Aerial Branch Sampling to Detect Forest Pathogens
Abstract
1. Introduction
1.1. Physical Branch Sample Collection and Retrieval
1.2. Sampling Application: Ceratocystis wilt of ‘ōhi‘a in Hawai’i
2. Materials and Methods
2.1. Determining the Minimum Branch Diameter for Detecting C. lukuohia
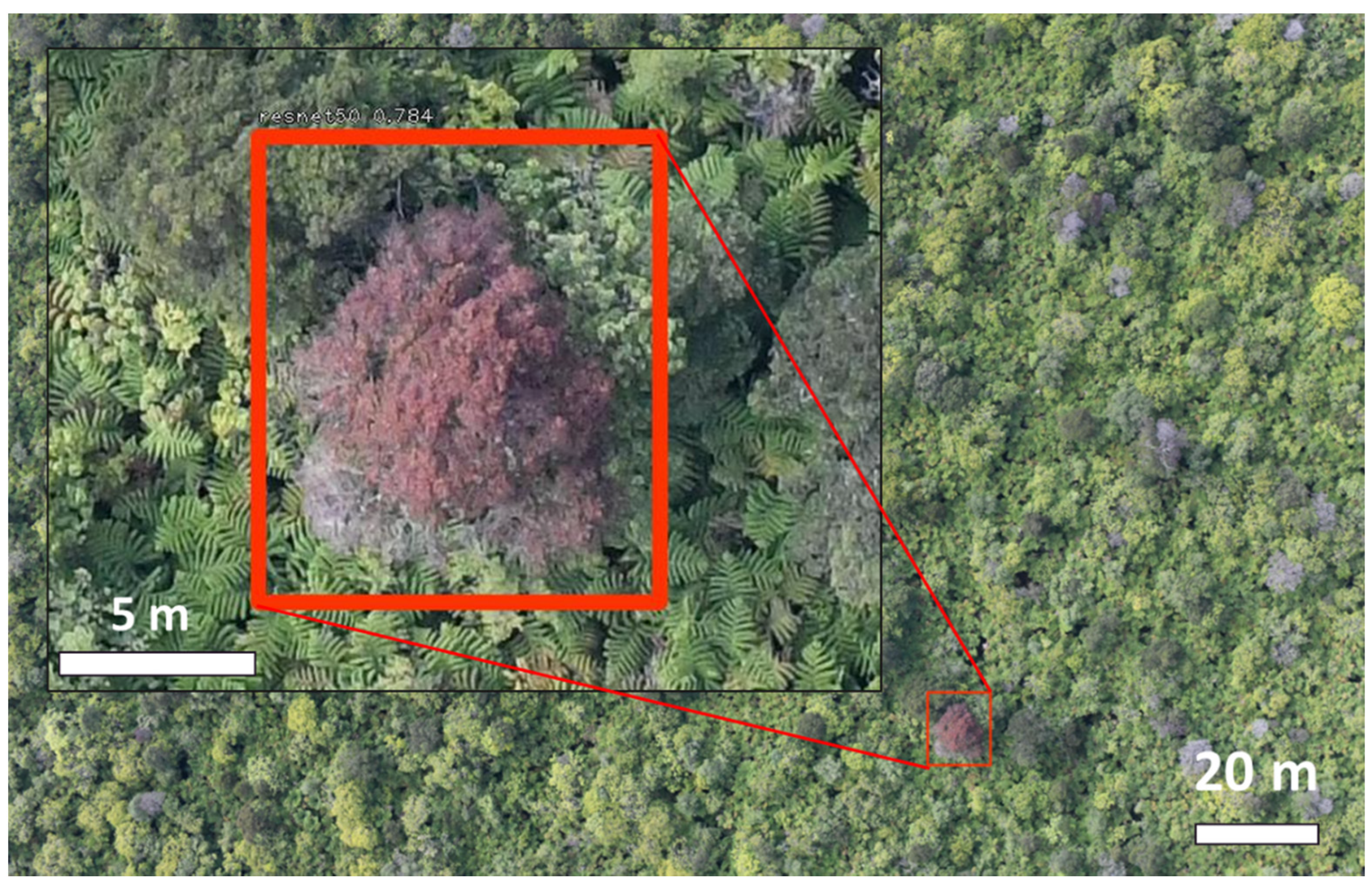
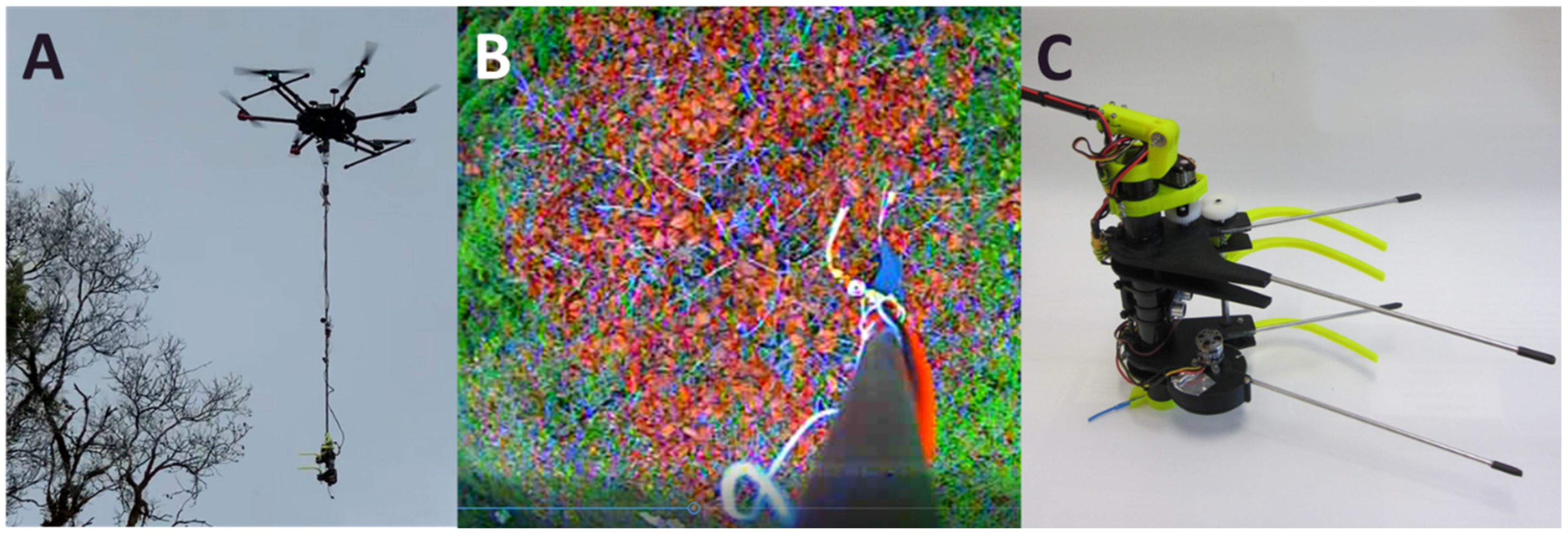
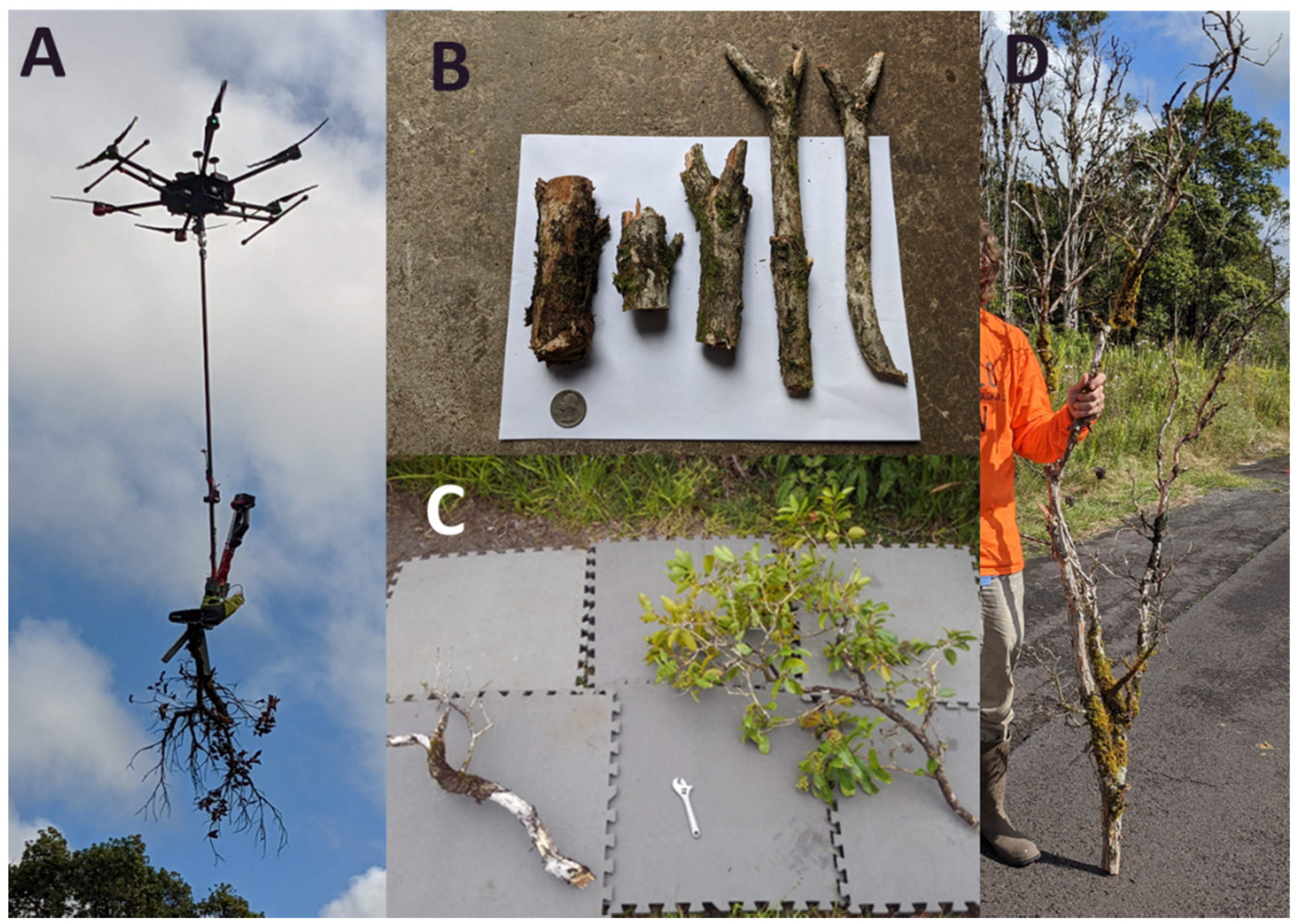
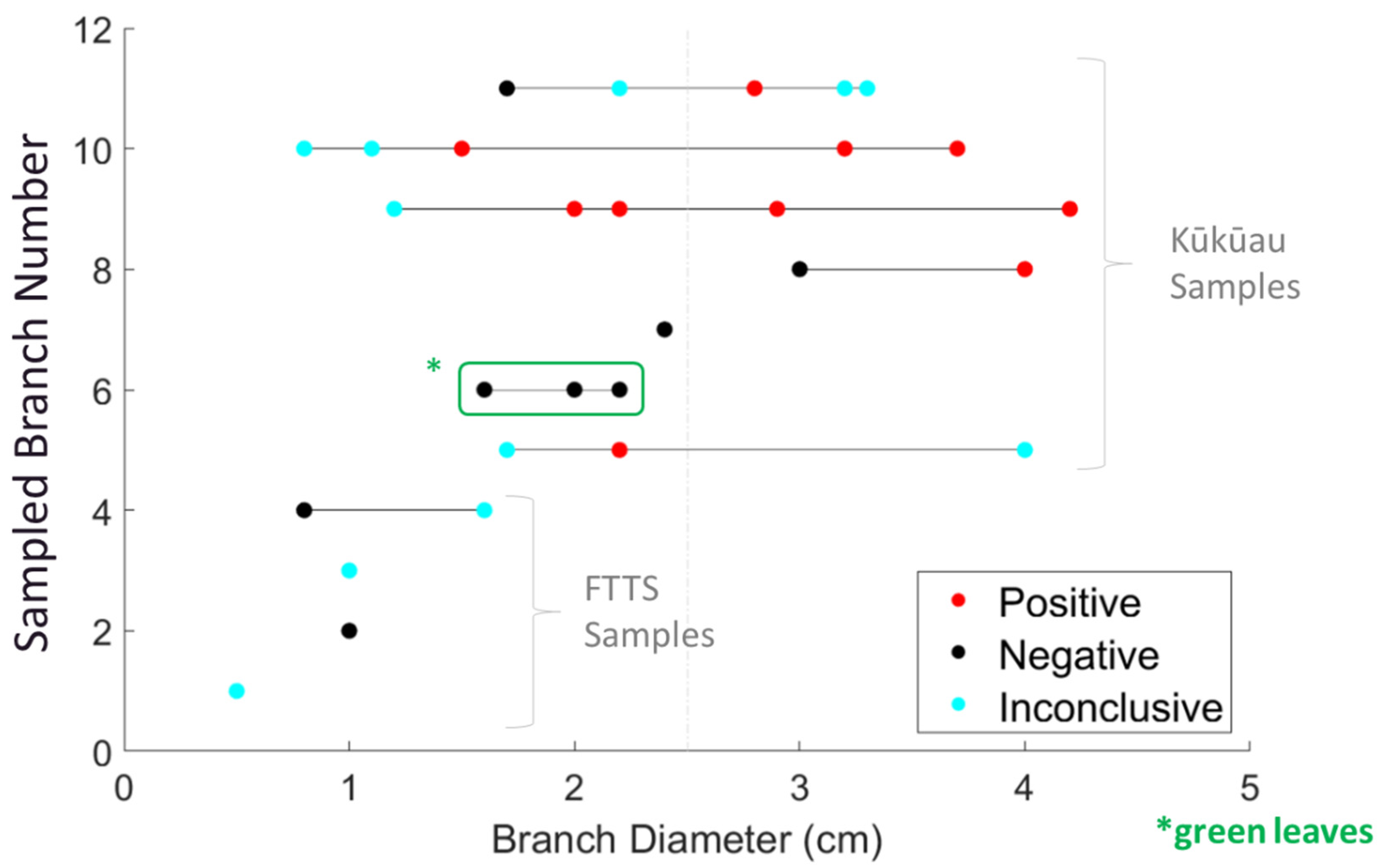
2.2. Aerial Branch Sampling with the FTTS
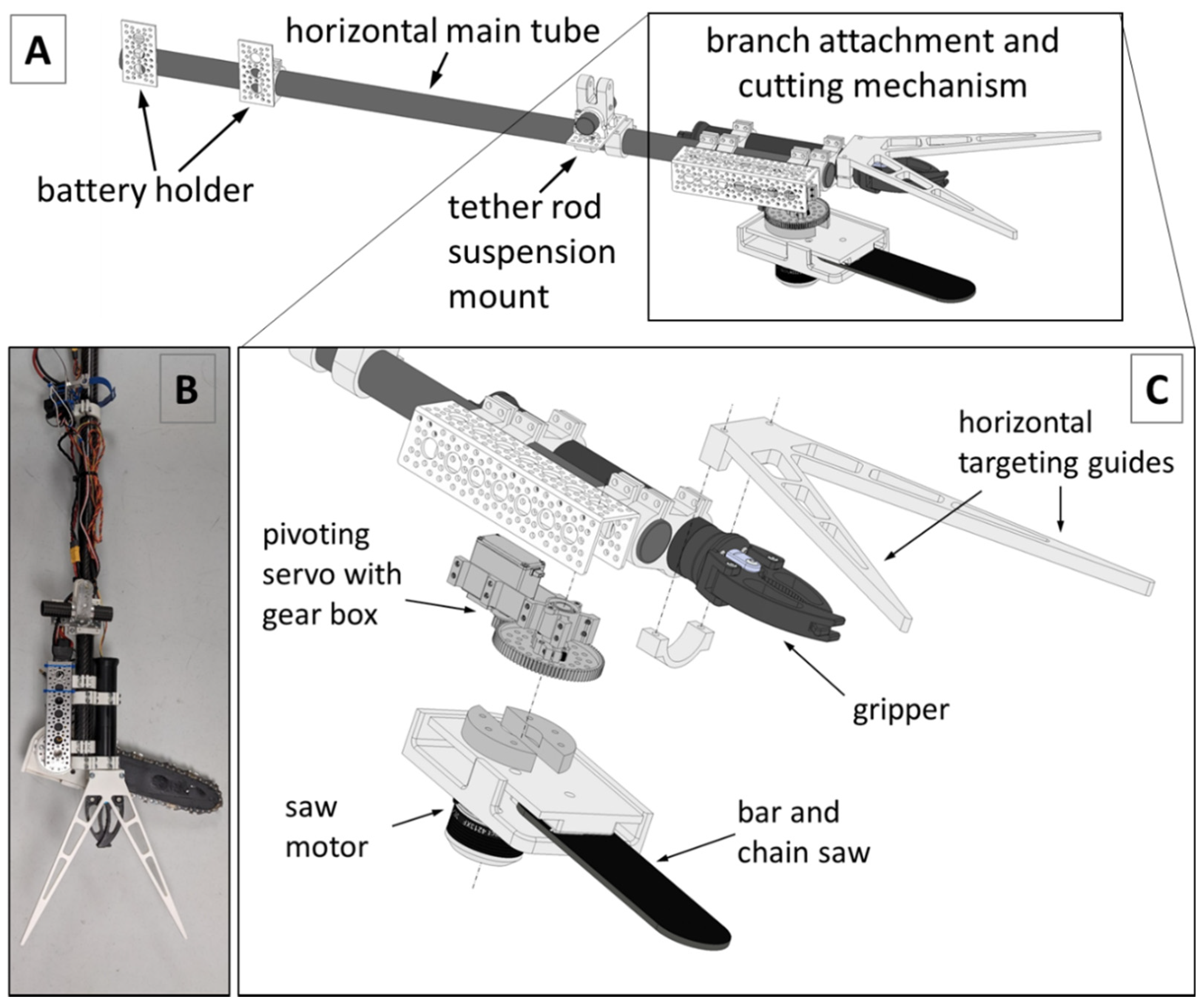
2.3. Development of the Kūkūau Branch Sampler
3. Results
3.1. Branch Diameter and C. lukuohia Detections
3.2. Aerial Branch Sampling Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Tree Sample | Diameter (cm) | CODE | Results |
|---|---|---|---|
| 211 | 1.70 | 1 | positive for C. lukuohia |
| 211 | 1.90 | 1 | positive for C. lukuohia |
| 211 | 2.50 | 0 | inconclusive |
| 211 | 2.80 | 1 | positive for C. lukuohia |
| 211 | 5.10 | 1 | positive for C. lukuohia |
| 211 | 5.10 | 1 | positive for C. lukuohia |
| 212 | 2.00 | 1 | positive for C. lukuohia |
| 212 | 2.30 | 0 | inconclusive |
| 212 | 3.40 | 0 | inconclusive |
| 212 | 4.40 | 1 | positive for C. lukuohia |
| 212 | 7.40 | 1 | positive for C. lukuohia |
| 214 | 1.00 | 0 | inconclusive |
| 214 | 1.60 | 1 | positive for C. lukuohia |
| 214 | 2.20 | 0 | inconclusive |
| 214 | 2.70 | 1 | positive for C. lukuohia |
| 214 | 4.40 | 0 | inconclusive |
| 214 | 5.50 | 1 | positive for C. lukuohia |
| 214 | 6.70 | 1 | positive for C. lukuohia |
