Elasmobranch Use of Nearshore Estuarine Habitats Responds to Fine-Scale, Intra-Seasonal Environmental Variation: Observing Coastal Shark Density in a Temperate Estuary Utilizing Unoccupied Aircraft Systems (UAS)
Abstract
1. Introduction
2. Materials and Methods
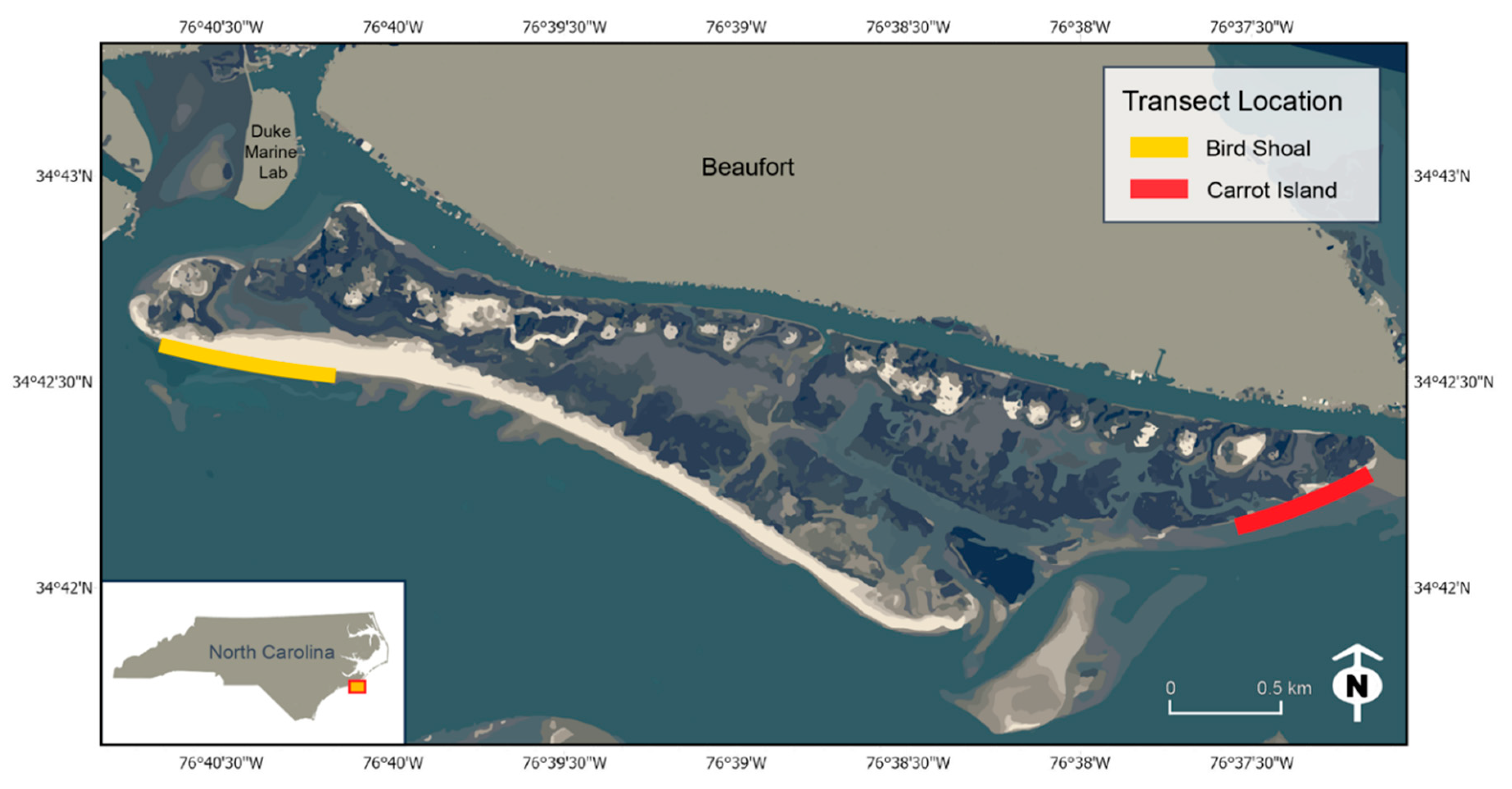
2.1. Study Area
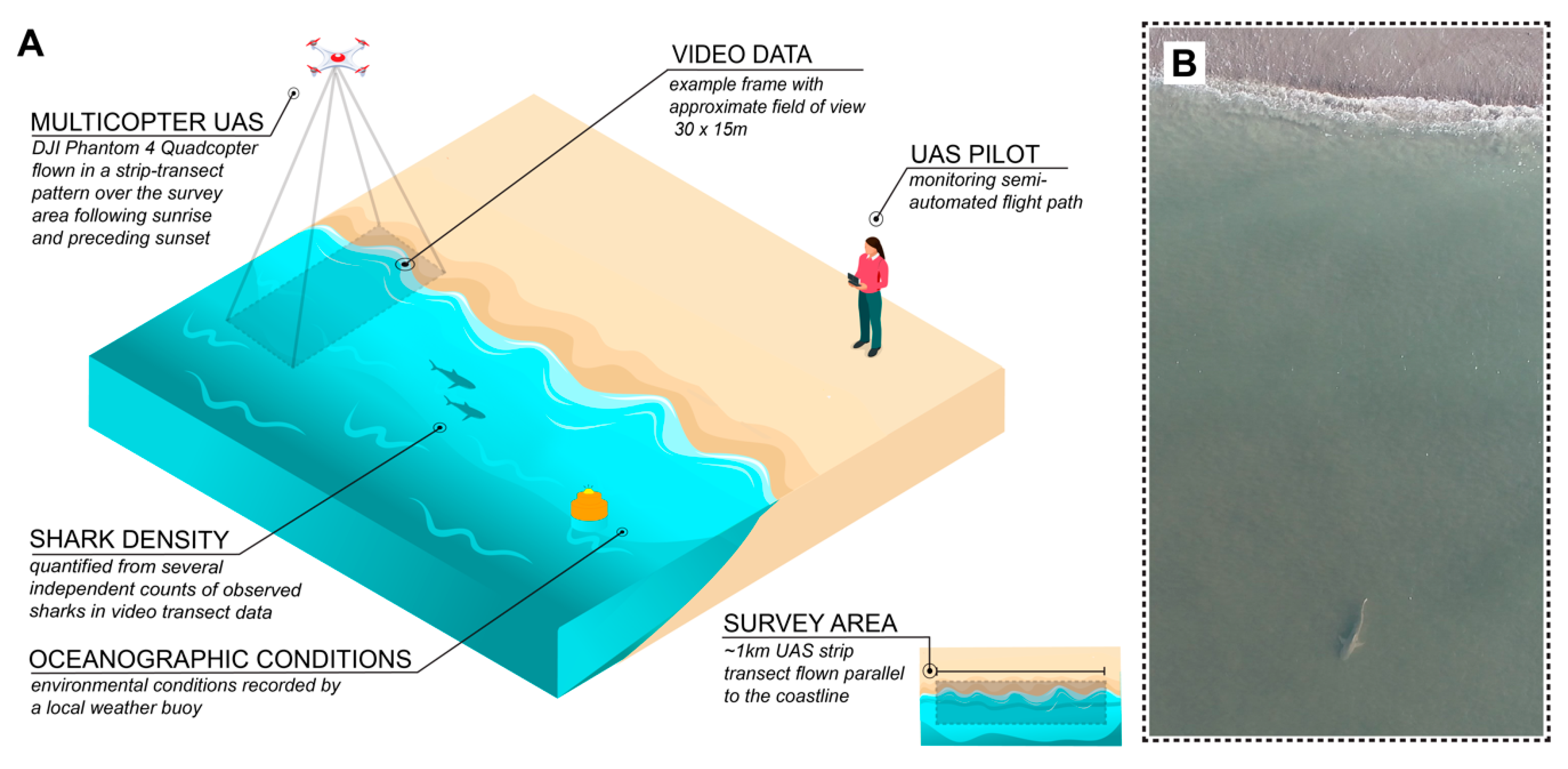
2.2. UAS Imagery and Collection
2.3. Video Observation
2.4. Environmental Data
2.5. Statistical Analyses
2.5.1. Correlation Analyses
2.5.2. Generalized Linear Model (GLM)
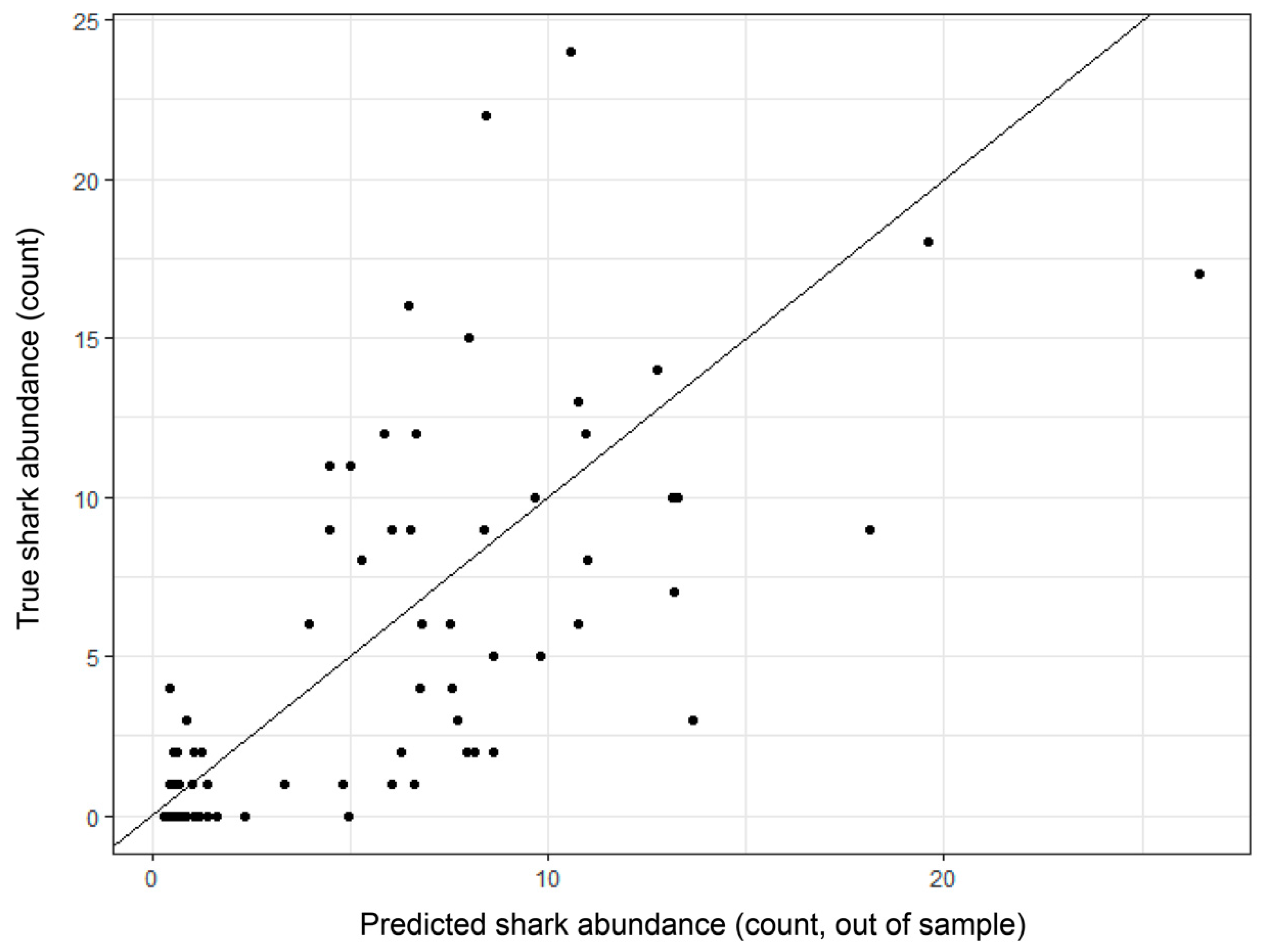
