identified, both those in the front office and those in the back office. This enabled the design of a stakeholder map, which put each stakeholder in the perspective of their impact on the system under study. The authors adopted the following criteria to classify the different actors in four tiers, from the inner ones of high interest to outer tiers of lower interest. The first, inner tier hosted users directly involved in the designed service. The second tier regarded staff that worked in the back office or in the pre-service actively supporting the process. The third tier encompassed the stakeholders that impacted or were impacted by the service. Finally, the fourth, outer tier grouped less directly involved stakeholders.
The research about UAS technologies was the foundation used to design a process flow of a UAS delivery service considering all the service touchpoints and the employed components, starting from the order placement to the order collection. Designing that process flow, the respective components and the involved users generated the ground knowledge to understand the main factors that needed to be considered when scaling a UAS delivery service in the reality that is the focus of the current study. A blueprint of the designed Aspirational service was produced and the stakeholder map was updated to include new involved entities.
Alongside the design of the service, information useful for the development and implementation of the needed technology was included, i.e., a hierarchy of KPIs for the considered case study, a set of constraints for the service implementation and additional requirements for the rollout of the conceptualized digital system.
Finally, exploring concepts of UAM disclosed elements (i.e., actors and external services) related to the pre-service and the back office that allowed for a safer implementation of a routine UAS service. As a result, a functional scheme of all the main actors of the envisioned UAM ecosystem and their interactions was produced.
2.3. Validation
The objective of this phase of the study comprised the following goals: first, to validate the researchers’ understanding of the current process flow; secondly, to identify unmet needs of prospective users; finally, to receive feedback on the designed drone service.
Based on the insights produced during the analysis phase, the tools used during interviews with prospective users were produced: a low-definition service blueprint in form of a storyboard of the current drug logistics and one of the designed UAS drug internal distribution service, with the respective stakeholder maps. Moreover, an interview script was defined to facilitate the interviews.
The pharmacy staff was the first group and the OU staff was the second group of prospective interviewed users. Each interview was divided in two phases: first, the validation of the As Is, and secondly, the examination and validation of the Aspirational service. The material used to structure the first interview allowed the researchers to update the blueprints with a more accurate workflow discussed in the second interview. Moreover, the script created for the nursing staff was almost the same used for the pharmacy staff, with more focus on the software used to place a drug order and questions on the functional and non-functional requirements of the service.
In order to stimulate interaction in the interviews, all the produced design tools were placed on a table, along with post-its and markers to ‘set the stage’ and stimulate the interaction. The interviews started with the presentation of the storyboard of the current internal distribution of pharmaceuticals from the formulation of the request from the clinical staff to the successful delivery to the OU. Each process phase, the respective touchpoints and actors were analyzed by asking questions if something appeared to be missing in the flow. Corrections and modifications on the flow were applied and, together with the interviewees, pain points and gain points were defined along the process.
At the end of the first phase of the interview, the researchers showed the stakeholder map of the As Is service. During the two interviews, the map was analyzed with the pharmacy staff and with the nursing staff. Consequently, the relationships between the stakeholders and their roles were adjusted according to their inputs. For example, additional stakeholders that were not previously considered emerged, while others were cancelled or prioritized differently.
The second phase of the interviews focused on the presentation of a storyboard of the Aspirational drone delivery service that was prototyped on the analysis phase. This storyboard, with the related stakeholder map, was a visualization tool intended to make the future service more understandable and to solicit feedback and observations about each process phase and the overall process. Such description considered each process phase individually, describing the differences between the new and the current process, including new stakeholders and touch points. This meetings with the users allowed the researchers to define pain points and limitations of the Aspirational service.
3. Results
The process flows of the internal distribution activities for urgent pharmaceutical delivery, i.e., healthcare logistics blueprint, is shown in detail in the
Supplementary Material to this article. The following sections describe the
As Is scenario and its limitations, and the
Aspirational scenario, i.e., the process of the designed service. The analysis of regulations and international standards allowed for the definition of the UAM ecosystem needed to support the operations’ safety and compliance with rules. Finally, the roles of the users and the involved entities are shown in the stakeholder map.
3.1. “As Is” Healthcare Logistics Blueprint
The interviews with the pharmacy and the OU staff focused on the ‘urgent delivery’, i.e., drugs that need to be delivered in a relatively short amount of time (from 30 min up to 4/5 h) out of the ordinarily scheduled medicine delivery. Such a need may occur on multiple occasions in one day for an OU, e.g., if the need of a medicine exceeds the limits set for ordinary delivery of that medicine, or if new inpatients need a pharmaceutical that is out of the ordinary for that OU. This can typically occur for certain categories of medicines, such as chemotherapeutics, diuretics, special antibiotics, antiblastics and anti-rejection drugs. Some of these medicines can be highly priced or prepared for the specific patient, hence, they can require careful management.
In the current state, the urgent delivery is managed by the hospital pharmacy and the single OU that together decide on the delivery options on a case-by-case basis. In fact, there are three urgent delivery options: (i) pneumatic tube, (ii) traditional urgent delivery and (iii) personal pickup. The pneumatic tube represents the quickest way to transport medicines to an OU, although it presents a few limitations. For example, this option may not be viable for certain pharmaceuticals (e.g., larger packages, fragile compounds), and it may often be too burdened to be used by the pharmacy since it is used by the majority of the hospital’s OUs for the delivery of diagnostic samples. Moreover, the buildings of recent additional constructions for the care of COVID-19 patients in San Raffaele Hospital are not served by the pneumatic tube, whose layout is quite rigid. When the pneumatic tube is not viable, the second option is traditional urgent delivery, in which hospital logistic operators collect drugs from the pharmacy and deliver them to all the OUs in a hospital building. However, the logistic flow of urgent deliveries is pre-determined and the orders are fulfilled at a pre-set time of the day (e.g., at 4 pm). Moreover, during high demand of this service, delivery staff may not be available in time for the needed drugs. In that case, personal pickup by the OU staff is the most responsive delivery option but diverts human resources from clinical activities to pick up drugs at the hospital pharmacy. This may of course disrupt patient care. The second disadvantage of this modality, highlighted by the interviewees, was the low traceability of the product, which can result in failure of the delivery.
The number of urgent requests to the pharmacy can reach over 50 units in a day, especially concentrated between 11 am and 4 pm. In fact, after 4 pm the delivery staff bring the medicines ordered before 11 am with the ‘urgent’ option to the OUs. The delivery options for all the medicines ordered after that time require a case-by-case negotiation between the pharmacy management and the OU.
