Synthesis New of Nucleoside of 1,3-bis-(2,3,5-tri-O-Benzoyl-β-D-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone †
Abstract
:1. Introduction
2. Experimental
2.1. General
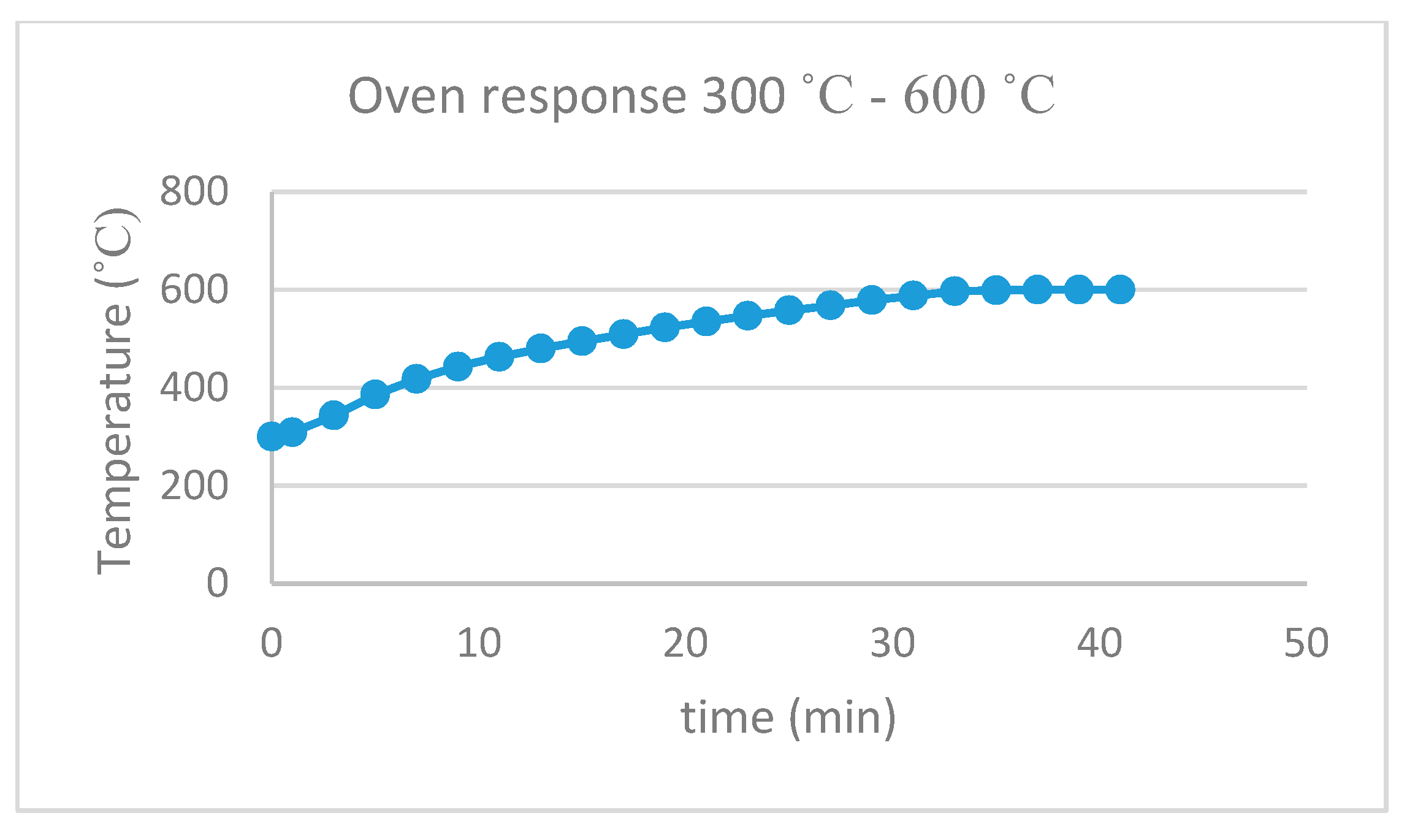
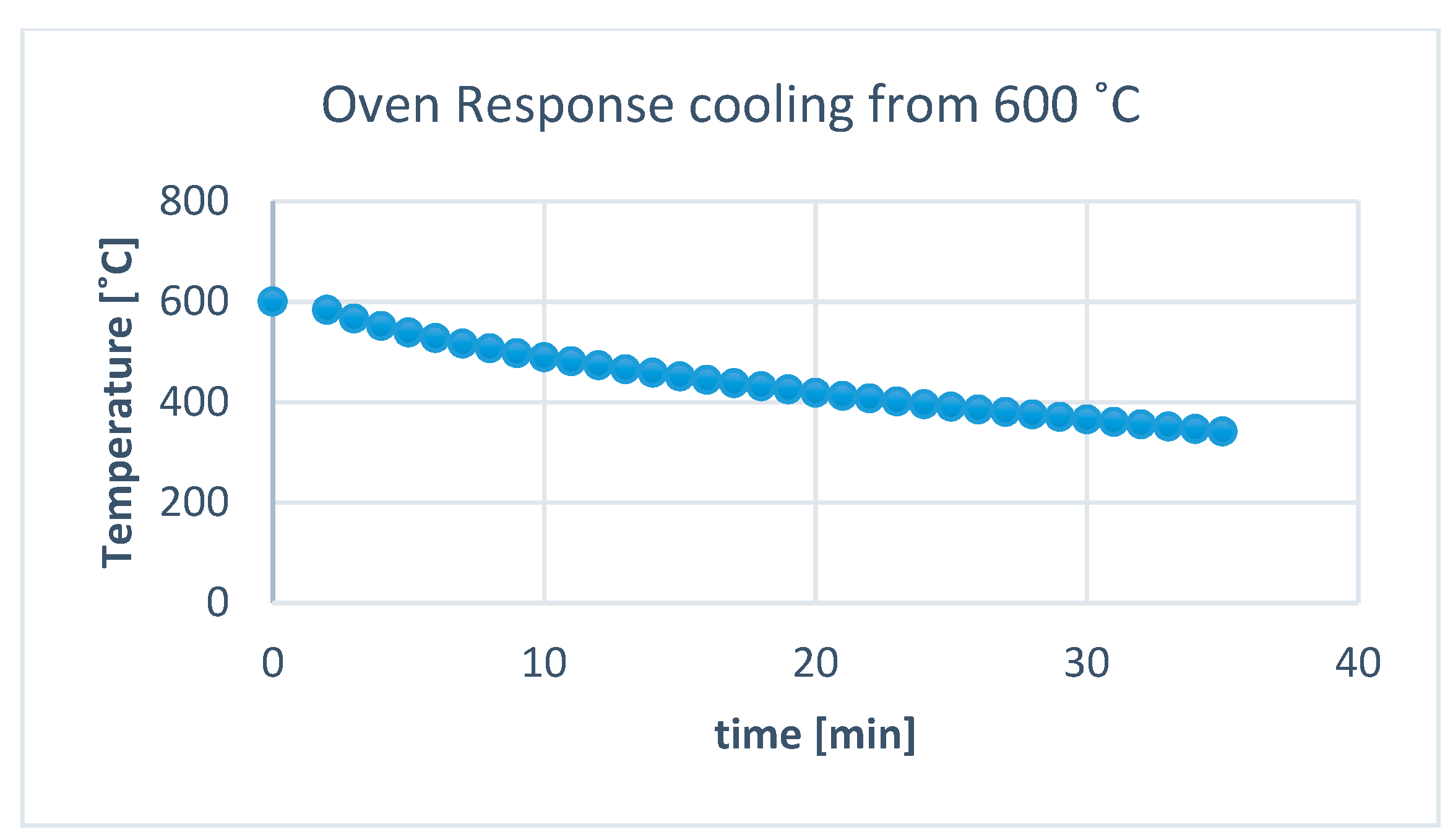
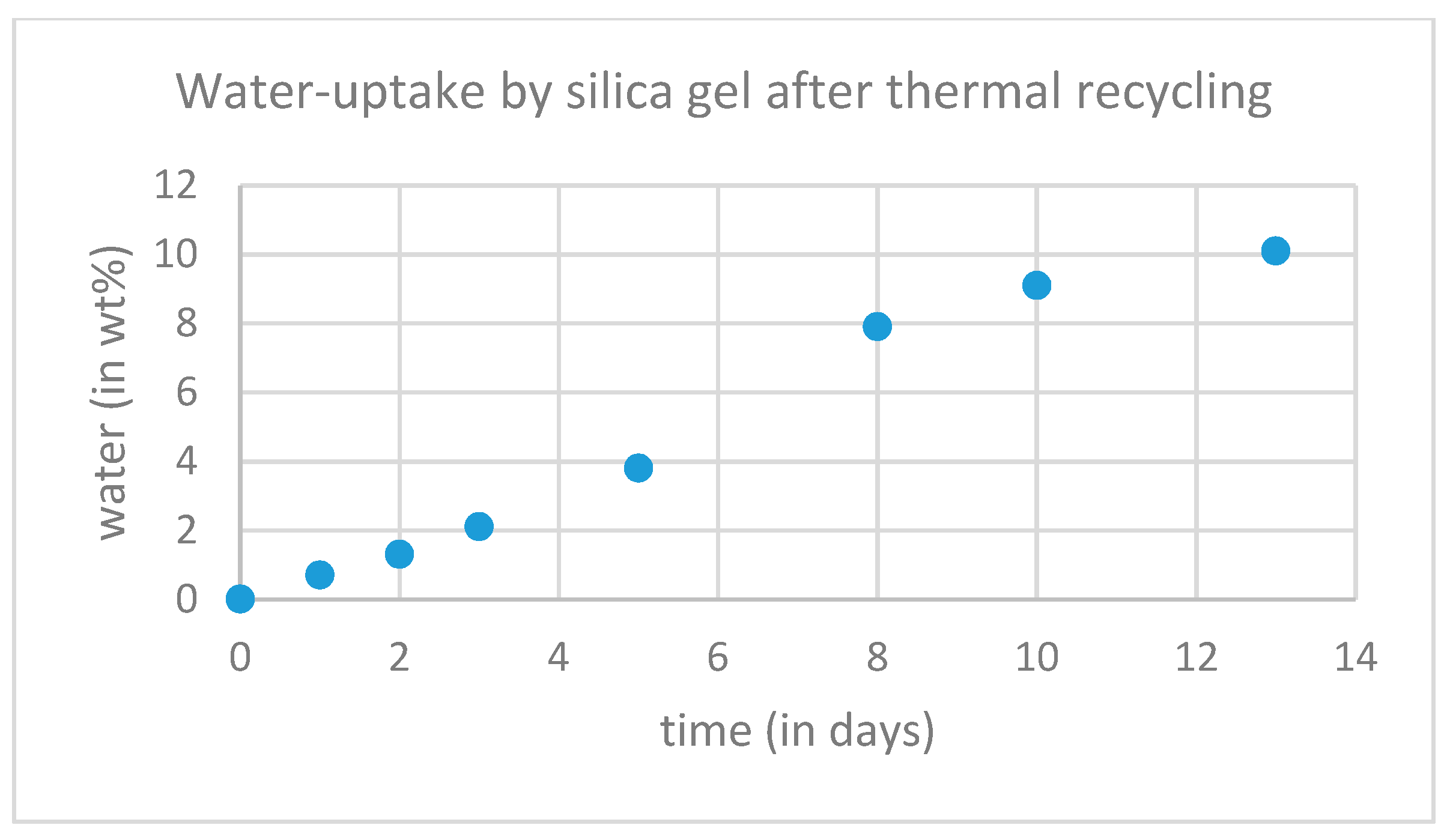
2.2. Thermal Recycling of the Silica Gel
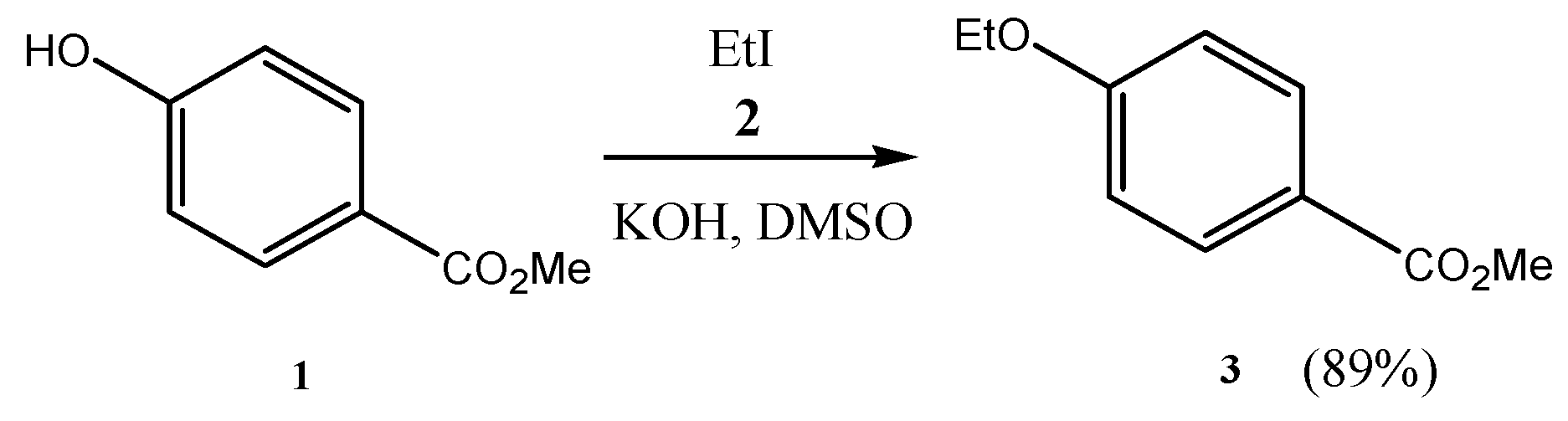
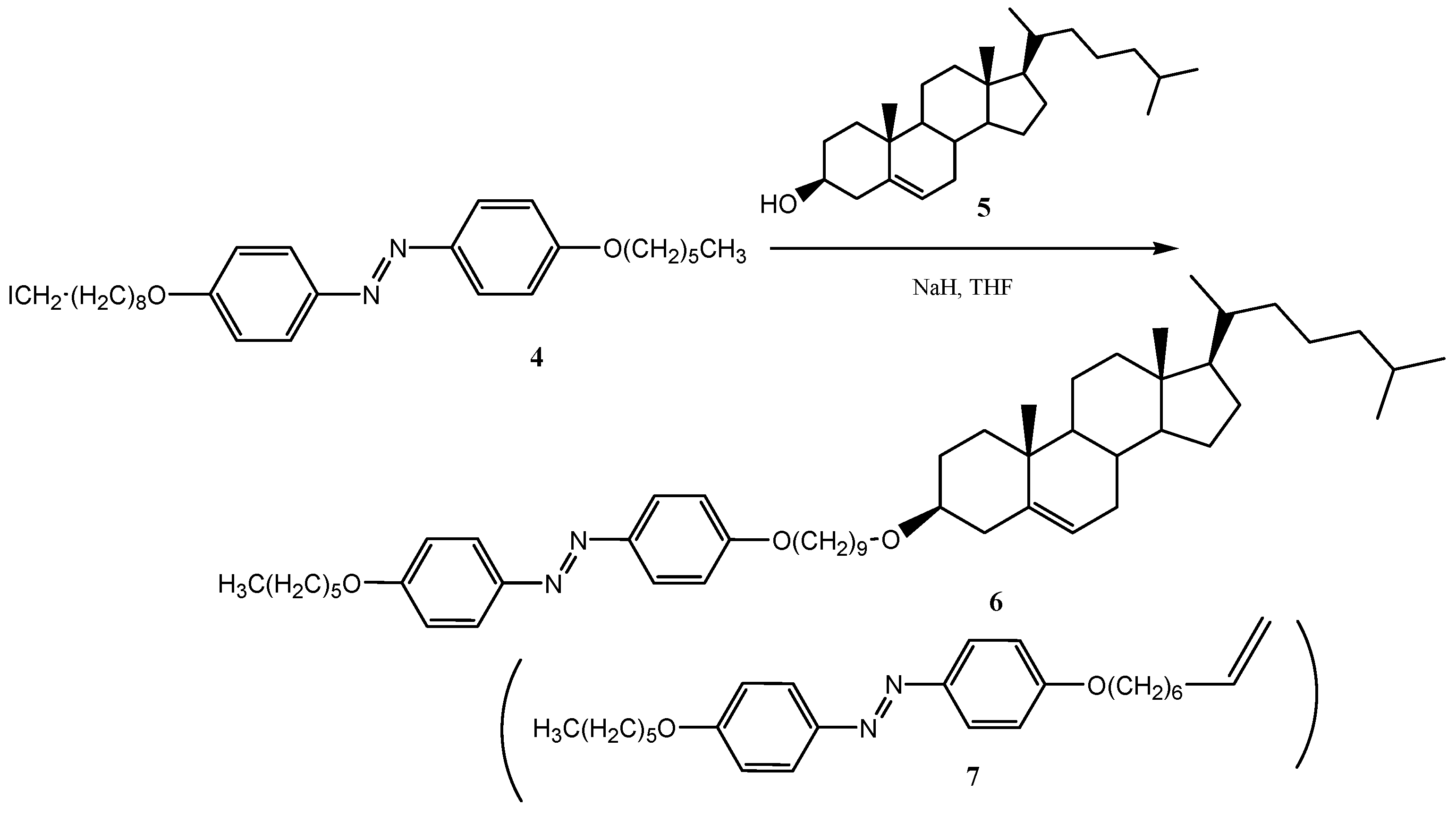
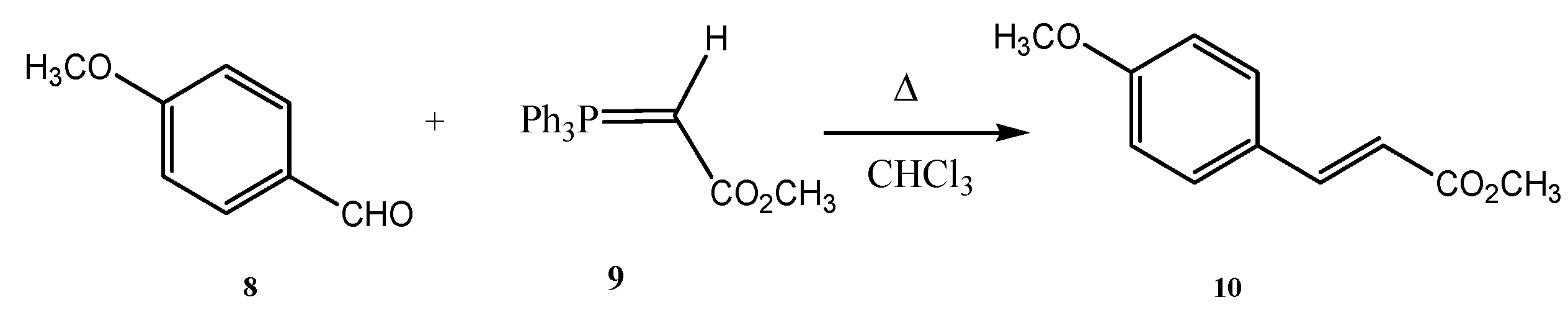
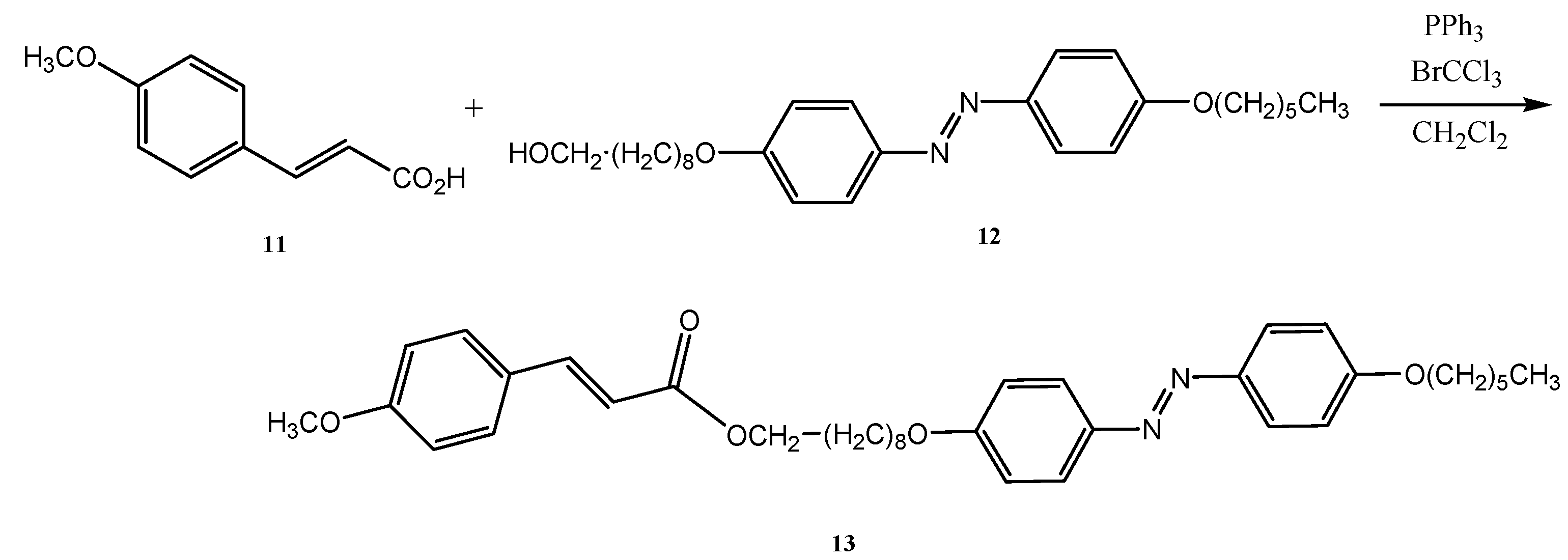
2.3. Preparation of Isopropyl 4-Hydroxybenzoate (16) Using Recycled Silica Gel in the Purification Step
3. Results and Discussion
4. Environmental Impact Assessment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loureiro, A.P.; de