3. Results
3.1. Species Identification
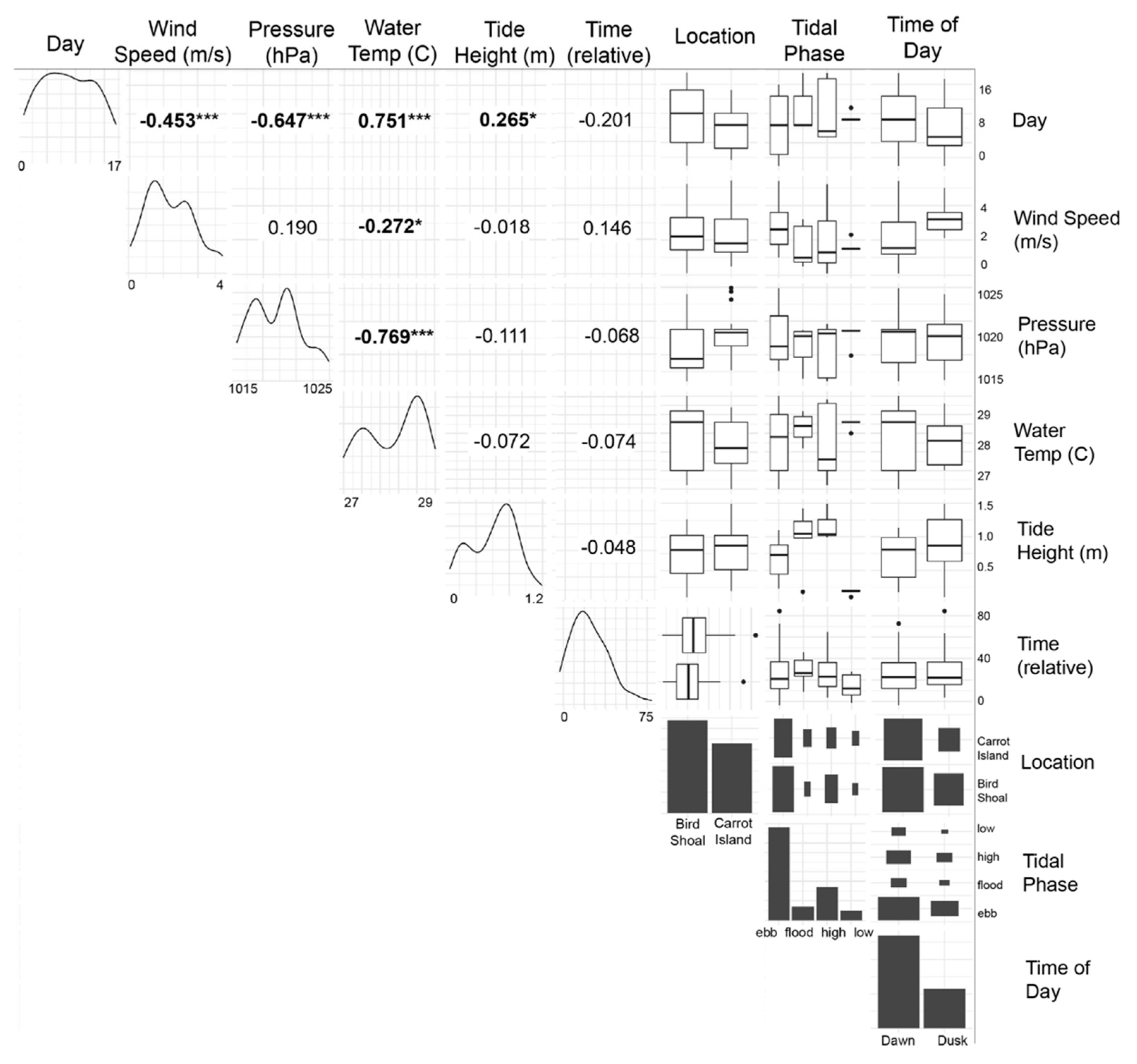
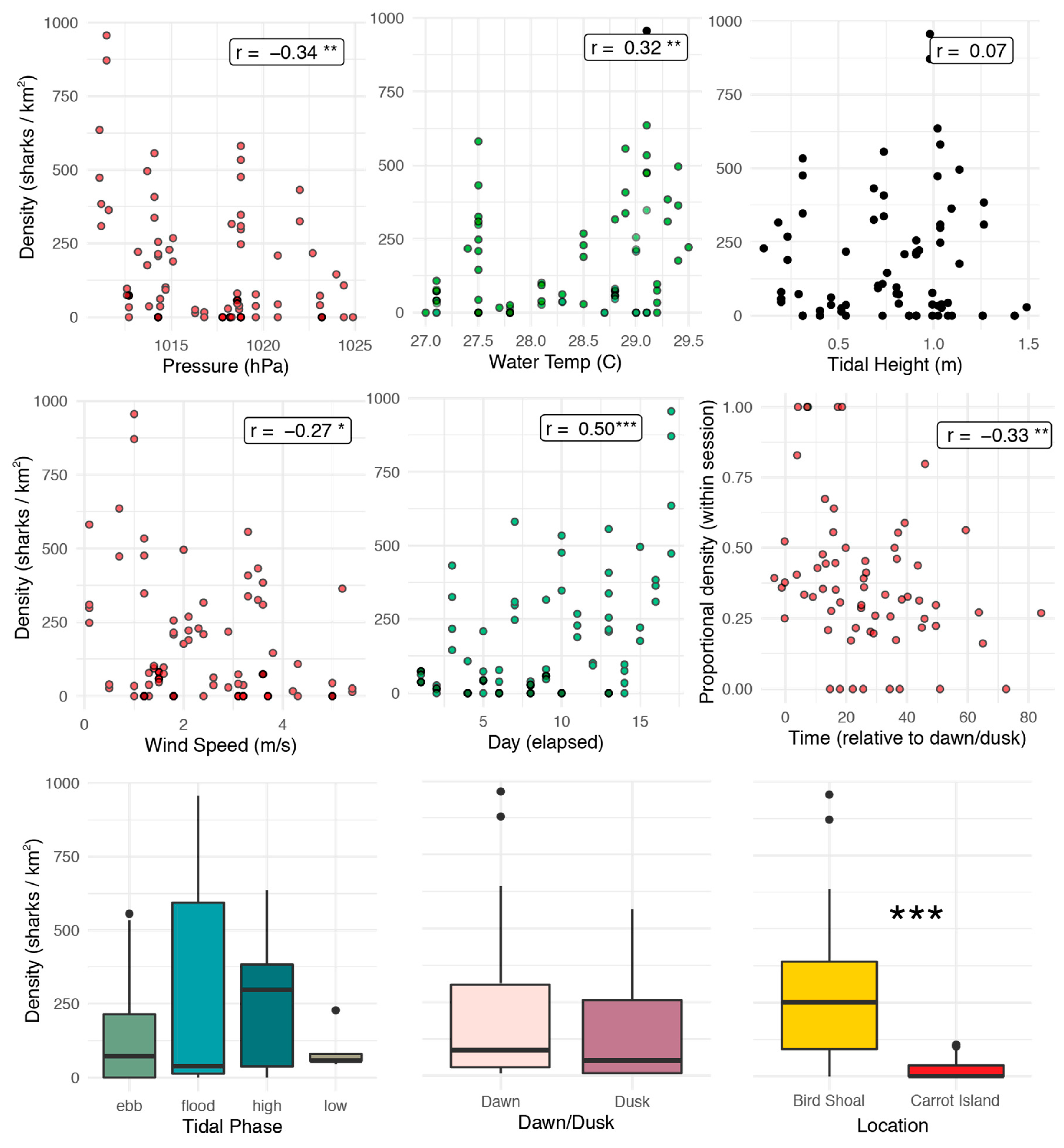
3.2. Correlation Analyses
3.3. Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noss, R.F.; Platt, W.J.; Sorrie, B.A.; Weakley, A.S.; Means, D.B.; Costanza, J.; Peet, R.K. How global biodiversity hotspots may go unrecognized: Lessons from the North American Coastal Plain. Divers. Distrib. 2015, 21, 236–244. [Google Scholar] [CrossRef]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Mehvar, S.; Filatova, T.; Dastgheib, A.; de Ruyter van