In
Table 2, the specifications of the cargo in terms of weight and range of dimensions are provided.
Our results indicated that the personal pickup delivery option is the most inefficient since it occasionally requires the use of precious clinical human resources to transport a single product. However, this option is often necessary when the pharmaceuticals are most urgent. The
As Is scenario (shown in
Figure S1 of Supplementary Material) refers to this delivery option and outlines the workflow from the order placement to the order delivery and filing.
Workflow of the As Is Scenario
The current logistic flow starts with the OU coordinator who needs specific pharmaceuticals out of the ordinary delivery schedule by the end of the day. To address this need, they directly call the pharmacy management staff for a rapid negotiation and possibly an order placement. After the negotiation with the pharmacy management staff and their approval, the OU coordinator can proceed with the coordination for the personal pickup. Hence, the OU coordinator asks one of the nurses or healthcare collaborators to personally go to the pharmacy to collect the needed drugs. In the meantime, the pharmacy management staff contact the pharmacy coordinator to warn them about the placed order and the personal pickup procedure.
While the OU healthcare collaborator directs to the pharmacy, the OU coordinator proceeds with the order placement into the Hospital Management Software. This consists of the selection of the urgent delivery option and the drugs that were previously approved by the pharmacy management staff during the call. The order placed by the OU coordinator is received by the pharmacy management staff to be approved digitally. The pharmacy management staff check the order and, after approving it, send the order directly to the pharmacy in order to be processed.
When the order arrives at the pharmacy, the pharmacy coordinator prints the delivery note and entrusts the drugs’ collection to one of their collaborators before the OU collaborator arrives.
The OU collaborator arrives at the pharmacy, collects the drugs from the pharmacy collaborator and signs the delivery note. Thereafter, the pharmacy collaborator brings back the signed delivery note to the pharmacy coordinator, who archives it. In the meantime, the OU collaborator goes back to the OU with the ordered lot.
3.2. ‘Aspirational’ Healthcare Logistics Blueprint
In the Aspirational scenario, the hospital would rely on a drone delivery service that can either be run by an external commercial drone operator (a contracted company that is legally responsible for the drones’ activities) or be a part of the hospital internal assets (i.e., the hospital would also be the drone operator). In this scenario, two actors who are part of the drone operator’s organization are introduced: the Fleet Manager (FM), who allocates the resources (crew and aircraft) for the operations, and the Remote Pilot (RP), who is in charge of the flight execution.
The delivery service is run by highly automated drones, which would have pre-installed routes or employ an AI-based software to calculate the best trajectories in terms of safety and efficiency. The trajectories would be from the drone base to the loading point in the proximity of the pharmacy warehouse (that ideally could coincide in a single vertiport), and then from the loading point to the landing pad installed at the floor of the point of care. After the delivery the drone would fly back to base. Drone delivery is one of the options that the OU staff can select for a drug order placement through the Hospital Management System. The users that are directly involved with the delivery have a dedicated software installed on their workstation or smartphone (i.e., a ‘Drone Delivery App’) that allows them to interact with the service: before the operation, the FM informs the users through the Drone Delivery App about the drone availability and delivery pickup time and place. During the operations, the users can monitor the state of the delivery consulting the same software and send confirmation of loading and unloading. This scenario would also require that the regular hospital staff involved with the drone operations are adequately trained, e.g., to ensure that the area is clear of people or animals that may be injured by the drone before loading/unloading confirmation. Moreover, to establish a shared terminology for clear communications to report any issues to the crew or for the crew to effectively instruct the hospital staff in case of need would be a good practice.
The
Aspirational blueprint can be graphically consulted in
Figure S2 of the Supplementary Material. The flow that was produced regards the process from order placement to the order delivery and filing, and involves drone operations that were previously deemed safe, compliant with rules and authorized by ENAC (i.e., the Italian Civil Aviation Authority, or CAA) in the pre-service phase.
3.2.1. Workflow of the Aspirational Scenario
The flow is triggered by the OU Coordinator who, during an emergency, needs a rapid delivery of a specific pharmaceutical. From their workstation, they select the urgent drug delivery option with the preference via drone and place the order with the needed drugs through the Hospital Management Software.
The pharmacy management staff receive the order and forward it to the pharmacy. Then, the pharmacy coordinator prints the delivery note and assigns it to one of their collaborators. While the collaborator collects the drugs listed in the delivery note, the pharmacy coordinator places the drone delivery request by using the Drone Delivery App.
The drone delivery request reaches the FM, who checks the drone’s and RP’s availability and allocates the resources for the operation. A notification confirms the drone availability and identification to the pharmacy manager and their collaborator. The latter reaches the drone base with the ordered drugs and the delivery note, which they load on the delivery container attached to the drone. By using the Drone Delivery App, they confirm that the drone has been loaded.
The RP performs the pre-flight checklist to ensure drone serviceability and safety (i.e., authorization to fly, assessment of weather conditions, consultation of available UTM services, check that drone parts are intact and unobstructed, battery charge, and possible communication interferences) and then initiates the flight. The drone turns on the motors, rises in altitude and flies headed to the OU pickup point by following pre-defined trajectories under the RP’s supervision. A notification of the Drone Delivery App informs the OU collaborators of the drone proximity. The nurse waits for the drone to land, unloads it and then confirms the drone unloading through the Drone Delivery App. The confirmation triggers the assigned drone, which automatically turns on the motors, rises in altitude and flies back to the base notifying the users involved in the delivery that the drugs have been delivered. When the drone lands at the drone base, a notification informs the FM about the completed delivery and the RP performs the post-flight checklist.
In summary, drone delivery would be just one more option available to the OU to request medicines. Should it prove effective and efficient, the service might be scaled up with more drones and more RPs. In any case, the pivotal point connecting the drone and the digital ecosystem would be the FM, whose work position may eventually be staffed 24/7.