| 215 | 0.8 | −1 | Ceratocystis not detected |
| 215 | 1.4 | 1 | Positive for C. lukuohia |
| 215 | 1.6 | 1 | Positive for C. lukuohia |
| 215 | 2.5 | 1 | Positive for C. lukuohia |
| 215 | 3.5 | 1 | Positive for C. lukuohia |
| 215 | 4.5 | 1 | Positive for C. lukuohia |
| 215 | 2.2 | 0 | inconclusive |
| 215 | 2.5 | 0 | inconclusive |
| 215 | 2.8 | 0 | inconclusive |
| 215 | 3.6 | 0 | inconclusive |
| 215 | 5.1 | 0 | inconclusive |
| 216 | 5.60 | −1 | no Ceratocystis detected |
| 216 | 6.00 | −1 | no Ceratocystis detected |
| 217 | 7.20 | −1 | no Ceratocystis detected |
| 217 | 9.60 | 1 | positive for C. lukuohia |
| 218 | 4.30 | −1 | no Ceratocystis detected |
| 218 | 5.60 | −1 | no Ceratocystis detected |
| 219 | 4.20 | −1 | no Ceratocystis detected |
| 219 | 7.40 | −1 | no Ceratocystis detected |
| 220 | 1.40 | 1 | Positive for C. lukuohia |
| 220 | 1.70 | −1 | no Ceratocystis detected |
| 220 | 2.60 | 1 | Positive for C. lukuohia |
| 220 | 3.40 | 0 | inconclusive |
| 220 | 5.20 | 1 | positive for C. lukuohia |
| 220 | 8.40 | 1 | positive for C. lukuohia |
| Tree 224 | 1.2 | 0 | inconclusive |
| Tree 224 | 1.5 | 0 | inconclusive |
| Tree 224 | 1.9 | 0 | inconclusive |
| Tree 224 | 2.2 | −1 | no Ceratocystis detected |
| Tree 224 | 3 | −1 | no Ceratocystis detected |
| Tree 224 | 3.4 | 1 | C. lukuohia detected |
| Tree 224 | 0.8 | −1 | no Ceratocystis detected |
| Tree 224 | 1.3 | −1 | no Ceratocystis detected |
| Tree 224 | 2.1 | −1 | no Ceratocystis detected |
| Tree 224 | 2.4 | −1 | no Ceratocystis detected |