Steveninck, E.; Ranasinghe, R. Quantifying economic value of coastal ecosystem services: A review. J. Mar. Sci. Eng. 2018, 6, 5. [Google Scholar] [CrossRef]
- Suchanek, T.H. Temperate coastal marine communities: Biodiversity and threats. Integr. Comp. Biol. 1994, 34, 100–114. [Google Scholar] [CrossRef]
- Knip, D.M.; Heupel, M.R.; Simpfendorfer, C.A. Sharks in nearshore environments: Models, importance, and consequences. Mar. Ecol. Prog. Ser. 2010, 402, 1–11. [Google Scholar] [CrossRef]
- Moxley, J.; Skomal, G.; Chisholm, J.; Halpin, P.; Johnston, D. Daily and seasonal movements of Cape Cod gray seals vary with predation risk. Mar. Ecol. Prog. Ser. 2020, 644, 215–228. [Google Scholar] [CrossRef]
- Dill, L.M.; Heithaus, M.R.; Walters, C.J. Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 2003, 84, 1151–1157. [Google Scholar] [CrossRef]
- Bangley, C.W.; Paramore, L.; Dedman, S.; Rulifson, R.A. Delineation and mapping of coastal shark habitat within a shallow lagoonal estuary. PLoS ONE 2018, 13, e0195221. [Google Scholar] [CrossRef]
- Haulsee, D.E.; Breece, M.W.; Miller, D.C.; Wetherbee, B.M.; Fox, D.A.; Oliver, M.J. Habitat selection of a coastal shark species estimated from an autonomous underwater vehicle. Mar. Ecol. Prog. Ser. 2015, 528, 277–288. [Google Scholar] [CrossRef]
- Dean Grubbs, R.; Musick, J.A. Spatial Delineation of Summer Nursery Areas for Juvenile Sandbar Sharks in Chesapeake Bay, Virginia. In Shark Nursery Grounds of the Gulf of Mexico and the East Coast Waters of the United States; American Fisheries Society: New York, NY, USA, 2007; Volume 50, pp. 63–86. Available online: https://fisheries.org/bookstore/all-titles/afs-symposia/54053p/ (accessed on 10 November 2020).