3.2.2. Hierarchy of KPIs for the Urgent Intra-Hospital Transport of Medicines
A hierarchy of KPIs for intra-hospital urgent delivery of pharmaceuticals was developed on the bases of prior research [
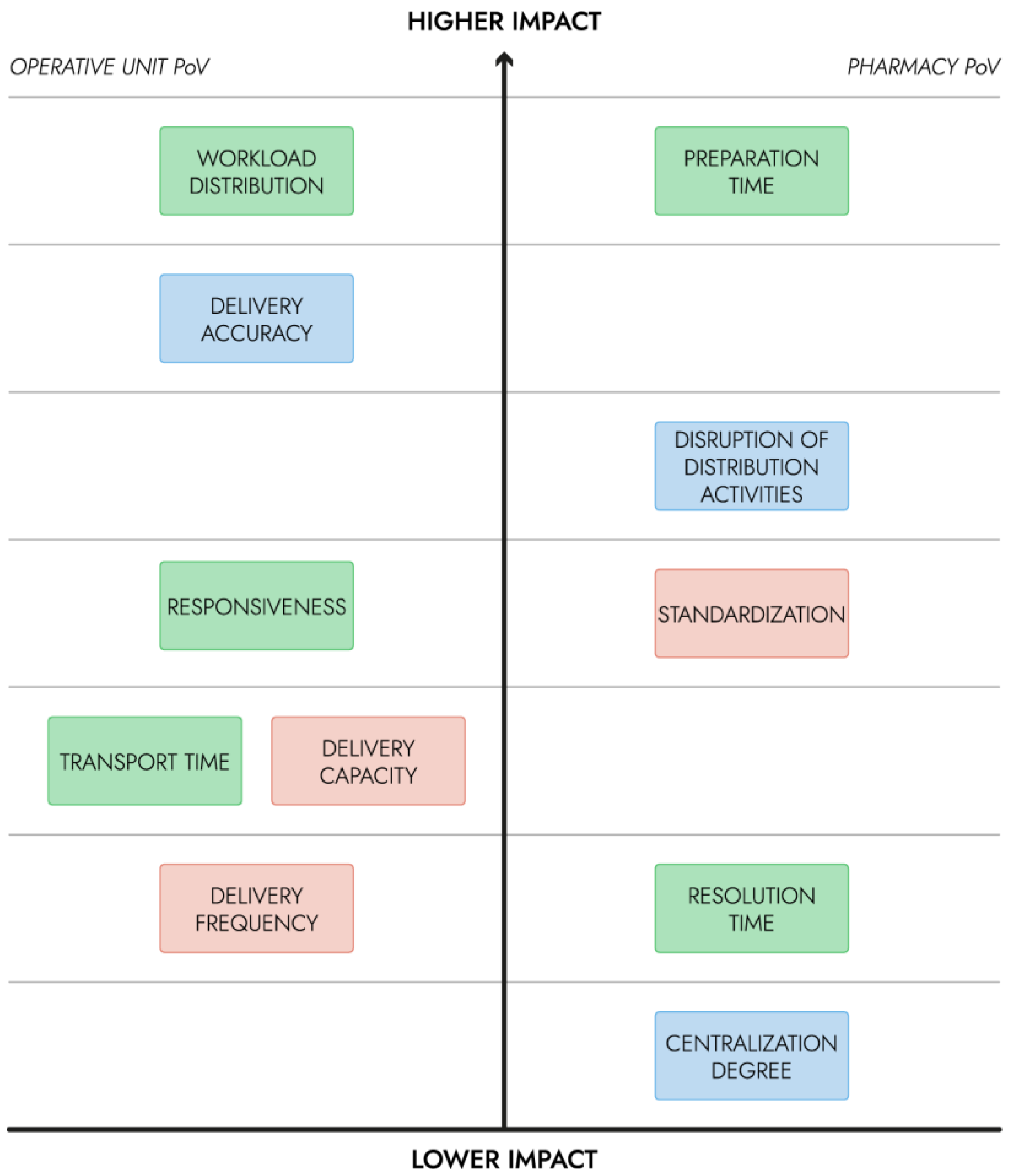
2]. In the current study, ‘responsiveness (on-time delivery)’ was subdivided in ‘preparation time’ and ‘transport time’ to address the specificity of the investigated scenarios. Responsiveness is a measure of the overall time from the start (i.e., intention of submitting an order) to the availability of the ordered products for the OU that made the request. Preparation time is measured from the start to the loading of the cargo. Transport time is the time employed to move the products from the pharmacy to the OU. Resolution time was added by the current study to measure the period needed in order to be able to respond to the next request of the same kind with the same resources after the previous order was fulfilled. This measure has no influence on the single delivery, but it is useful to assess the continuity of the running service.
The hierarchy was created to guide the development and the correct scalability of such a service for a hospital of medium to high dimensions (e.g., for the correct sizing of the drone fleet, drone and charging requirements and delivery container requirements). The prospective benefit for the main users was the main factor that was considered in the KPI hierarchy. The financial constraints of the ‘distribution cost’ KPI were considered separately. In
Figure 2, the hierarchy of KPIs is shown: those in higher positions are the ones that are expected to have a greater impact on the users, and consequently on patient care. On the left side, the KPIs with greater impact relatively to the OU staff’s point of view (PoV) were reported, while those having a greater impact on the pharmacy users were reported on the right.
The developed hierarchy pertains to the specific use case tackled by the present study. The designed service would be integrated into the pharmacy’s daily activities, hence the KPIs concerning the efficiency and the accuracy of logistics activities should have the highest priority. In the current study, the decrease in the employment of the clinical staff on non-clinical activities is considered the main advantage. Hence, the workload distribution is measured as the time spent on the delivery activity by the clinical staff and has the highest priority, as they would be significantly affected by the introduction of the drone service. Preparation time has the highest impact on the pharmacy staff’s workload, and potentially on other OUs that may make an order.
KPIs on lower ranks would have a low to moderate impact; in fact, the use case revolves around medicines that are needed out of the ordinary schedule and may be required by the OU in a few hours, hence, responsiveness and delivery frequency were not highly prioritized. In
Table 3, the KPIs of the time category identified by [
2] and introduced by the current study are estimated for the
Aspirational scenario and compared to those of the
As Is scenario.
