Automated Sorting System for Skeletal Deformities in Cultured Fishes †
Abstract
1. Introduction
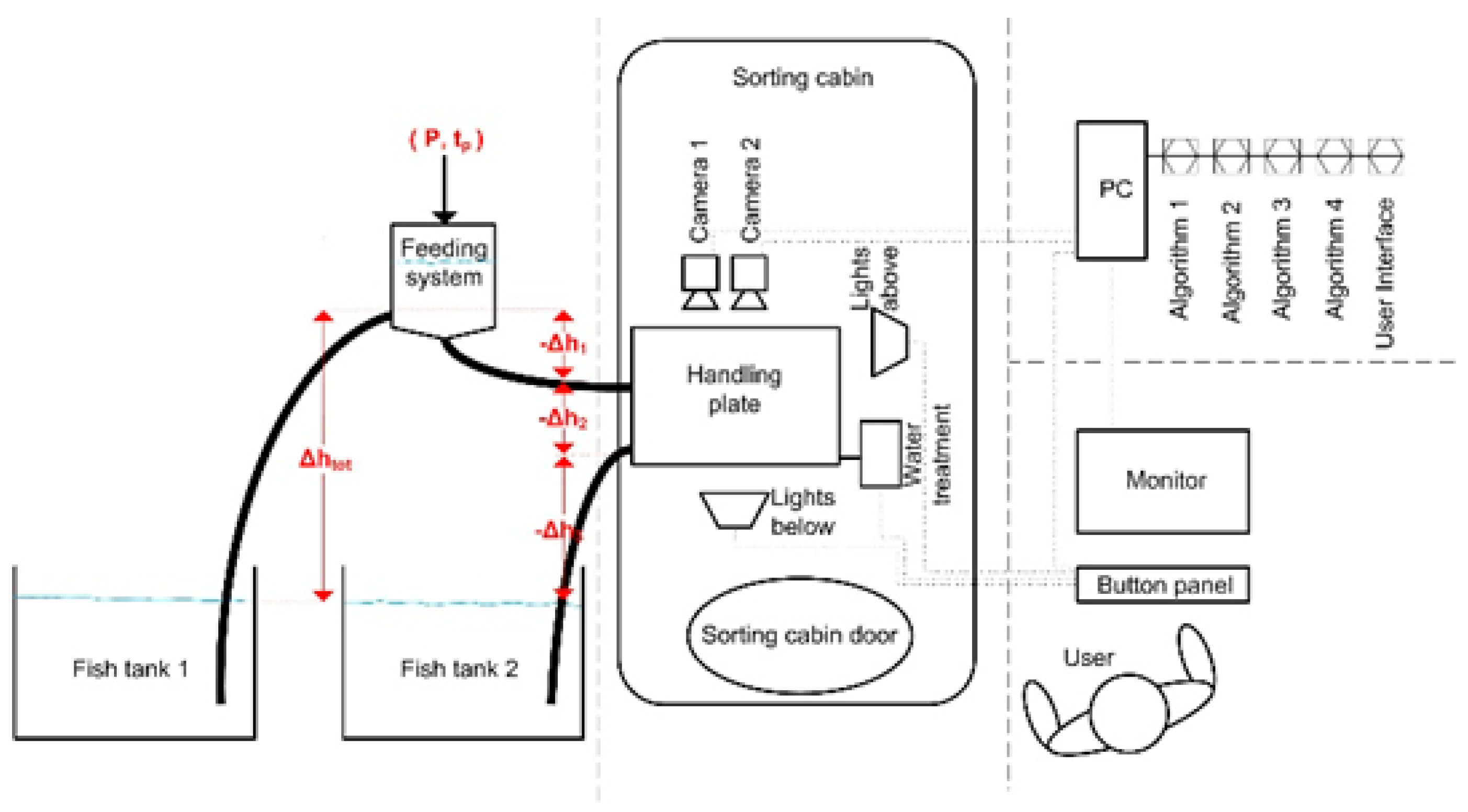
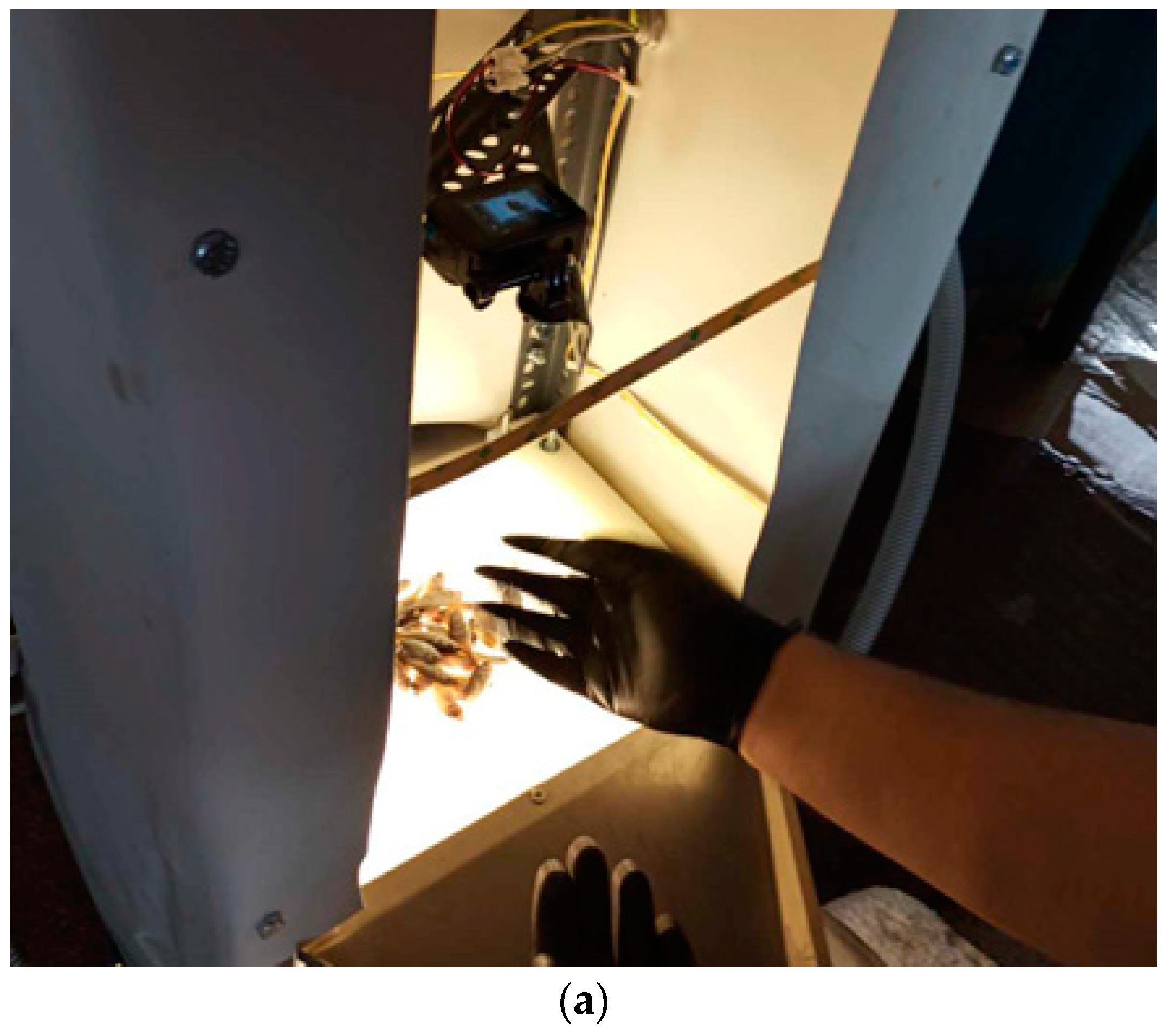
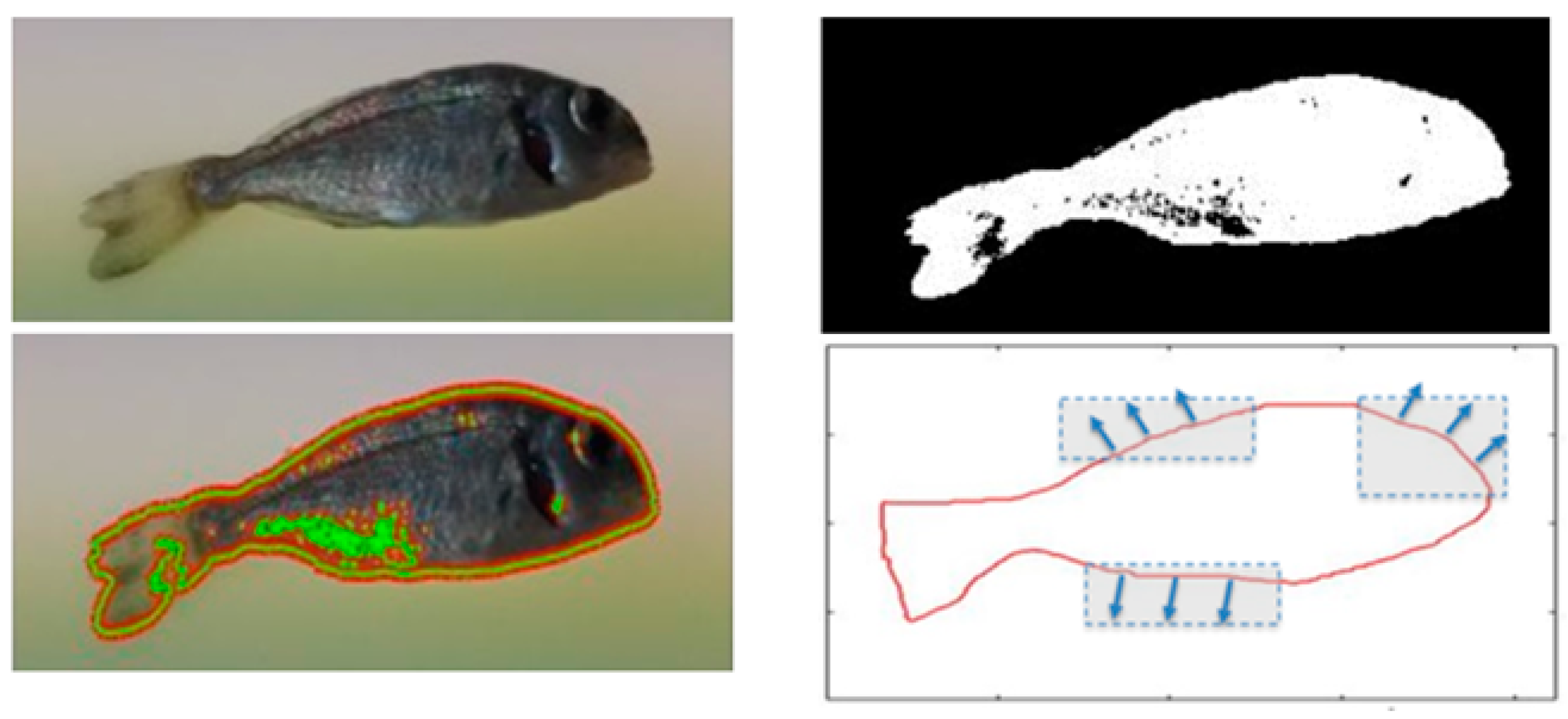
2. Methods
3. Results
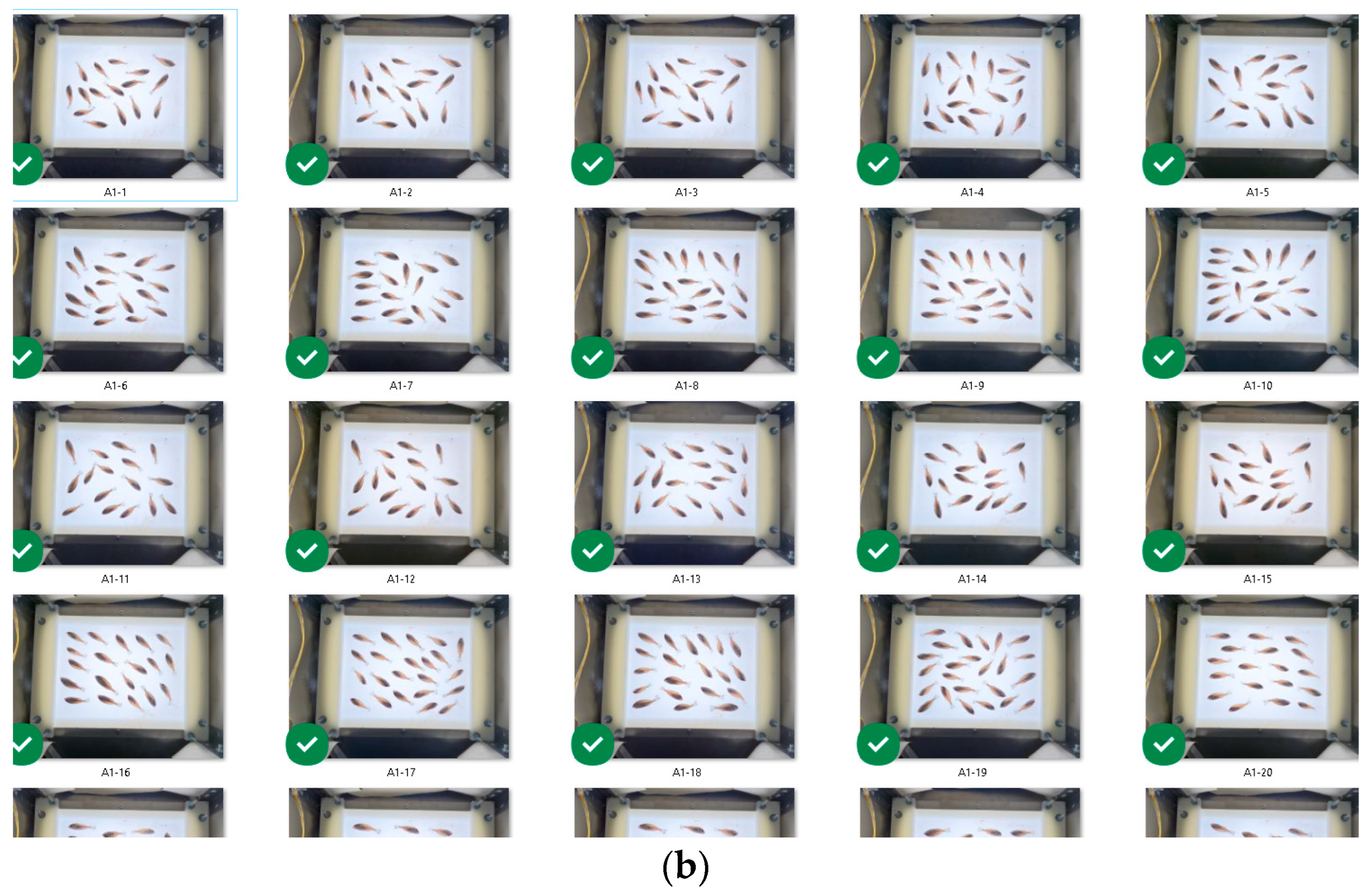
- Step 1: A batch of a random number of fish was acquired, and the fish were manually counted to achieve 1394 fish.
- Step 2: The batch was fed to the system, and 124 fish were classified as “discard”. Also, the total number of the fish was automatically counted to 1394, which was valid.
- Step 3: The fish that were classified as “discard” were manually separated and examined by an experienced worker, and two (2) were sorted as “acceptable”.
- Step 4: The initial batch was formed again by mixing fish and then was manually sorted by an experienced worker. The number of fish that were found to be non-acceptable was 138. This led to the conclusion that the device had an 88.4% recognition success rate.
- Step 5: The manually pre-sorted batch that contained 1256 acceptable fish was fed to the device, and three were classified as “discard”.
- Step 6: The manually pre-sorted batch that contained 138 non-acceptable fish was fed to the device, and 123 of them were classified as “discard”, which led to the conclusion of an 89.1% recognition success rate, which is slightly higher than that in the previous test.