Souza, J.A.; Aparecido, D.; Fenrandes, J.B. Recuperação de silica gel: Nova alternative. Química Nova 1991, 14, 112–112. [Google Scholar]
- Andreão, P.S.S.; Giacomini, R.A.; Stumbo, A.M.; Waldman, W.R.; Braz-Filho, R.; Ligiéro, C.B.P.; Miranda, P.C.M.L. Utilização e recuperação de sílica gel impregnada com nitrato de prata. Química Nova 2010, 33, 212–215. [Google Scholar] [CrossRef]
- Da Silva Riehl, A.B.; Da Cunha Pinto, A. Sílica gel: Uma alternativa. Química Nova 1988, 11, 329–330. [Google Scholar]
- Teixeira, S.C.G.; Mathias, L.; Canela, M.C. Recuperação de silica-gel utilizando processos oxidativos avançados: Uma alternative simples de baixo custo. Química Nova 2003, 26, 931–933. [Google Scholar] [CrossRef]
- Neves, G.M.; Lenza, R.F.S.; Vasconcelos, W.L. Evaluation of influence of microwaves in the structure of silica gels. Mater. Res. 2002, 5, 447–451. [Google Scholar] [CrossRef]
- Christy, A.A.; Egeberg, P.K. Quantitative determination of surface silanol groups in silica gel by deuterium exchange combined with infrared spectroscopy and chemometrics. Analyst 2005, 130, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Christy, A.A. Near infrared spectroscopic characterization of surface hydroxyl groups on hydrothermally treated silica gel. Int. J. Chem. Environ. Eng. 2011, 2, 27–32. [Google Scholar]
- Moribe, K.; Masaki, M.; Kinoshita, R.; Zhang, J.; Limwikrant, W.; Higasdhi, K.; Tozuka, Y.; Oguchi, T.; Yamamoto, K. Guest molecular size-dependent inclusion complexation of parabens with cholic acid by cogrinding. Int. J. Pharm. 