3.2.3. Constraints for the Drone Service Implementation
The ‘distribution cost’ KPI was considered separately to address the problem of the financial constraints for the introduction of the designed service. Since it was not conceived with the purpose of generating profit, but rather to improve the hospital’s efficiency and healthcare outcomes, that are hardly quantifiable economically, outsourcing such a service may not be the most convenient choice. In fact, the gain may be expressed in terms of innovation, time-saving for the transport and higher patient care. Moreover, the present use case involves simple flights of one or two drones in the airspace limited by the hospital’s perimeter. Hence, it would be feasible for a hospital to manage the drone service in-house. This arrangement would involve facing initial costs tied to the certification of the organization as a drone operator and the operative costs for one or two employees that execute the RP’s and the FM’s functions. Additional initial costs to be considered are the purchase of the drone fleet, of appropriate delivery containers, of the physical infrastructure (e.g., landing pads on the OUs floors, bird nests for drone automatic recharge) and of the IT platform (which includes the fleet management software and the Drone Delivery App). Operative costs that need to be considered are the employees’ training and costs tied to the regulatory compliance. Marginal costs are those for electricity and internet services (which can be cut down by properly ensuring wireless networking coverage on the flight corridors).
In order to ensure the service’s reliability, it is appropriate to ensure that the drone fleet is always charged to be responsive to a new request. To that extent, the use case constraints to be considered are: (i) the peak frequency of delivery requests, (ii) the average distance and (iii) the average weight cargo. From these factors, it is possible to select the most convenient technical specifications for the drone fleet, including: the number of drones, the drone autonomy, the drone delivery speed and the drones’ batteries recharge rate. Different strategies can be applied: the staff can have charged batteries available when needed, or in more advanced scenarios, bird nests for automated drones’ recharge can be envisaged.
For the following evaluation, the researchers exemplified the use case with a DJI Matrice 300. Such a choice is due to the readily available technical data and the widespread use of DJI drones. A drone autonomy of 30 min was considered because, according to the DJI official data, this is the drone autonomy with the maximum loadable weight (i.e., 2.7 kg), that must also include the delivery container [
22]. A flight speed of 3 m/s can be assumed for safety. By estimating that the average flight path distance from the drone base to the OU and back would be approximately 500 m at San Raffaele Hospital, a delivery would require the drone less than 3 min of activity in total. Such a timespan does not take into account the time spent in the loading and the unloading phase because the drone would be turned off. It can be concluded that a drone could safely perform at least ten deliveries with a pair of batteries. The battery recharger of the considered drone model can recharge two pairs of batteries (for two battery changes) in almost 60 min. Considering that a drone can be busy in the operations for a delivery for 11 to 20 min (see
Figure S2), the time for battery charge is sufficient for the activities of a single drone.
The design of the solution must also take into consideration the risks linked to the loss of regulated and/or expensive pharmaceuticals. Such risks were identified and evaluated based on the respective probability and impact levels. For each, the authors identified features of the service that would offer acceptable mitigations to those risks (see
Table 4). Likelihood was assigned low if it was estimated to be a rare event (i.e., lower than 0.1%) and medium if it may happen in between 0.1% and 1% of the cases. Impact was considered low when the consequence of a case was a minor delay of the delivery, medium if the case would result in a longer wait for the medicine (from 30 min to hours) and high when the consequence would be the loss of a medicine and/or potential damage to bystanders or hospital assets. The Drone Delivery App could facilitate risk management by including an ‘error reporting’ feature for all the users involved to request the intervention of the relevant actors.
3.2.4. Requirements for the Rollout of the Drone Delivery App
As emerged from the prioritization of KPIs for the present use case (see
Figure 2), the main priority is given to the minimization of the time spent on delivery activities, both for the pharmacy and the OU staff. Hence, the digital resources allowing for accurate and responsive exchange of information has a central role in the
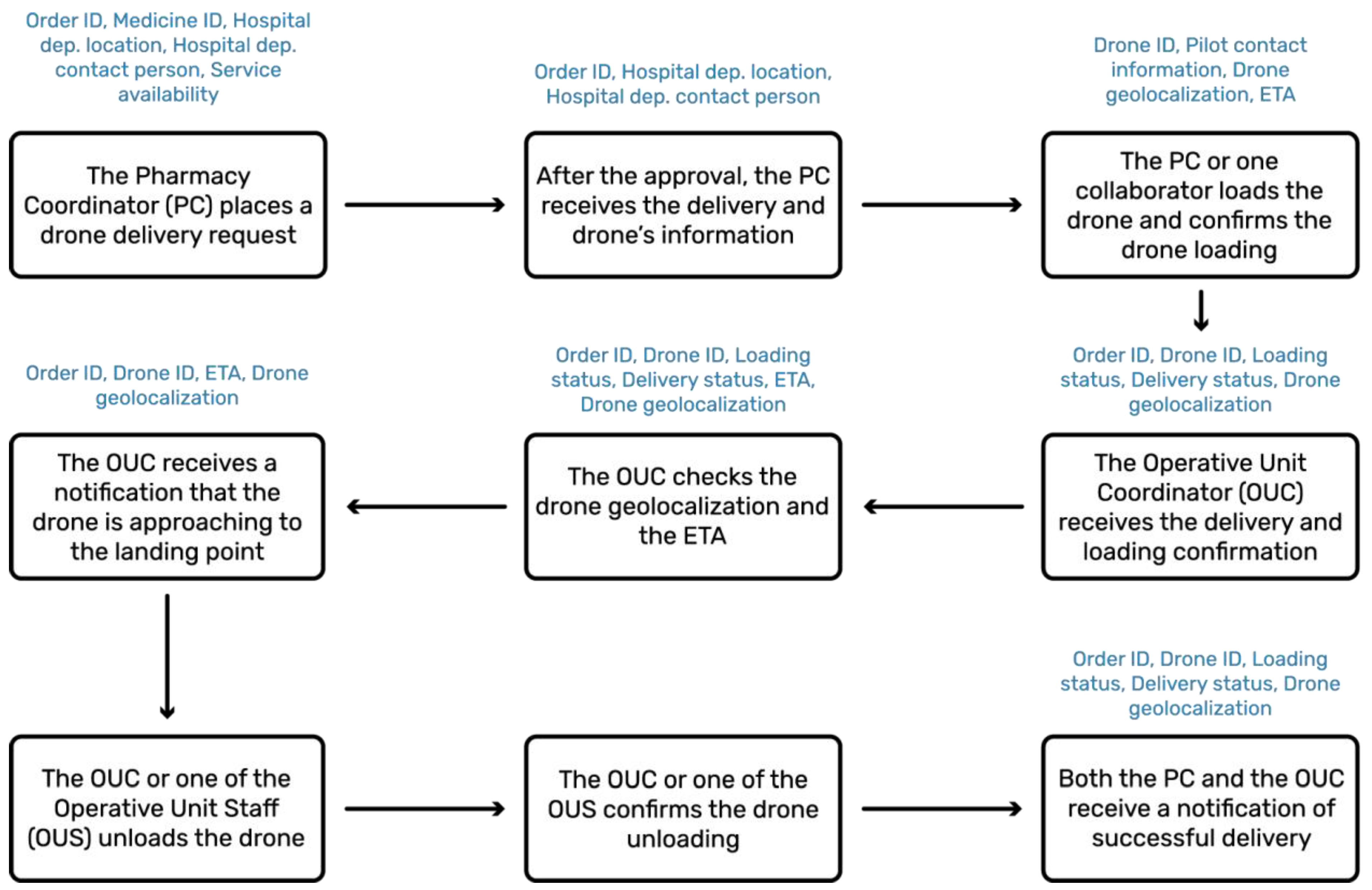
Aspirational scenario. In the designed service, these and more functions are performed through the ‘Drone Delivery App’. Starting from the designed logistics blueprint (see
Figure S2), the service flow revolving around such software was constructed to highlight the information that the system would need to use and exchange (see
Figure 3).
