Abstract
This work reports on printed organic PEDOT:PSS sensors for the detection of hydrogen peroxide (H2O2) vapors. Compared to commercial devices, the proposed sensors are thin and flexible, allowing their simple integration into an industrial process for multilocation decontamination monitoring. The sensors show a reproducible behavior in a laboratory environment using chambers containing a concentration of 130 ppm of H2O2, measuring an increase in resistance of ~1200% for 1 h exposure. The results highlight the importance of contact interfaces and their encapsulation for reproducible sensing behavior. These devices are promising as low-cost disposable devices for decontamination process monitoring.
1. Introduction
Hydrogen peroxide (H2O2) decontamination processes are widely used in the pharmaceutical and healthcare industry for their robust microbial inactivation effect. Commercially available H2O2 vapor concentration sensor systems are quite bulky and do not allow multilocation mapping experiments. Mapping studies are performed with chemical and biological indicators, which act as integrators and lack the ability to provide in-process real-time data. A low-cost, low-profile and disposable sensor able to measure H2O2 vapor concentration at multiple locations in real time would enable significant improvements in H2O2 vapor process development and qualification strategies.
2. Materials and Methods
Poly(3,4-ethylene dioxythiophene):polystyrene sulfonate (PEDOT:PSS) is one of the most studied organic materials thanks to its stability, high conductivity, printability, and sensitivity to multiple stimulus. Here we implemented PEDOT:PSS-based resistors on flexible polyimide substrates by inkjet printing (Dimatix DMP, Santa Clara, CA, USA) for H2O2 vapor detection, as shown in Figure 1. The PEDOT:PSS solution (1.3 wt. %, Sigma Aldrich, St-Louis, MO, USA) was mixed with 5% vol of DMSO before printing two layers of material on the gold evaporated contacts. The responses of the devices to hydrogen peroxide vapor were tested using a glass chamber (400) and adding 30 mL of liquid H2O2 (Sigma Aldrich 30%). A commercial sensor (Vaisala HPP272, Vantaa, Finland) was added to monitor the formation and concentration of H2O2 vapor reaching a stable concentration of ~130 ppm after ~5 min (RS ~95% at 24.8 °C). Different contact configurations were tested as they are influential on the sensing behavior. For measuring the PEDOT:PSS resistors, a silver glue was employed for connecting Teflon-coated electrical wires to the gold contacts, optionally covering them partially (i.e., only on the silver glue) or fully (i.e., covering gold contact and overlapping on PEDOT:PSS with a protective epoxy (Epo-Tek H70E-2, Billerica, MA, USA). Another configuration involved u-shaped PEDOT:PSS resistors with the electrical contacts taken outside of the chamber using a ZIF connector. The real-time variations in resistance of the sensors under H2O2 vapor exposure were monitored with a custom-made LABVIEW program.
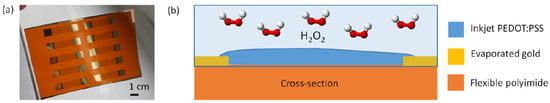
Figure 1.
Printed PEDOT:PSS resistors. (a) The fabricated flexible sensors and (b) schematic of the cross-section of the device, including materials used and the application for H2O2 detection.
3. Results and Discussion
The simultaneous measurement of two sensors in the test chamber was performed during 1 h, and variations in the resistance observed are presented in Figure 2. The resistance in all cases increased in the presence of H2O2 in a non-linear way over time. With the contacts partially covered with the epoxy, a variation of resistance of 1160 ± 652% (n = 6) was measured in the tested conditions with a large deviation between the sensors. When fully covering the contacts with the epoxy (arriving at the PEDOT:PSS interface), a lower variation of 624 ± 50% (n = 2) was measured, but with more reproducible results between the sensors. When using a different device design with the contacts placed outside the chamber, a lower variation in resistance was also measured, equal to 434 ± 133% (n = 2, Figure 2f). This indicates that the contributions to the sensor response came not only from the interaction of H2O2 with the PEDOT:PSS resistor, but also with the contact heterojunction. Sensors have been successfully tested for the monitoring of an industrial H2O2 decontamination process.
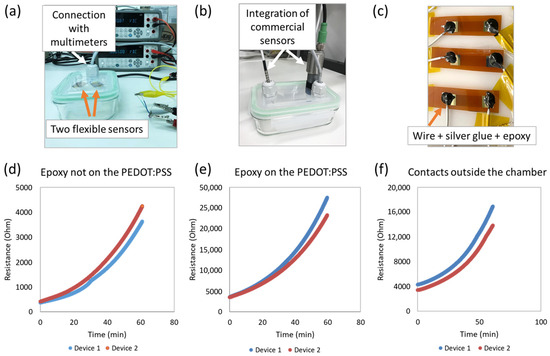
Figure 2.
Setup and results. (a) Picture of the test setup. (b) Image of sensors with wiring. (c) Image of a sensor with ZIF connector. (d–f) Response to H2O2 vapor for sensors with different connections.
Author Contributions
Sensors design and fabrication, S.D., J.K. and D.B.; sensors testing, S.D., J.K., D.B. and M.N.; supervision, D.B., M.N. and G.H.; writing—original draft, S.D. and D.B.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SKAN AG.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Martin Novák and Gregor Hommes was employed by SKAN AG. The authors declare that this study received funding from SKAN AG. The funder had the following involvement with the study: the funder contributed to the study by providing test equipment.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).