| Tree 224 | 2.5 | −1 | no Ceratocystis detected |
| Tree 224 | 2.6 | −1 | no Ceratocystis detected |
| Tree 224 | 3 | −1 | no Ceratocystis detected |
| Tree 224 | 4.8 | 1 | C. lukuohia detected |
| Tree 225 | 1.40 | 0 | inconclusive |
| Tree 225 | 1.80 | −1 | No Ceratocystis detected |
| Tree 225 | 2.20 | 0 | inconclusive |
| Tree 225 | 2.30 | −1 | No Ceratocystis detected |
| Tree 225 | 3.00 | −1 | No Ceratocystis detected |
| Tree 225 | 3.90 | 1 | Positive for C. lukuohia |
| Tree 225 | 4.20 | −1 | No Ceratocystis detected |
| Tree 226 | 1.30 | 1 | Positive for C. lukuohia (weak positive) |
| Tree 226 | 1.60 | 0 | inconclusive |
| Tree 226 | 2.00 | 1 | Positive for C. lukuohia (ohia internal marker was not detected in sample) |
| Tree 226 | 2.50 | 1 | Positive for C. lukuohia |
| Tree 226 | 2.70 | 1 | Positive for C. lukuohia |
| Tree 226 | 3.60 | 1 | Positive for C. lukuohia |
| Tree 226 | 5.00 | 1 | Positive for C. lukuohia |
| Tree 226_Rot_Core | 5.10 | 1 | Positive for C. lukuohia |
| Tree 226 | 7.40 | 0 | inconclusive |
| 228 | 1.50 | −1 | No Ceratocystis detected |
| 228 | 2.20 | 1 | positive for C. lukuohia |
| 228 | 2.40 | −1 | No Ceratocystis detected |
| 228 | 4.30 | 1 | positive for C. lukuohia |
| 228 | 4.30 | 1 | positive for C. lukuohia |
| 228 | 6.30 | 1 | positive for C. lukuohia |
| 228 | 6.30 | 1 | positive for C. lukuohia |
References
- Baena, S.; Boyd, D.S.; Moat, J. UAVs in Pursuit of Plant Conservation—Real World Experiences. Ecol. Inform. 2018, 47, 2–9. [Google Scholar] [CrossRef]