2011, 420, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Li, Q.-L.; Chen, Z.-P.; Jin, H.-X.; Liu, B. Thermolysis parameter and kinetic research in copolyamide 66 containing triarylphosphine oxide. High Perform. Polym. 2018, 25, 502–507. [Google Scholar]
- Yang, X.-F.; Li, Q.-L.; Chen, Z.-P.; Zhang, L.; Zhou, Y. Mechanism studies of thermolysis process in copolyamide 66 containing triarylphosphine oxide. J. Therm. Anal. Calorim. 2013, 112, 567–571. [Google Scholar] [CrossRef]
- Takamura, T.; Yoshida, H.; Inazuka, K. Infrared characteristic bands of highly dispersed silica. Langmuir 1987, 3, 960–967. [Google Scholar] [CrossRef]
- Zhdanov, S.; Kosheleva, L.S.; Titova, T.I. IR study of hydroxylated silica. Langmuir 1987, 3, 960–967. [Google Scholar] [CrossRef]
- Riegel, B.; Hartmann, I.; Kiefer, W.; Groß, J.; Fricke, J. Raman spectroscopy on silica aerogels. J. Non-Cryst. Solids 1997, 211, 294–298. [Google Scholar] [CrossRef]
- Warring, S.L.; Beattie, D.A.; McQuillan, A.J. Surficial siloxane-tosilanol interconversion during room-temperature hydration/dehydration of amorphous silica films observed by ATR-IR and TIR-Raman spectroscopy. Langmuir 2016, 32, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Al-Azani, M.; al-Sulaibi, M.; Al Soom, N.; Al Jasem, Y.; Bugenhagen, B.; Al Hindawi, B.; Thiemann, T. The use of BrCCl3-PPh3 in Appel type transformations to esters, O-acyloximes, amides, and acid anhydrides. C. R. Chim. 2016, 19, 921–932. [Google Scholar] [CrossRef]