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katavic, I. Diet Involvement in Mass Mortality of Sea Bass (Dicentrarchus labrax) Larvae. Aquaculture 1986, 58, 45–54. [Google Scholar] [CrossRef]
- Foscarini, R. A review: Intensive farming procedure for red sea bream (Pagrus major) in Japan. Aquaculture 1988, 72, 191–246. [Google Scholar] [CrossRef]
- Carmichael, G.J.; Tomasso, J.R. Swim bladder stress syndrome in largemouth bass. Tex. J. Sci. 1984, 35, 315321. [Google Scholar]
- Divanach, P.; Papandroulakis, N.; Anastasiadis, P.; Koumoundouros, G.; Kentouri, M. Effect of water currents during postlarval and nursery phase on the development of skeletal deformities in sea bass (Dicentrarchus labrax L.) with functional swim-bladder. Aquaculture 1997, 156, 145–155. [Google Scholar] [CrossRef]
- Browder, A.J.; McClellan, D.B.; Harper, D.E.; Michael, G. Kandrashoff, M.G.; Walter Kandrashoff, W. A major developmental defect observed in several Biscayne Bay, Florida, fish species. Environ. Biol. Fishes 1993, 37, 181–188. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Boglione, C.; Cataudella, S.; Kentouri, M. Normal and abnormal osteological development of caudal fin in Sparus aurata L. fry. Aquaculture 1997, 149, 215–226. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Oran, G.; Divanach, P.; Stefanakis, S.; Kentouri, M. The opercular complex deformity in intensive gilthead sea bream (Sparus aurata L.) larviculture. Moment of apparition and description. Aquaculture 1997, 156, 165–177. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Divanach, P.; Savaki, A.; Kentouri, M. Effects of three preservation methods on the evolution of swimbladder radiographic appearance in sea bass and sea bream juveniles. Aquaculture 2000, 182, 17–25. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Sfakianakis, S.; Maingot, E.; Divanach, P.; Kentouri, M. Osteological development of the vertebral column and of the fins in Diplodus sargus (Teleostei: Perciformes: Sparidae). Mar. Biol. 2001, 139, 853–862. [Google Scholar]
- Corti, M.; Loy, A.; Cataudella, S. Form changes in the sea bass, Dicentrarchus labrax (Moronidae: Teleostei), after acclimation to freshwater: An analysis using shape coordinates. Environ. Biol. Fishes 1996, 47, 165–175. [Google Scholar] [CrossRef]
- Korwin-Kossakowski, M.; Myszkowski, L.; Kaminski, R. A simple method to detect body morphological abnormalities in juvenile cyprinid fish—A case study on ide Leuciscus idus. Aquaculture 2017, 25, 915–925. [Google Scholar] [CrossRef]
- Boglione, C.; Gisbert, E.; Gavaia, P.; Witten, P.E.; Moren, M.; Fontagné, S.; Koumoundouros, G. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev. Aquac. 2013, 5, 121–167. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellis, G.; Papaggelos, P.; Vlachogianni, E.; Laleas, I.; Moustos, S.; Patas, T.; Poulios, S.; Tzioumakis, N.; Giakas, G.; Bokas, D.; et al. Automated Sorting System for Skeletal Deformities in Cultured Fishes. Proceedings 2024, 94, 58. https://doi.org/10.3390/proceedings2024094058
Bellis G, Papaggelos P, Vlachogianni E, Laleas I, Moustos S, Patas T, Poulios S, Tzioumakis N, Giakas G, Bokas D, et al. Automated Sorting System for Skeletal Deformities in Cultured Fishes. Proceedings. 2024; 94(1):58. https://doi.org/10.3390/proceedings2024094058
Chicago/Turabian StyleBellis, George, Paris Papaggelos, Evangeli Vlachogianni, Ilias Laleas, Stefanos Moustos, Thanos Patas, Sokratis Poulios, Nikos Tzioumakis, Giannis Giakas, Dimitris Bokas, and et al. 2024. "Automated Sorting System for Skeletal Deformities in Cultured Fishes" Proceedings 94, no. 1: 58. https://doi.org/10.3390/proceedings2024094058
APA StyleBellis, G., Papaggelos, P., Vlachogianni, E., Laleas, I., Moustos, S., Patas, T., Poulios, S., Tzioumakis, N., Giakas, G., Bokas, D., Kokkotis, C., & Tsaopoulos, D. (2024). Automated Sorting System for Skeletal Deformities in Cultured Fishes. Proceedings, 94(1), 58. https://doi.org/10.3390/proceedings2024094058