- Guimarães, N.; Pádua, L.; Marques, P.; Silva, N.; Peres, E.; Sousa, J.J. Forestry Remote Sensing from Unmanned Aerial Vehicles: A Review Focusing on the Data, Processing and Potentialities. Remote Sens. 2020, 12, 1046. [Google Scholar] [CrossRef]
- Sharma, J.B. Applications of Small Unmanned Aircraft Systems: Best Practices and Case Studies; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-52085-3. [Google Scholar]
- Käslin, F.; Baur, T.; Meier, P.; Koller, P.; Buchmann, N.; D’Odorico, P.; Eugster, W. Novel Twig Sampling Method by Unmanned Aerial Vehicle (UAV). Front. For. Glob. Change 2018, 1, 2. [Google Scholar] [CrossRef]
- Lucas, J.A. Plant Pathology and Plant Pathogens; John Wiley & Sons: New York, NY, USA, 2020; ISBN 978-1-118-89386-9. [Google Scholar]
- West, J.S.; Canning, G.G.; Perryman, S.A.; King, K. Novel Technologies for the Detection of Fusarium Head Blight Disease and Airborne Inoculum. Trop. Plant Pathol. 2017, 42, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Szczecińska, M.; Sramko, G.; Wołosz, K.; Sawicki, J. Genetic Diversity and Population Structure of the Rare and Endangered Plant Species Pulsatilla Patens (L.) Mill in East Central Europe. PLoS ONE 2016, 11, e0151730. [Google Scholar] [CrossRef]
- Luna, T. Native Plant Restoration on Hawai‘i. Native Plants J. 2018, 19, 58–69. [Google Scholar] [CrossRef]