- Sagarese, S.R.; Frisk, M.G.; Cerrato, R.M.; Sosebee, K.A.; Musick, J.A.; Rago, P.J. Application of generalized additive models to examine ontogenetic and seasonal distributions of spiny dogfish (Squalus acanthias) in the Northeast (US) shelf large marine ecosystem. Can. J. Fish. Aquat. Sci. 2014, 71, 847–877. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A.; Freitas, G.G.; Wiley, T.R.; Heupel, M.R. Distribution and habitat partitioning of immature bull sharks (Carcharhinus leucas) in a southwest Florida estuary. Estuaries 2005, 28, 78–85. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Britten, G.L.; Bethea, D.M.; Carlson, J.K. Characterizing and predicting essential habitat features for juvenile coastal sharks. Mar. Ecol. 2015, 36, 419–431. [Google Scholar] [CrossRef]
- Froeschke, J.; Stunz, G.W.; Wildhaber, M.L. Environmental influences on the occurrence of coastal sharks in estuarine waters. Mar. Ecol. Prog. Ser. 2010, 407, 279–292. [Google Scholar] [CrossRef]
- Ellis, J.R.; McCully Phillips, S.R.; Poisson, F. A review of capture and post-release mortality of elasmobranchs. J. Fish Biol. 2017, 90, 653–722. [Google Scholar] [CrossRef] [PubMed]
- Hueter, R.E.; Manire, C.A.; Tyminski, J.P.; Hoenig, J.M.; Hepworth, D.A. Assessing Mortality of Released or Discarded Fish Using a Logistic Model of Relative Survival Derived from Tagging Data. Trans. Am. Fish. Soc. 2006, 135, 500–508. [Google Scholar] [CrossRef]
- Rulifson, R.A. Spiny Dogfish Mortality Induced by Gill-Net and Trawl Capture and Tag and Release. N. Am. J. Fish. Manag. 2007, 27, 279–285. [Google Scholar] [CrossRef]
- Huish, M.T.; Benedict, C. Sonic Tracking of Dusky Sharks in the Cape Fear River, North Carolina. J. Elisha Mitchell Scien. Soc. 1978, 93, 21–26. [Google Scholar]
- Hammerschlag, N.; Gallagher, A.J.; Lazarre, D.M. A review of shark satellite tagging studies. J. Exp. Mar. Biol. Ecol. 2011, 398, 1–8. [Google Scholar] [CrossRef]
- Jewell, O.J.D.; Wcisel, M.A.; Gennari, E.; Towner, A.V.; Bester, M.N.; Johnson, R.L.; Singh, S. Effects of smart position only (SPOT) tag deployment on white sharks carcharodon carcharias in South Africa. PLoS ONE 2011, 6, e27242. [Google Scholar] [CrossRef]
- Rowat, D.; Gore, M.; Meekan, M.G.; Lawler, I.R.; Bradshaw, C.J.A. Aerial survey as a tool to estimate whale shark abundance trends. J. Exp. Mar. Biol. Ecol. 2009, 368, 1–8. [Google Scholar] [CrossRef]
- Kessel, S.T.; Gruber, S.H.; Gledhill, K.S.; Bond, M.E.; Perkins, R.G. Aerial Survey as a Tool to Estimate Abundance and Describe Distribution of a Carcharhinid Species, the Lemon Shark, Negaprion brevirostris. J. Mar. Biol. 2013, 2013, 597383. [Google Scholar] [CrossRef]
- Acuña-Marrero, D.; Smith, A.; Salinas-de-León, P.; Harvey, E.; Pawley, M.; Anderson, M. Spatial patterns of distribution and relative abundance of coastal shark species in the Galapagos Marine Reserve. Mar. Ecol. Prog. Ser. 2018, 593, 73–95. [Google Scholar] [CrossRef]
- Osgood, G.J.; McCord, M.E.; Baum, J.K. Using baited remote underwater videos (BRUVs) to characterize chondrichthyan communities in a global biodiversity hotspot. PLoS ONE 2019, 14, e0225859. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Simpfendorfer, C.A.; Tobin, A.J.; Heupel, M.R. Application of baited remote underwater video surveys to quantify spatial distribution of elasmobranchs at an ecosystem scale. J. Exp. Mar. Biol. Ecol. 2013, 448, 281–288. [Google Scholar] [CrossRef]
- Johnston, D.W. Unoccupied aircraft systems in marine science and conservation. Annu. Rev. Mar. Sci. 2019, 11, 439–463. [Google Scholar] [CrossRef] [PubMed]
- Koski, W.R.; Allen, T.; Ireland, D.; Buck, G.; Smith, P.R.; Macrender, A.M.; Halick, M.A.; Rushing, C.; Sliwa, D.J.; McDonald, T.L. Evaluation of an unmanned airborne system for monitoring marine mammals. Aquat. Mamm. 2009, 35, 347–357. [Google Scholar] [CrossRef]
- Loughlin, T.R.; Perlov, A.S.; Vladimirov, V.A. Range-wide survey and estimation of total number of steller sea lions in 1989. Mar. Mammal Sci. 1992, 8, 220–239. [Google Scholar] [CrossRef]
- Marsh, H.; Sinclair, D.F. An experimental evaluation of dugong and sea turtle aerial survey techniques. Wildl. Res. 1989, 16, 639–650. [Google Scholar] [CrossRef]
- Hensel, E.; Wenclawski, S.; Layman, C.A. Using a small, consumer-grade drone to identify and count marine megafauna in shallow habitats. Lat. Am. J. Aquat. Res. 2018, 46, 1025–1033. [Google Scholar] [CrossRef]
- Kiszka, J.; Mourier, J.; Gastrich, K.; Heithaus, M. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- NC DEQ: Rachel Carson Reserve. Available online: https://deq.nc.gov/about/divisions/coastal-management/nc-coastal-reserve/reserve-sites/rachel-carson-reserve (accessed on 10 November 2020).