Software development should take into consideration the applicable regulatory requirements. In Europe, Medical Devices Regulation 2017/745 (“MDR”) states how the technology must be CE marked and what legal obligations must be respected by technology providers and distributors. The European Commission provided a guide that defines the criteria for a software to fall into the scope of MDR [
23]. In the view of such guidelines, the Drone Delivery App conceived by the present study would not qualify as a medical device software (MDSW), as a software must have a medical purpose on its own to be qualified as an MDSW. However, hospitals should implement a quality management system compliant with ISO standard 9001:2015, already in practice, for example, for pharmaceutical and laboratory analysis quality assurance, which also applies to logistics management and the relative software [
24]. Suppliers of such software must satisfy all the criteria for ISO certification. Guidelines for the interpretation of such a standard for software development, supply and maintenance were provided in 2018 [
25].
For the purpose of developing an App such as the one proposed by the current study, the authors proposed the co-creation methodology for the requirements’ collection and validation. Each of the later stages of development should take into account the requirements and the functions elicited in the first stages (see
Table 5).
3.3. The UAM Ecosystem
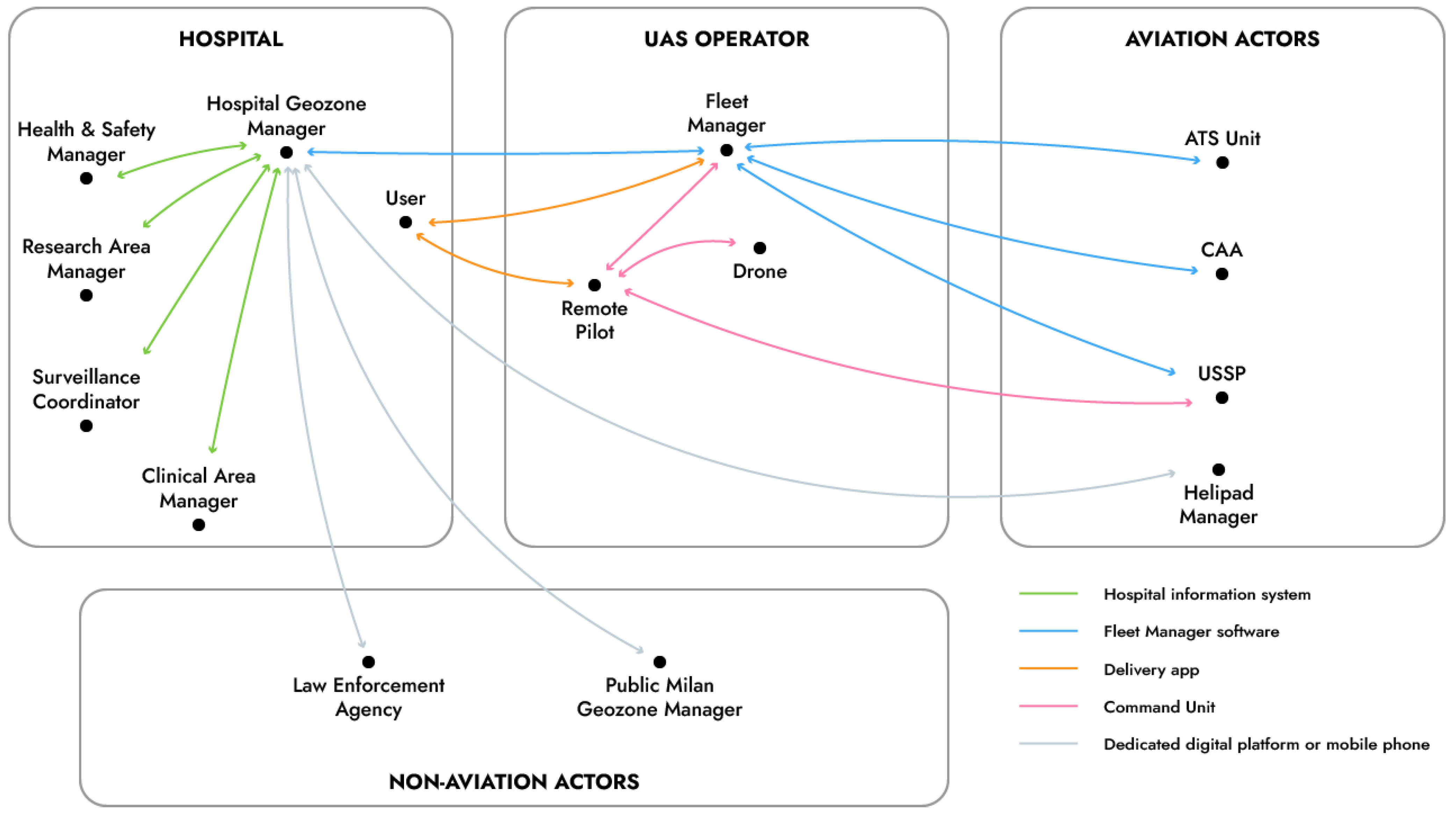
The designed service is possible only if supported by a digital infrastructure that ensures not only safety and compliance with rules but also efficiency of the service. In fact, users must be able to rapidly exchange information among them before, during and after the delivery. Several of these IT actors are not directly involved in flying the drone.
Figure 4 shows the functional scheme of the exchange of information between all identified actors and the components used to achieve such communication, called a UAM ecosystem. Such a digital ecosystem comprises actors pertaining to the large end-user organization of the service (i.e., the hospital), to the UAS operator and also both aviation and non-aviation actors.
