ZrO2/g-C3N4 Hybrid Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Trimethylsilyl Protection of Hydroxyl Groups and Synthesis of α-Aminophosphonates †
Abstract
:1. Introduction
2. Experimental
2.1. General
2.2. Preparation of ZrO2
2.3. Preparation of ZrO2/g-C3N4 Nanocomposite
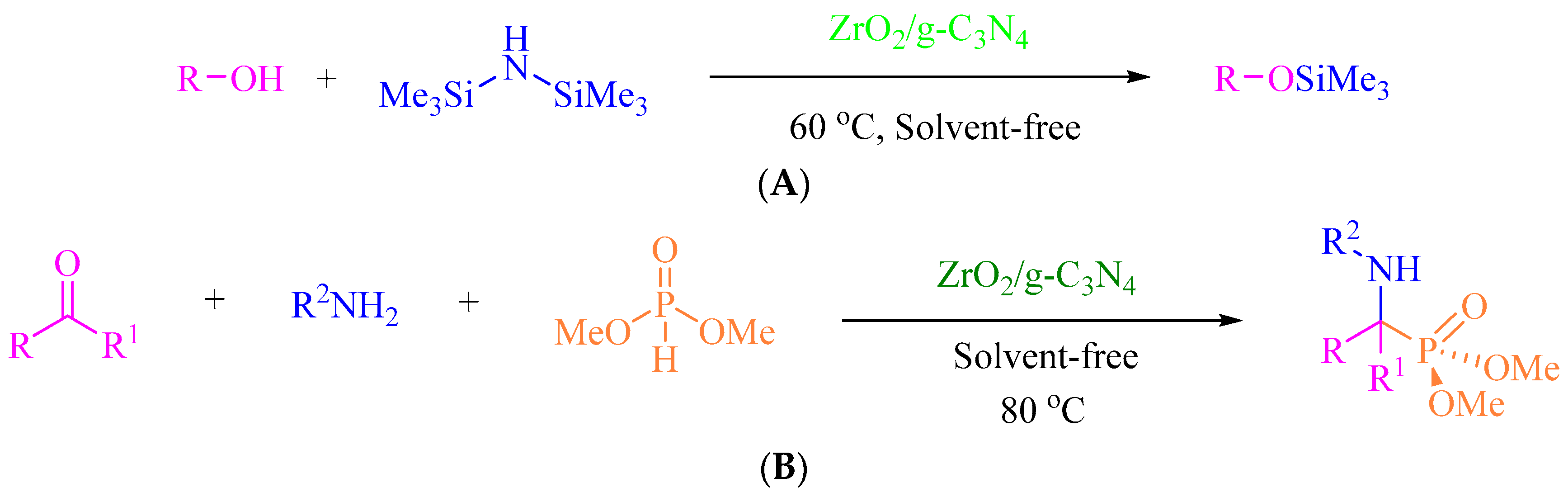
2.4. General Procedure for the Protection of Hydroxyl Groups Using Hexamethyldisilazane (HMDS)
2.5. General Procedure for the Synthesis of α-Aminophosphonates
2.6. Spectral Data
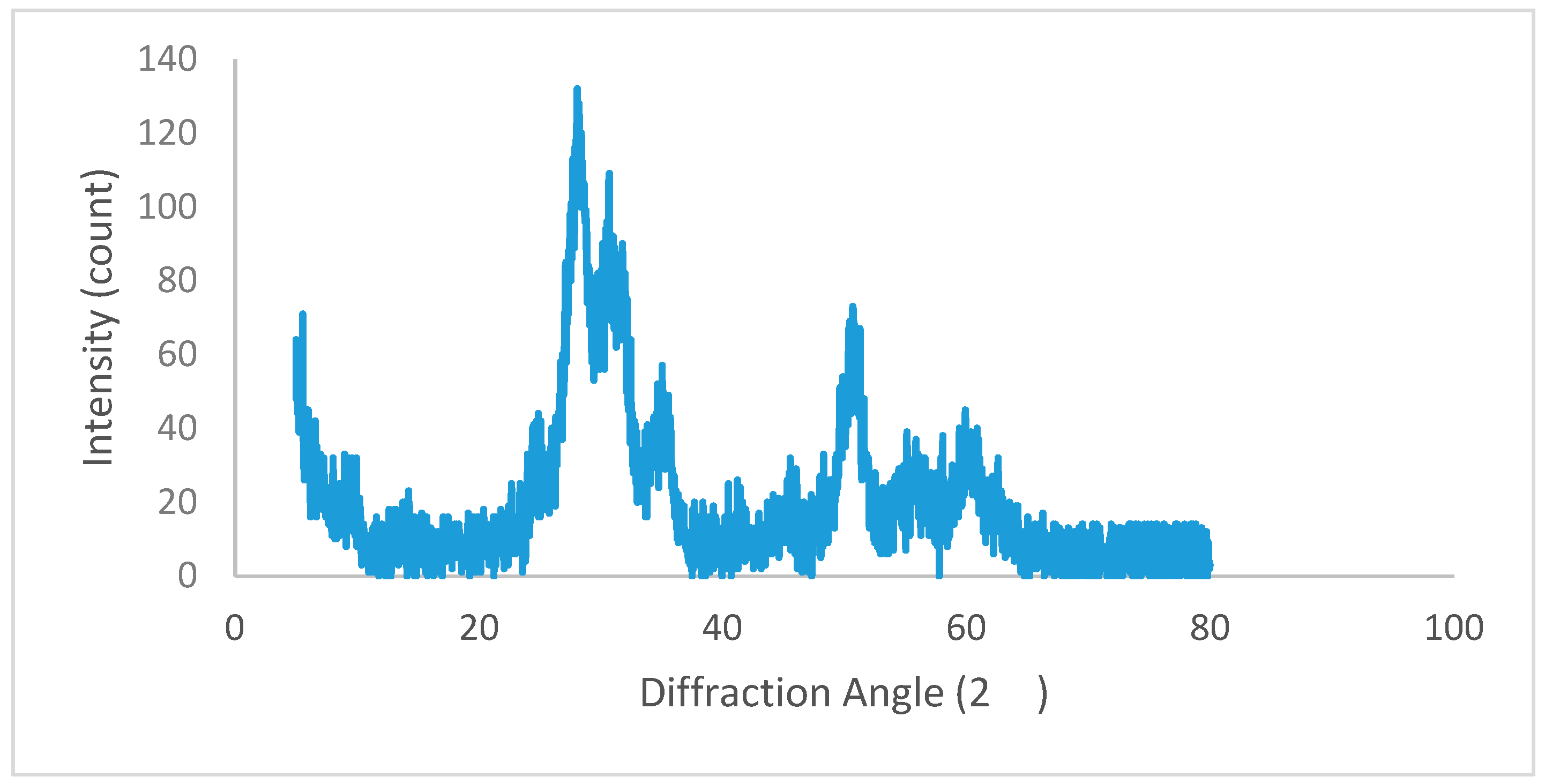
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Gupta, P.; Paul, S. Solid acids: Green alternatives for acid catalysis. Catal. Today 2014, 236, 153–170. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-assembly of metal oxides into three-dimensional nanostructures: Synthesis and application in catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, P.; Luo, S.; Tu, X.; Cao, Q.; Shu, M. Synthesis of novel nanocomposite Fe3O4/ZrO2/chitosan and its application for removal of nitrate and phosphate. Appl. Surf. Sci. 2013, 284, 942–949. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of a ZrO 2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Mu, X.-J.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Microwave-assisted solvent-free and catalyst-free Kabachnik–Fields reactions for α-amino phosphonates. Tetrahedron Lett. 2006, 47, 1125–1127. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; Khorassani, S.M.H.; Hazeri, N.; Rostamizadeh, M.; Sajadikhah, S.S.; Shahkarami, Z.; Maleki, N. An efficient synthesis of α-Amino phosphonates using silica sulfuric acid as a heterogeneous catalyst. Heteroat. Chem. 2009, 20, 316–318. [Google Scholar] [CrossRef]