- Weisenberger, L.A.; Weller, S.G.; Sakai, A.K. Remnants of Populations Provide Effective Source Material for Reintroduction of an Endangered Hawaiian Plant, Schiedea Kaalae (Caryophyllaceae). Am. J. Bot. 2014, 101, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Reuter, D.; Robinson, J.B. Plant Analysis: An Interpretation Manual; Csiro Publishing: Clayton, Australia, 1997; ISBN 978-0-643-10126-5. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Tupayachi, R.; Anderson, C.B.; Sinca, F.; Carranza-Jiménez, L.; Martinez, P. Amazonian Functional Diversity from Forest Canopy Chemical Assembly. Proc. Natl. Acad. Sci. USA 2014, 111, 5604–5609. [Google Scholar] [CrossRef] [PubMed]
- Lira-Martins, D.; Humphreys-Williams, E.; Strekopytov, S.; Ishida, F.Y.; Quesada, C.A.; Lloyd, J. Tropical Tree Branch-Leaf Nutrient Scaling Relationships Vary With Sampling Location. Front. Plant Sci. 2019, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Serbin, S.P.; McNeil, B.E.; Kingdon, C.C.; Townsend, P.A. Imaging Spectroscopy Algorithms for Mapping Canopy Foliar Chemical and Morphological Traits and Their Uncertainties. Ecol. Appl. 2015, 25, 2180–2197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, T.; Darvishzadeh, R.; Skidmore, A.K.; Jones, S.; Suarez, L.; Woodgate, W.; Heiden, U.; Heurich, M.; Hearne, J. Vegetation Indices for Mapping Canopy Foliar Nitrogen in a Mixed Temperate Forest. Remote Sens. 2016, 8, 491. [Google Scholar] [CrossRef]
- Nakamura, A.; Kitching, R.L.; Cao, M.; Creedy, T.J.; Fayle, T.M.; Freiberg, M.; Hewitt, C.N.; Itioka, T.; Koh, L.P.; Ma, K.; et al. Forests and Their Canopies: Achievements and Horizons in Canopy Science. Trends Ecol. Evol. 2017, 32, 438–451. [Google Scholar] [CrossRef]