It is worthy of note that, while geozone managers may be appointed by the public administrations (e.g., the Prefect of Milan for flights inside the restricted airspace volume above the metropolitan area), large infrastructures (e.g., university campuses and hospitals) may have their additional geozone manager to coordinate activities of all actors inside the perimeter of the respective establishment. This role would just be one more IT entity in the digital ecosystem, which in fact does not have a pre-defined and limited number of actors.
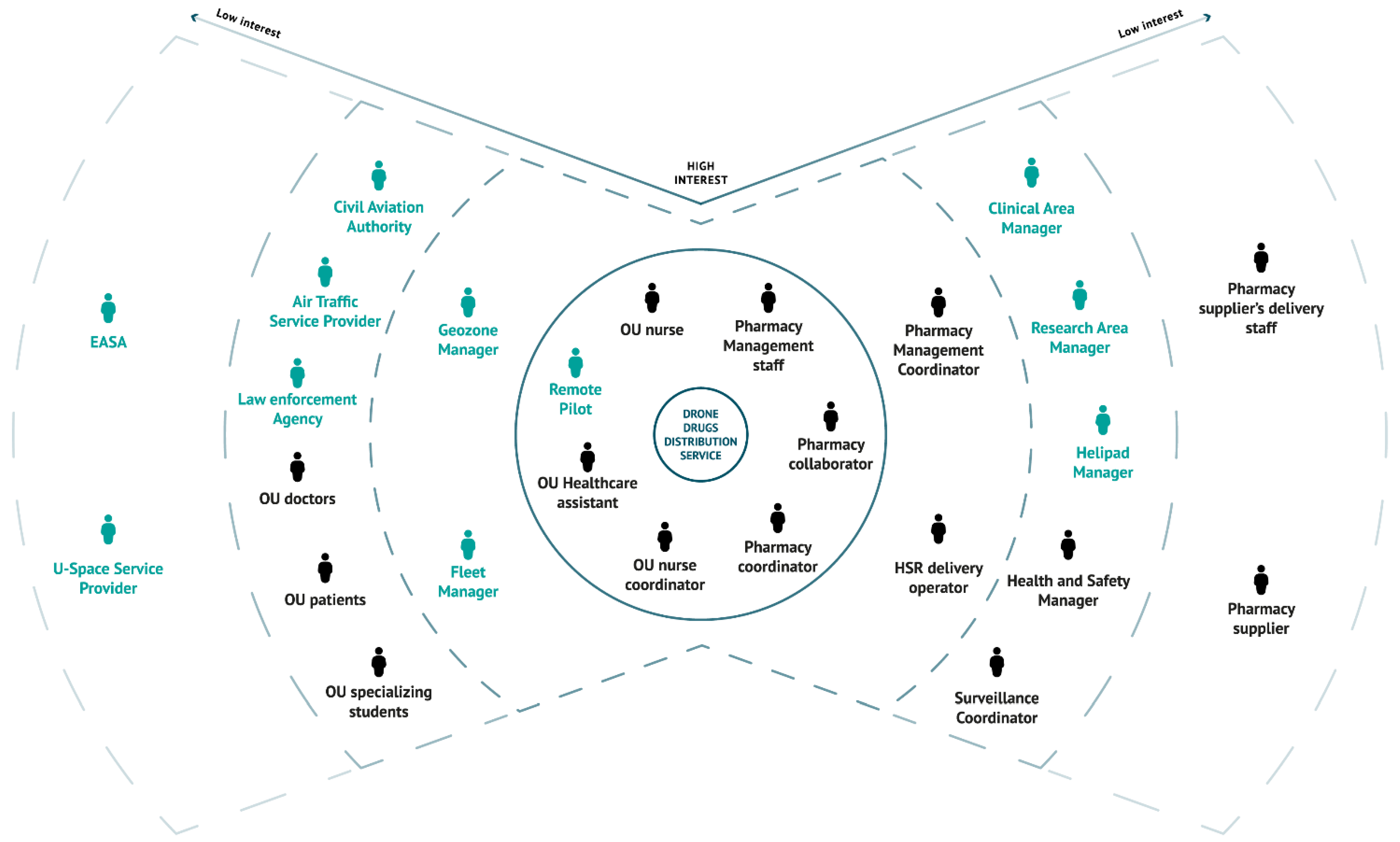
3.4. Stakeholder Map
The stakeholders of the pharmaceutical delivery service were subdivided in increasing degrees of interest.
In
Figure 5, the stakeholder map is shown. The first tier hosts the main users of the service, i.e., the staff of the OU, the staff of the pharmacy and the RP, who are directly involved in the delivery. The second tier regards staff whose work would change significantly if the new solution was introduced and the staff working in coordination with the main users (i.e., pharmacy management coordinator, HSR delivery operator, the FM and the geozone manager). The third tier of the stakeholder map encompasses hospital’s personnel and executives, domain enterprises and others who routinely interact with the most affected users and who can have an impact on the daily service availability and functioning. In the
As Is scenario, the involved stakeholders are doctors, specializing students, patients, the Health and Safety (H&S) manager, who is accountable for the safe movement of people in the hospital premises, and the surveillance coordinator, who receives and implements safety measures. The
Aspirational scenario introduces additional stakeholders to this tier. On the hospital side, the clinical area manager and the research area manager are the roles that can withdraw the approval of the airspace use above the healthcare and research buildings, respectively. Moreover, the
Aspirational scenario introduces aviation actors, i.e., CAA, which is the regulatory body that authorizes the operations and which could also interact digitally with the FM, as well as the nearest Air Traffic Service Unit (e.g., control tower at Linate airport) today involved through a procedural interface with the FM to de-conflict the flight from other airspace users during the strategic planning phase, in case of flight into controlled airspace (‘CTR zone’). In the future, the information would be digitally coordinated with the relevant ATS Units, even in case of contingencies. In fact, normal operations would be planned below the obstacle limitation surfaces around Linate airport, which means in a volume of airspace in which there are no operations of traditional commercial air transport. The law enforcement agency and the helipad manager are actors introduced to implement safe UAM, since they also progressively would become actors in the digital ecosystem.
Finally, on the fourth tier, the stakeholders are suppliers, regulators and others not directly involved with the use case (i.e., pharmacy suppliers and the pharmacy suppliers’ delivery staff) but who are distantly involved in the service operations. Moreover, two actors are introduced by the use of the drone service, i.e., EASA and one or more USSPs (U-Space Service Providers).