- Lukanov, L.; Venkov, A. One-pot synthesis of dialkyl arylaminomethyl-and (arylamino) arylmethylphosphonates and their N-acylated derivatives. Synthesis 1992, 1992, 263–264. [Google Scholar] [CrossRef]
- Manjula, A.; Rao, B.V.; Neelakantan, P. One-pot synthesis of α-aminophosphonates: An inexpensive approach. Synth. Commun. 2003, 33, 2963–2969. [Google Scholar] [CrossRef]
| Entry | Substrate | Product | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | 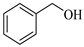 | 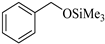 1a | 15 | 90 |
| 2 | 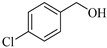 | 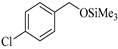 1b | 15 | 95 |
| 3 | 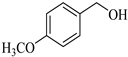 | 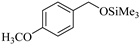 1c | 20 | 90 |
| 4 | 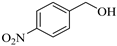 | 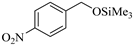 1d | 30 | 90 |
| 5 | 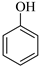 | 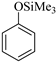 1e | 40 | 92 |
| 6 | 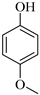 | 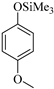 1f | 10 | 95 |
| 7 | 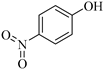 | 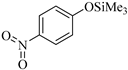 1g | 40 | 75 |
| Entry | Aldehydes | Amines | Products | Time (min) | Yield (%) | Mp (°C) (Observed) | Mp (°C) |
|---|---|---|---|---|---|---|---|
| 1 | PhCHO | Aniline | 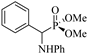 2a | 40 | 95 | 90–92 | [6] |