- Malenovský, Z.; Homolová, L.; Lukeš, P.; Buddenbaum, H.; Verrelst, J.; Alonso, L.; Schaepman, M.E.; Lauret, N.; Gastellu-Etchegorry, J.-P. Variability and Uncertainty Challenges in Scaling Imaging Spectroscopy Retrievals and Validations from Leaves Up to Vegetation Canopies. Surv. Geophys. 2019, 40, 631–656. [Google Scholar] [CrossRef]
- Cannon, C.H.; Borchetta, C.; Anderson, D.L.; Arellano, G.; Barker, M.; Charron, G.; LaMontagne, J.M.; Richards, J.H.; Abercrombie, E.; Banin, L.F.; et al. Extending Our Scientific Reach in Arboreal Ecosystems for Research and Management. Front. For. Glob. Change 2021, 4, 160. [Google Scholar] [CrossRef]
- Jackaman, W.; Sacco, D.A. Reconnaissance Biogeochemical Survey Using Spruce Tops in the West Road (Blackwater) River Area, Fraser Plateau, Central British Columbia (Parts of NTS 093C/14, /15, 093F/02, /03). 2015, 4. Available online: https://cdn.geosciencebc.com/pdf/SummaryofActivities2015/SoA2015_Jackaman.pdf (accessed on 7 August 2022).
- Hildes. Turanich-Noyen Aerochem: An Introduction and Comparison with Traditional Stream Sediment Sampling. Available online: https://www.semanticscholar.org/paper/Aerochem-%3A-An-Introduction-and-Comparison-with-Hildes-Turanich-Noyen/1b36eccf3d2cbc3bb4dac4c5544740e10809e467 (accessed on 11 December 2020).
- UC Berkeley Forest Pathology and Mycology Lab Sampler Drones for Forestry Reseach. Available online: https://nature.berkeley.edu/garbelottowp/?p=1801 (accessed on 14 December 2020).