In
Table A1 of
Appendix A, the identified stakeholders are reported along with their role description and level of interest.
4. Discussion
The proposed Aspirational scenario was designed with the aim of improving the workflow of intra-hospital delivery of pharmaceuticals through the use of drones. With this aim in mind, the main prospective end-users were interviewed with a co-creation approach.
According to the authors’ estimates, all the Time KPIs would significantly improve with the proposed design. In particular, it was estimated that drones would reduce the response time of the service effectively. In fact, transport time would be directly reduced by the use of drones instead of appointed walkers, who are slower and can face bottlenecks, e.g., due to elevators. Preparation time would also benefit by the reduction in human interactions between the pharmacy and the OU staff. It must, hence, be noticed that maximum efficiency gains would be achieved not just because of using a drone, but because the drone and its operator would be integrated into a service-oriented digital ecosystem.
The Quality KPIs are the delivery accuracy, centralization degree and disruption of distribution activities [
2]. Delivery accuracy is the percentage of successful deliveries on the total number of operations. Centralization degree, which is the aggregation of all the logistic activities under a single organizational unit, and disruption of distribution activities are not expected to undergo significant changes in the proposed scenario since the service is meant not to substitute current transport modes, but to offer an additional reliable and fast option for an intra-hospital delivery.
In the Productivity category of KPIs, there are three factors to take into account: delivery capacity, delivery frequency and standardization [
2]. Delivery capacity is an important parameter in logistics. From the interviews, it emerged that urgent orders usually contain the request for a single item, which does not exceed 4 L of volume and whose largest dimension does not exceed 25 cm, as shown in
Table 1. This research does not focus on delivery containers as the subject would require a dedicated study, but it is assumed that the drone service could ensure such delivery capacity and that the weight of the ordered lot would not constitute an issue in the vast majority of the urgent requests. Delivery frequency of urgent drug requests in the investigated case study is estimated, on average, to be one request every thirty minutes in times of low demand, to around eight minutes, in the case of high demand (see
Table 1). These requests are filled with the use of the pneumatic tube, with the staff dedicated on intra-hospital delivery and personal pickup from the OUs depending on the availability of the first two options. A feasible delivery frequency with a drone service would depend on how many drones composed the fleet. According to the time estimates, one drone would be able to fulfil a delivery request every 11–20 min. Hence, it would be sensible to envisage at least 2–3 drones to increase availability and reliability of the service. Finally, standardization degree has to be taken into account for performance. From our interviews, it resulted that there are precise rules on how the pharmacy management prioritizes the different modes of transport. The decision over the mode of transport is entirely dependent on the availability of the pneumatic tube and that of the delivery staff, other than the particular medicine to be transported. However, the digital forms of the requests are filled only after a vocal negotiation between the OU staff and the pharmacy management. This can lead to ambiguity and low time efficiency with respect to a completely digitized system.
The current study provided the main financial constraints for the implementation of the proposed service managed in-house. It was argued that the economic gain would be hardly quantifiable since the main aim would be increasing hospital efficiency and patient care. However, it is possible to argue that the introduction of a drone service for pharmaceutical delivery could be more cost effective if the same UAS was used on other services during low demand. Examples of other use cases in a hospital are the transport of diagnostic samples and other medical supplies, monitoring infrastructures (e.g., HVAC systems’ integrity, thermal bridges in buildings) and surveillance (e.g., strengthening security during night shifts, detecting intrusions). That is why the role of the FM becomes pivotal, while several services may be implemented though more than one drone and more than one RP.
The proposed design would also bring a few added benefits, such as the possible reduction in nosocomial infections, since it would reduce possible vectors of infection by minimizing movements of people between clinical and non-clinical areas. Moreover, it would specifically reduce the burden on clinical activities since the pharmaceuticals would directly reach the point of care. The product traceability would be ensured by the association of each order to the appointed drone identifier and by the pre-defined design of each possible drone’s route. Traceability and security would be further ensured if a tamper-evident seal with the order identifier printed on the strip was applied to the container, which is a system already in use for hospital valuable goods. These would be further assured by the application of a mechatronic lock controlled by the users through the Drone Delivery App on the delivery container.
It is worthy to mention that the implementation of drone services should not disregard public acceptance at any step. Ethical feasibility should always be assessed along with legal and technological feasibility, as UAM may also affect citizens’ privacy, the environment and the authorities’ involvement [
26].
4.1. Regulatory Challenges Surrounding Automated Flight
In summary, the designed Aspirational scenario would be able to satisfy the unmet needs of the users of the service and to improve its efficiency. However, the safety and the regulatory compliance of such a service would require careful consideration.
One of the main safety concerns would be that the designed scenario proposes a high level of system automation. Joint Authorities for Rulemaking on Unmanned Systems (JARUS) identified six possible levels of UAS automation, which included: (i) manual UAS, i.e., an RP controls the flight continuously manipulating the flight controls; (ii) four levels of progressively enhanced automation, where some functions may be automated, but the human is still tasked to supervise and able to intervene at any moment to change the trajectory of the aircraft; (iii) fully autonomous UAS, that is, a completely independent, self-governing system [
27]. In this very ambitious level, the human is neither any longer necessary to supervise the flight nor able to change the trajectory of the aircraft. In other words, in fully autonomous operations the RP is no longer necessary, which does not exclude a continuing role of the FM to plan the operation, command its initiation and activate the emergency response plan in case of need. Other classifications of automation/autonomy do exist, sometimes using the terms ‘human in the loop’, ‘human on the loop’ or ‘human out of the loop’. For example, ASTM, EASA and ECA (European Cockpit Association) also distinguish six levels of automation/autonomy [
28]. Theoretically, in Europe autonomous drones are allowed in the ‘specific’ category [
29], but the means of compliance for the technical, safety and operational requirements are more stringent and require considerably more resources than solutions with less autonomy. Moreover, fully autonomous civil unmanned UAS are not being considered by the international and national organizations that are working on integration into controlled airspace [
30]. In conclusion, there is general consensus over the fact that completely autonomous solutions will not be available in the short- or mid-term for commercial application [
28]. In fact, almost all developers of eVTOL for the carriage of passengers envisage progressive steps:
Pilot is on board;
Pilot becomes remote but still one remote pilot governing only one eVTOL at any given time;
Remote Pilot governing simultaneously more than one eVTOL;
Finally, no longer a Remote Pilot, but a Fleet Manager able to coordinate the flights of an entire fleet of eVTOL.