| 2 | 2-(Cl)C6H4CHO | Aniline | 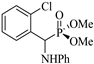 2b | 30 | 93 | 128–129 | [7] |
| 3 | 4-(Cl)C6H4CHO | Aniline | 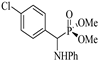 2c | 25 | 95 | 139–140 | [8] |
| 4 | 2,4-(Cl)2C6H3CHO | Aniline | 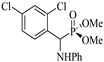 2d | 40 | 89 | 110–112 | [7] |
| 5 | 2,6-(Cl)2C6H3CHO | Aniline | 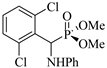 2e | 80 | 85 | 98–100 | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafuri, H.; Jafari, G.; Goodarzi, N.; Rashidizadeh, A. ZrO2/g-C3N4 Hybrid Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Trimethylsilyl Protection of Hydroxyl Groups and Synthesis of α-Aminophosphonates. Proceedings 2019, 9, 51. https://doi.org/10.3390/ecsoc-22-05652
Ghafuri H, Jafari G, Goodarzi N, Rashidizadeh A. ZrO2/g-C3N4 Hybrid Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Trimethylsilyl Protection of Hydroxyl Groups and Synthesis of α-Aminophosphonates. Proceedings. 2019; 9(1):51. https://doi.org/10.3390/ecsoc-22-05652
Chicago/Turabian StyleGhafuri, Hossein, Ghazaleh Jafari, Nahal Goodarzi, and Afsaneh Rashidizadeh. 2019. "ZrO2/g-C3N4 Hybrid Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Trimethylsilyl Protection of Hydroxyl Groups and Synthesis of α-Aminophosphonates" Proceedings 9, no. 1: 51. https://doi.org/10.3390/ecsoc-22-05652
APA StyleGhafuri, H., Jafari, G., Goodarzi, N., & Rashidizadeh, A. (2019). ZrO2/g-C3N4 Hybrid Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Trimethylsilyl Protection of Hydroxyl Groups and Synthesis of α-Aminophosphonates. Proceedings, 9(1), 51. https://doi.org/10.3390/ecsoc-22-05652





