Zn-Based Triphenylene Metal–Organic Frameworks as a Chemiresistive Platform for Methane Detection †
Abstract
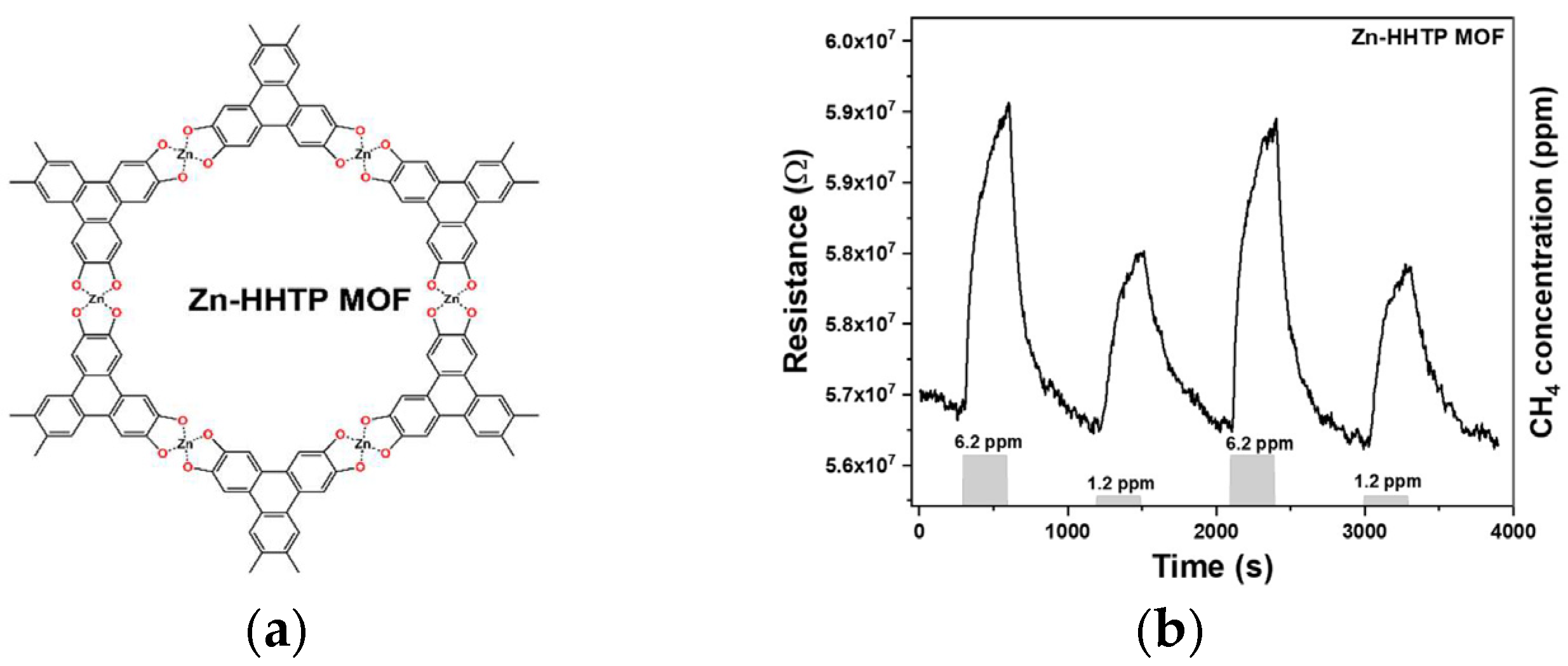
1. Introduction
2. Materials and Methods
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bezdeka, M.; Luo, S.; Ku, K.; Swager, T. A chemiresistive methane sensor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022515118. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Koo, W.; Jang, J.; Kim, L. Metal-organic frameworks for chemiresistive sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Lan, X.; Thoning, K.W.; Dlugokencky, E.J. Trends in globally-averaged CH4, N2O, and SF6 determined from NOAA Global Monitoring Laboratory measurements. Version 2024-03. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navale, S.; Fort-Grandas, I.; Mendoza, Y.; Pellegrino, P.; Moreno, M.; Sainz, D.; Vidal-Ferran, A.; Romano-Rodriguez, A. Zn-Based Triphenylene Metal–Organic Frameworks as a Chemiresistive Platform for Methane Detection. Proceedings 2024, 97, 137. https://doi.org/10.3390/proceedings2024097137
Navale S, Fort-Grandas I, Mendoza Y, Pellegrino P, Moreno M, Sainz D, Vidal-Ferran A, Romano-Rodriguez A. Zn-Based Triphenylene Metal–Organic Frameworks as a Chemiresistive Platform for Methane Detection. Proceedings. 2024; 97(1):137. https://doi.org/10.3390/proceedings2024097137
Chicago/Turabian StyleNavale, Sachin, Ignasi Fort-Grandas, Yuzelfy Mendoza, Paolo Pellegrino, Mauricio Moreno, Daniel Sainz, Anton Vidal-Ferran, and Albert Romano-Rodriguez. 2024. "Zn-Based Triphenylene Metal–Organic Frameworks as a Chemiresistive Platform for Methane Detection" Proceedings 97, no. 1: 137. https://doi.org/10.3390/proceedings2024097137
APA StyleNavale, S., Fort-Grandas, I., Mendoza, Y., Pellegrino, P., Moreno, M., Sainz, D., Vidal-Ferran, A., & Romano-Rodriguez, A. (2024). Zn-Based Triphenylene Metal–Organic Frameworks as a Chemiresistive Platform for Methane Detection. Proceedings, 97(1), 137. https://doi.org/10.3390/proceedings2024097137