- Kutia, J. Aerial Manipulation for Canopy Sampling. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2019. [Google Scholar]
- Charron, G.; Robichaud-Courteau, T.; Vigne, H.L.; Weintraub, S.; Hill, A.; Justice, D.; Bélanger, N.; Desbiens, A.L. The DeLeaves: A UAV Device for Efficient Tree Canopy Sampling. J. Unmanned Veh. Syst. 2020, 8, 245–264. [Google Scholar] [CrossRef]
- Krisanski, S.; Taskhiri, M.S.; Montgomery, J.; Turner, P. Design and Testing of a Novel Unoccupied Aircraft System for the Collection of Forest Canopy Samples. Forests 2022, 13, 153. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Desbiens, A.L.; Charron, G.; Vigne, H.L.; Laliberté, E. Foliar Sampling with an Unmanned Aerial System (UAS) Reveals Spectral and Functional Trait Differences within Tree Crowns. Can. J. For. Res. 2020, 50, 966–974. [Google Scholar] [CrossRef]
- Barnes, I.; Fourie, A.; Wingfield, M.J.; Harrington, T.C.; McNew, D.L.; Sugiyama, L.S.; Luiz, B.C.; Heller, W.P.; Keith, L.M. New Ceratocystis Species Associated with Rapid Death of Metrosideros Polymorpha in Hawai’i. Persoonia Mol. Phylogeny Evol. Fungi 2018, 40, 154–181. [Google Scholar] [CrossRef]
- Camp, R.J.; LaPointe, D.A.; Hart, P.J.; Sedgwick, D.E.; Canale, L.K. Large-Scale Tree Mortality from Rapid Ohia Death Negatively Influences Avifauna in Lower Puna, Hawaii Island, USA. Condor 2019, 121, duz007. [Google Scholar] [CrossRef]
- Fortini, L.B.; Kaiser, L.R.; Keith, L.M.; Price, J.; Hughes, R.F.; Jacobi, J.D.; Friday, J.B. The Evolving Threat of Rapid ‘Ōhi‘a Death (ROD) to Hawai‘i’s Native Ecosystems and Rare Plant Species. For. Ecol. Manag. 2019, 448, 376–385. [Google Scholar] [CrossRef]
- Hughes, M.A.; Juzwik, J.; Harrington, T.C.; Keith, L.M. Pathogenicity, Symptom Development, and Colonization of Metrosideros Polymorpha by Ceratocystis Lukuohia. Plant Dis. 2020, 104, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.M.; Hughes, R.F.; Sugiyama, L.S.; Heller, W.P.; Bushe, B.C.; Friday, J.B. First Report of Ceratocystis Wilt on ˋŌhiˋa (Metrosideros Polymorpha). Plant Dis. 2015, 99, 1276. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Keith, L.M.; Heller, W.P.; Hughes, M.A.; Vaughn, N.R.; Hughes, R.F.; Balzotti, C. A Spectral Mapping Signature for the Rapid Ohia Death (ROD) Pathogen in Hawaiian Forests. Remote Sens. 2018, 10, 404. [Google Scholar] [CrossRef]
- Vaughn, N.R.; Asner, G.P.; Brodrick, P.G.; Martin, R.E.; Heckler, J.W.; Knapp, D.E.; Hughes, R.F. An Approach for High-Resolution Mapping of Hawaiian Metrosideros Forest Mortality Using Laser-Guided Imaging Spectroscopy. Remote Sens. 2018, 10, 502. [Google Scholar] [CrossRef]
- Perroy, R.L.; Hughes, M.; Keith, L.M.; Collier, E.; Sullivan, T.; Low, G. Examining the Utility of Visible Near-Infrared and Optical Remote Sensing for the Early Detection of Rapid ‘Ōhi‘a Death. Remote Sens. 2020, 12, 1846. [Google Scholar] [CrossRef]
- Heller, W.P.; Keith, L.M. Real-Time PCR Assays to Detect and Distinguish the Rapid ʻŌhiʻa Death Pathogens Ceratocystis Lukuohia and C. Huliohia. Phytopathology 2018, 108, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M. (Hawaii Department of Land and Natural Resources, Honolulu, Hawaii, USA). Personal communication. 2020. [Google Scholar]
- Kunert, N.; Aparecido, L.M.T.; Wolff, S.; Higuchi, N.; dos Santos, J.; de Araujo, A.C.; Trumbore, S. A Revised Hydrological Model for the Central Amazon: The Importance of Emergent Canopy Trees in the Forest Water Budget. Agric. For. Meteorol. 2017, 239, 47–57. [Google Scholar] [CrossRef]
- Giambelluca, T.W.; Chen, Q.; Frazier, A.G.; Price, J.P.; Chen, Y.-L.; Chu, P.-S.; Eischeid, J.K.; Delparte, D.M. Online Rainfall Atlas of Hawai‘i. Bull. Am. Meteorol. Soc. 2013, 94, 313–316. [Google Scholar] [CrossRef]
- Frazier, A.G.; Giambelluca, T.W. Spatial Trend Analysis of Hawaiian Rainfall from 1920 to 2012-Frazier-2017-International Journal of Climatology—Wiley Online Library. Available online: https://rmets.onlinelibrary.wiley.com/doi/abs/10.1002/joc.4862 (accessed on 9 September 2022).