In the case under study, flight is automated but supervised by an RP, who would be able to govern more than one vehicle at a time. An important requirement to ensure safety in this scenario is that the RP should always be able to take control of the drone at any time. In European countries, a case such as the one under study would not be allowed in the ‘open’ category since it requires the transportation of medical material in highly populated areas. Drone operators who fly drones in the ‘specific’ category need to be authorized by the CAA, which requires a risk analysis that can be carried out with the Specific Operations Risk Assessment (SORA) methodology, as recommended by AMC 1 of EASA to art. 11 of Regulation 2019/947 [
31]. Such analysis evaluates the ground risk, which includes the risk of harming bystanders. In order to reach an acceptable level of risk for the operation, both technological and operational mitigations can be applied. The appropriate mitigations depend on the operations, but a few practical examples can be made: designing the drone path over the top of buildings (this practice was applied in the present case study in
Figure S3), equipping the drone with a parachute for safe emergency landing and equipping the drone with a Flight Termination System (FTS), this being completely independent to avoid common mode failure. For higher risk operations, it may be required that the introduced mitigations adhere to specific technical standards. In that regard, the Horizon 2020 project AW-Drones aims to collect all the applicable technical standards to meet each mitigation. In conclusion, it is worthy to mention that the UAS operator is the legal entity that is responsible for the safety of the operations. Operators should implement a safety management system (SMS) as standard practice, the requirements of which are described by ISO standard 21384-3 (Unmanned aircraft systems—Part 3: Operational procedures) [
20]. Moreover, JARUS Guidelines for SORA constitute further guidance for risk assessment [
32]. In case of accidents, the operator is required to enact an ‘emergency response plan’ by step 3 of SORA. This plan contains measures that must be known to the pilot and depend on the specific geographic location.
4.2. Envisioning the UAM Ecosystem
As anticipated, when more than one operator is expected to fly drones in the same area, UTM will be necessary to avoid conflicts between different airborne vehicles. Fleet management and its functions would be sufficient whenever an area is restricted to use by one operator. The current study explores the scenario where more than one operator is allowed to execute flights in the hospital airspace in order to design a scalable solution. Hence, this scenario also allows the study of the feasibility of this kind of service on a larger scale, for example, the use of the airspace over a large hospital from drones of different operators and with different purposes.
In order to mitigate the mentioned risks, a digital infrastructure and a robust operational organization are needed. UAM involves the coordinated exchange of information among various actors to guarantee the safety of the operations. This underlies the concept of the UAM ecosystem integrating various actors of the ‘smart city’ operating in the urban environment. For example, the geozone manager can enforce a halt to the drones’ activities on the airspace under their authority for a certain amount of time. Moreover, coordination can be agreed with the air ambulance service in order to avoid any possible conflict with emergency helicopters landing at the nearby helipad.
In summary, it emerged that multiple actors need to interface to perform functions on the whole ecosystem. For example, the same software offers an interface that allows clinical staff to make an order request to the pharmacy, and a different interface to the pharmacy staff to check for the drones’ availability. Hence, this elicits the need for communication to be established between the hospital staff and the FM. However, it is fair to expect that a single vendor would not provide all the different kinds of software and interfaces that enable this use case. Therefore, interoperability of the technological components is an essential requirement to make this scenario possible and adaptable to different needs in the future.
4.3. Limitations
The current study presents a few limitations. To assess the generalizability of the results, it would be sensible to inquire whether other healthcare organizations utilize the same or similar processes for last-mile pharmaceutical delivery. To the authors’ knowledge, the system adopted to prioritize and manage different types of delivery requests is common practice in other hospitals of northern Italy. Further research could validate the generalizability of these findings by interviewing representatives of other hospitals to be able to generalize the results. The hierarchy of KPIs, the identified constraints to the introduction of the service and the requirements of the Drone Delivery App are probably generalizable to similar institutions willing to implement the same use case (i.e., urgent intra-hospital delivery of pharmaceuticals), although they may also be valid for additional use cases, such as for medical supplies and diagnostic samples. However, further research is needed to validate this hypothesis with prospective users.
The delivery container’s design was not considered in the current study. Currently, there is not a shared standard by aviation authorities or standardization organizations (e.g., ICAO Standards and Recommended Practices) regarding the specifications of a container for the transport of potentially dangerous materials by drones. Product integrity and the safety of third parties must be considered for an effective design, but the requirements may vary depending on the substance or the form in which it is transported [
33]. Monitoring acceleration forces, temperature and humidity of the content would enhance the reliability of the product’s integrity, but prior to this, it is essential to know drug stability to solicitations and physical changes that could apply during a drone flight, as was the focus of recent studies [
33,
34,
35]. In fact, it was argued that each pharmaceutical should undergo a study following pharmacopeia standards to be assessed suitable for drone transport [
33].
Furthermore, the current study does not address the technological requirements and the infrastructure required to support the drone service. In any case, the drone base should be a tamper-proof vertiport and possibly allow for the recharge of the drones automatically in order to ensure the continuity of the service. This vertiport could be managed by the hospital or by the drone operator, but, in any case, it would not be open to public use and therefore beyond the scope of both ICAO and EASA.
5. Conclusions
UAM has to face numerous challenges before turning into a reality in ‘smart cities’. Flying Forward 2020 aims to be an initiative that pushes towards this goal by conducting tests in five European cities. Among others, San Raffaele Hospital designed a drone service for pharmaceutical delivery as an initial proof-of-concept that in the future might be used as a base to study its possible scalability to more advanced use cases, such as inter-hospital transport of medical goods. Such a scenario would maximize the utility of the drone service as it would allow urban traffic to be avoided and consistently save local CO2 emissions.
The current study showed how the healthcare logistics sector can benefit from the introduction of a drone delivery service that reduces the burden of logistic flows on foot. The main benefit for a well-established healthcare organization would be the increase in hospital efficiency, which would result in better healthcare outcomes. However, it poses substantial technological and regulatory challenges that need to be addressed before it comes into reality.
In the vision expressed by the present research, the prototyped service will be enabled in the future by a UAM ecosystem that involves the exchange of information through a federated set of digital platforms that can be managed by different actors. In that regard, UTM (called U-space in Europe) services are expected to be needed to coordinate drones controlled by different operators in the same airspace.