- Barnas, A.F.; Chabot, D.; Hodgson, A.J.; Johnston, D.W.; Bird, D.M.; Ellis-Felege, S.N. A standardized protocol for reporting methods when using drones for wildlife research. J. Unmanned Veh. Syst. 2020, 8, 89–98. [Google Scholar] [CrossRef]
- TOOLS—GSD Calculator—Support. Available online: https://support.pix4d.com/hc/en-us/articles/202560249-TOOLS-GSD-calculator (accessed on 10 November 2020).
- Lindén, A.; Mäntyniemi, S. Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 2011, 92, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Moreno, A.; Pérez-Casany, M.; Puig, P.; Morante, M.; Castells, E. What does a zero mean? Understanding false, random and structural zeros in ecology. Methods Ecol. Evol. 2019, 10, 949–959. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B.; Carlin, J.; Stern, H.; Rubin, D.; Dunson, D. Bayesian Data Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Stan Development Team. “RStan: The R Interface to Stan.” R Package Version 2.21.2. 2020. Available online: http://mc-stan.org/ (accessed on 10 November 2020).
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. 2020. Available online: http://www.rstudio.com/ (accessed on 10 November 2020).
- Pamlico Sound Independent Gill Net Survey [2009: July 1–2014: June 30]—State Publications II—North Carolina Digital Collections. Available online: https://digital.ncdcr.gov/digital/collection/p16062coll9/id/170689/rec/7 (accessed on 10 November 2020).
- Schwartz, F.J. Occurrence, Abundance, and Biology of the Blacknose Shark, Carcharhinus acronotus, in North Carolina. Northeast Gulf Sci. 1984, 7, 29–47. [Google Scholar] [CrossRef][Green Version]
- Schlaff, A.M.; Heupel, M.R.; Simpfendorfer, C.A. Influence of environmental factors on shark and ray movement, behaviour and habitat use: A review. Rev. Fish Biol. Fish. 2014, 24, 1089–1103. [Google Scholar] [CrossRef]
- Elliott, A.; Aj, E. Wind-Driven Flow in a Shallow Estuary. Oceanologica Acta 1982, 5, 7–10. [Google Scholar]
- Benavides, M.T.; Fodrie, F.J.; Johnston, D.W. Shark detection probability from aerial drone surveys within a temperate estuary. J. Unmanned Veh. Syst. 2020, 8, 44–56. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A.; Hueter, R.E. Running before the storm: Blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle. J. Fish Biol. 2003, 63, 1357–1363. [Google Scholar] [CrossRef]
- Andrews, K.S.; Williams, G.D.; Farrer, D.; Tolimieri, N.; Harvey, C.J.; Bargmann, G.; Levin, P.S. Diel activity patterns of sixgill sharks, Hexanchus griseus: The ups and downs of an apex predator. Anim. Behav. 2009, 78, 525–536. [Google Scholar] [CrossRef]
- Nelson, D.R.; McKibben, J.N.; Strong, W.R.; Lowe, C.G.; Sisneros, J.A.; Schroeder, D.M.; Lavenberg, R.J. An acoustic tracking of a megamouth shark, Megachasma pelagios: A crepuscular vertical migrator. Environ. Biol. Fishes 1997, 49, 389–399. [Google Scholar] [CrossRef]
- Gorkin, R.; Adams, K.; Berryman, M.J.; Aubin, S.; Li, W.; Davis, A.R.; Barthelemy, J. Sharkeye: Real-Time Autonomous Personal Shark Alerting via Aerial Surveillance. Drones 2020, 4, 18. [Google Scholar] [CrossRef]
- Brooks, E.; Sloman, K.; Sims, D.; Danylchuk, A. Validating the use of baited remote underwater video surveys for assessing the diversity, distribution and abundance of sharks in the Bahamas. Endanger. Species Res. 2011, 13, 231–243. [Google Scholar] [CrossRef]

| Variable | Expected Relationship with Shark Density |
|---|---|
| Tidal Height | + |
| Tidal Phase | High: +, Ebb: −, Low: −, Flood: + |
| Water Temperature | + |
| Wind Speed | − |
| Barometric Pressure | + |
| Time of Day | Dawn: NA, Dusk: NA |