- Moral, J.; Agustí-Brisach, C.; Pérez-Rodríguez, M.; Xaviér, C.; Raya, M.C.; Rhouma, A.; Trapero, A. Identification of Fungal Species Associated with Branch Dieback of Olive and Resistance of Table Cultivars to Neofusicoccum Mediterraneum and Botryosphaeria Dothidea. Plant Dis. 2017, 101, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Beier, G.L.; Blanchette, R.A. Xylem Characteristics in Ulmus Americana Cultivars and Their Potential Use as a Preliminary Screening Method for Dutch Elm Disease Resistance. For. Pathol. 2020, 50, e12638. [Google Scholar] [CrossRef]
- Oren, E.; Klingeman, W.; Gazis, R.; Moulton, J.; Lambdin, P.; Coggeshall, M.; Hulcr, J.; Seybold, S.J.; Hadziabdic, D. A Novel Molecular Toolkit for Rapid Detection of the Pathogen and Primary Vector of Thousand Cankers Disease. PLoS ONE 2018, 13, e0185087. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.C. Ceratocystis Diseases. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 230–255. ISBN 978-1-78064-040-2. [Google Scholar]
- Lemoine, D.; Jacquemin, S.; Granier, A. Beech ( Fagus Sylvatica L.) Branches Show Acclimation of Xylem Anatomy Andhydraulic Properties to Increased Light after Thinning. Ann. For. Sci. 2002, 59, 761–766. [Google Scholar] [CrossRef]
- Jourez, B.; Riboux, A.; Leclercq, A. Anatomical Characteristics Of Tension Wood And Opposite Wood In Young Inclined Stems Of Poplar (Populus Euramericana Cv ’Ghoy’). IAWA J. 2001, 22, 133–157. [Google Scholar] [CrossRef]
- Yamamoto, F.; Kozlowski, T.T. Effects of Flooding, Tilting of Stems, and Ethrel Application on Growth, Stem Anatomy and Ethylene Production of Pinus Densiflora Seedlings. J. Exp. Bot. 1987, 38, 293–310. [Google Scholar] [CrossRef]
- Weingarten, E.; Martin, R.E.; Hughes, R.F.; Vaughn, N.R.; Shafron, E.; Asner, G.P. Early Detection of a Tree Pathogen Using Airborne Remote Sensing. Ecol. Appl. 2022, 32, e2519. [Google Scholar] [CrossRef]
- Lee, T.; Mckeever, S.; Courtney, J. Flying Free: A Research Overview of Deep Learning in Drone Navigation Autonomy. Drones 2021, 5, 52. [Google Scholar] [CrossRef]
- da Silva, D.Q.; dos Santos, F.N.; Sousa, A.J.; Filipe, V.; Boaventura-Cunha, J. Unimodal and Multimodal Perception for Forest Management: Review and Dataset. Computation 2021, 9, 127. [Google Scholar] [CrossRef]
- Salles, R.N.; de Campos Velho, H.F.; Shiguemori, E.H. Automatic Position Estimation Based on Lidar × Lidar Data for Autonomous Aerial Navigation in the Amazon Forest Region. Remote Sens. 2022, 14, 361. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Slippers, B.; Wingfield, B.D.; Barnes, I. The Unified Framework for Biological Invasions: A Forest Fungal Pathogen Perspective. Biol. Invasions 2017, 19, 3201–3214. [Google Scholar] [CrossRef]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of Emerging Fungal Diseases of Forest Trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Howard, P.L. Human Adaptation to Invasive Species: A Conceptual Framework Based on a Case Study Metasynthesis. Ambio 2019, 48, 1401–1430. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgen, B.; Richardson, D.; Higgins, S.I. Integrated Control of Invasive Alien Plants in Terrestrial Ecosystems. Land Use Water Resour. Res. 2001, 1, 1732-2016-140256. [Google Scholar] [CrossRef]
- Molina, J.; Hirai, S. Aerial Pruning Mechanism, Initial Real Environment Test. Robot. Biomim. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]



| Felled Inoculated Tree Branches | Aerially Sampled Branches | |||||
|---|---|---|---|---|---|---|
| Branch Diameter (cm) | # of Samples | # Positive for C. lukuohia | % Positive for C. lukuohia | # of Samples | # Positive for C. lukuohia | % Positive for C. lukuohia |
| <1.0 | 2 | 0 | 0% | 3 | 0 | 0% |
| 1.0–2.5 | 28 | 12 | 43% | 16 | 4 | 25% |
| 2.51–5.0 | 20 | 13 | 65% | 10 | 6 | 60% |
| 5.1–10.0 | 13 | 10 | 77% | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perroy, R.L.; Meier, P.; Collier, E.; Hughes, M.A.; Brill, E.; Sullivan, T.; Baur, T.; Buchmann, N.; Keith, L.M. Aerial Branch Sampling to Detect Forest Pathogens. Drones 2022, 6, 275. https://doi.org/10.3390/drones6100275
Perroy RL, Meier P, Collier E, Hughes MA, Brill E, Sullivan T, Baur T, Buchmann N, Keith LM. Aerial Branch Sampling to Detect Forest Pathogens. Drones. 2022; 6(10):275. https://doi.org/10.3390/drones6100275
Chicago/Turabian StylePerroy, Ryan L., Philip Meier, Eszter Collier, Marc A. Hughes, Eva Brill, Timo Sullivan, Thomas Baur, Nina Buchmann, and Lisa M. Keith. 2022. "Aerial Branch Sampling to Detect Forest Pathogens" Drones 6, no. 10: 275. https://doi.org/10.3390/drones6100275
APA StylePerroy, R. L., Meier, P., Collier, E., Hughes, M. A., Brill, E., Sullivan, T., Baur, T., Buchmann, N., & Keith, L. M. (2022). Aerial Branch Sampling to Detect Forest Pathogens. Drones, 6(10), 275. https://doi.org/10.3390/drones6100275