- European Commission. Integrated Pollution Prevention and Control. In Reference Document on Best Available Techniques for the Manufacture of large Volume Inorganic Chemicals—Solids and Others; Bref 0907; 2007. [Google Scholar]
- Roes, A.L.; Tabak, L.B.; Shen, L.; Nieuwlaar, E.; Patel, M.K. Influence of using nanoobjects as filler on functionality-based energy use of nanocomposites. J. Nanopart. Res. 2010, 12, 2011–2028. [Google Scholar] [CrossRef]

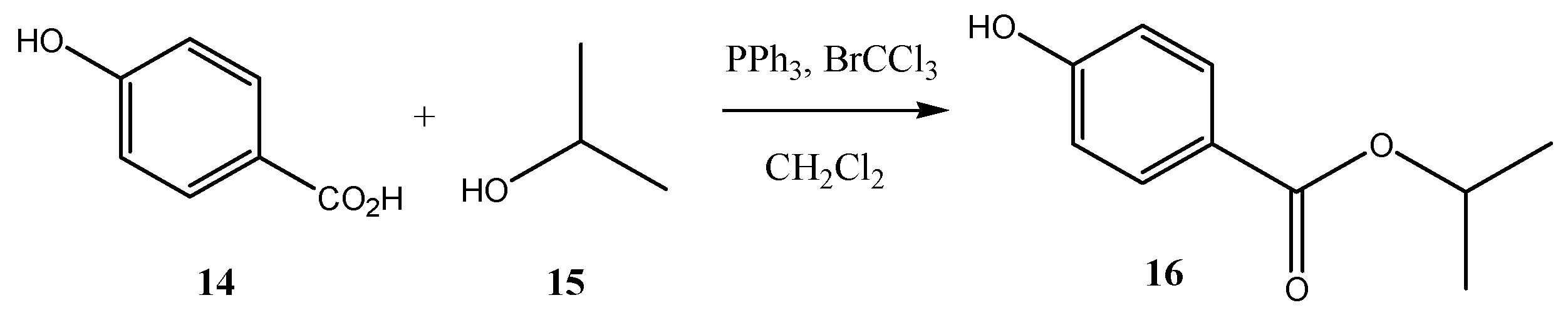
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Break, L.M.; Al-harthi, W.S. Synthesis New of Nucleoside of 1,3-bis-(2,3,5-tri-O-Benzoyl-β-D-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone. Proceedings 2019, 9, 57. https://doi.org/10.3390/ecsoc-22-05694
Break LM, Al-harthi WS. Synthesis New of Nucleoside of 1,3-bis-(2,3,5-tri-O-Benzoyl-β-D-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone. Proceedings. 2019; 9(1):57. https://doi.org/10.3390/ecsoc-22-05694
Chicago/Turabian StyleBreak, Laila Mohammed, and Wafa Saad Al-harthi. 2019. "Synthesis New of Nucleoside of 1,3-bis-(2,3,5-tri-O-Benzoyl-β-D-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone" Proceedings 9, no. 1: 57. https://doi.org/10.3390/ecsoc-22-05694
APA StyleBreak, L. M., & Al-harthi, W. S. (2019). Synthesis New of Nucleoside of 1,3-bis-(2,3,5-tri-O-Benzoyl-β-D-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone. Proceedings, 9(1), 57. https://doi.org/10.3390/ecsoc-22-05694