| Time (minutes elapsed from dawn/dusk) | − |
| Day Count | − |
| Location | Bird Shoal: +, Carrot Island: − |
| Variable | Source | Recording Interval | Data Details |
|---|---|---|---|
| Tidal Height (m) | NOAA Tide & Currents, Tides/Water Levels Station ID: 8656483, Duke Marine Lab, Beaufort, NC | 6 min | Water level relative to Mean Lower Low Water (MLLW) |
| Tidal Phase | NOAA Tide & Currents, Tides/Water Levels Station ID: 8656483, Duke Marine Lab, Beaufort, NC | 1 min | Defined as: High: within ± 1 h of reported high tide Low: within ± 1 h of reported low tide Ebb: Water level decreasing, from high to low tide Flood: Water level increasing, from low to high tide |
| Water temperature (°C) | NOAA Tide & Currents, Meteorological Observations Station ID: 8656483, Duke Marine Lab, Beaufort, NC | 6 min | |
| Wind speed (m/s) | NOAA Tide & Currents, Meteorological Observations Station ID: 8656483, Duke Marine Lab, Beaufort, NC | 6 min | |
| Pressure (hPa) | NOAA Tide & Currents, Meteorological Observations Station ID: 8656483, Duke Marine Lab, Beaufort, NC | 6 min | |
| Time of Day | UAS Aircraft Log: Time | NA | Defined as: Dawn: Transects following sunrise Dusk: Transects preceding sunset |
| Time relative to dawn/dusk (minutes) | UAS Aircraft Log: Time | 1 min | Calculated as: time elapsed since sunrise (Dawn transects) time remaining until sunset (Dusk transects) |
| Location | UAS Aircraft Log: GPS | NA | Bird Shoal (34.708503, −76.677384) Carrot Island (34.704096, −76.620851) (See Figure 1) |
| Day Count | UAS Aircraft Log: Date | NA | Defined as days elapsed from the start of sampling (dayCount = 1) to the end of sampling (dayCount = 17). To highlight temporal trends. |
| Covariate | Parameter | Mean | Median | Standard Deviation | 2.5th Percentile | 97.5th Percentile |
|---|---|---|---|---|---|---|
| Intercept | Β1 | 0.754 | 0.759 | 0.191 | 0.366 | 1.111 |
| Wind Speed | Β2 | −0.218 | −0.218 | 0.149 | −0.517 | 0.074 |
| Water Temperature | Β3 | 0.118 | 0.121 | 0.165 | −0.214 | 0.435 |
| Time Relative to Dawn/Dusk | Β4 | −0.256 | −0.256 | 0.073 | −0.401 | −0.114 |
| Location (Carrot Island) | Β5 | −2.564 | −2.554 | 0.375 | −3.310 | −1.861 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiGiacomo, A.E.; Harrison, W.E.; Johnston, D.W.; Ridge, J.T. Elasmobranch Use of Nearshore Estuarine Habitats Responds to Fine-Scale, Intra-Seasonal Environmental Variation: Observing Coastal Shark Density in a Temperate Estuary Utilizing Unoccupied Aircraft Systems (UAS). Drones 2020, 4, 74. https://doi.org/10.3390/drones4040074
DiGiacomo AE, Harrison WE, Johnston DW, Ridge JT. Elasmobranch Use of Nearshore Estuarine Habitats Responds to Fine-Scale, Intra-Seasonal Environmental Variation: Observing Coastal Shark Density in a Temperate Estuary Utilizing Unoccupied Aircraft Systems (UAS). Drones. 2020; 4(4):74. https://doi.org/10.3390/drones4040074
Chicago/Turabian StyleDiGiacomo, Alexandra E., Walker E. Harrison, David W. Johnston, and Justin T. Ridge. 2020. "Elasmobranch Use of Nearshore Estuarine Habitats Responds to Fine-Scale, Intra-Seasonal Environmental Variation: Observing Coastal Shark Density in a Temperate Estuary Utilizing Unoccupied Aircraft Systems (UAS)" Drones 4, no. 4: 74. https://doi.org/10.3390/drones4040074
APA StyleDiGiacomo, A. E., Harrison, W. E., Johnston, D. W., & Ridge, J. T. (2020). Elasmobranch Use of Nearshore Estuarine Habitats Responds to Fine-Scale, Intra-Seasonal Environmental Variation: Observing Coastal Shark Density in a Temperate Estuary Utilizing Unoccupied Aircraft Systems (UAS). Drones, 4(4), 74. https://doi.org/10.3390/drones4040074







