Development of Novel API-ILs for the Optimization of Anti-Alzheimer Drugs †
Abstract
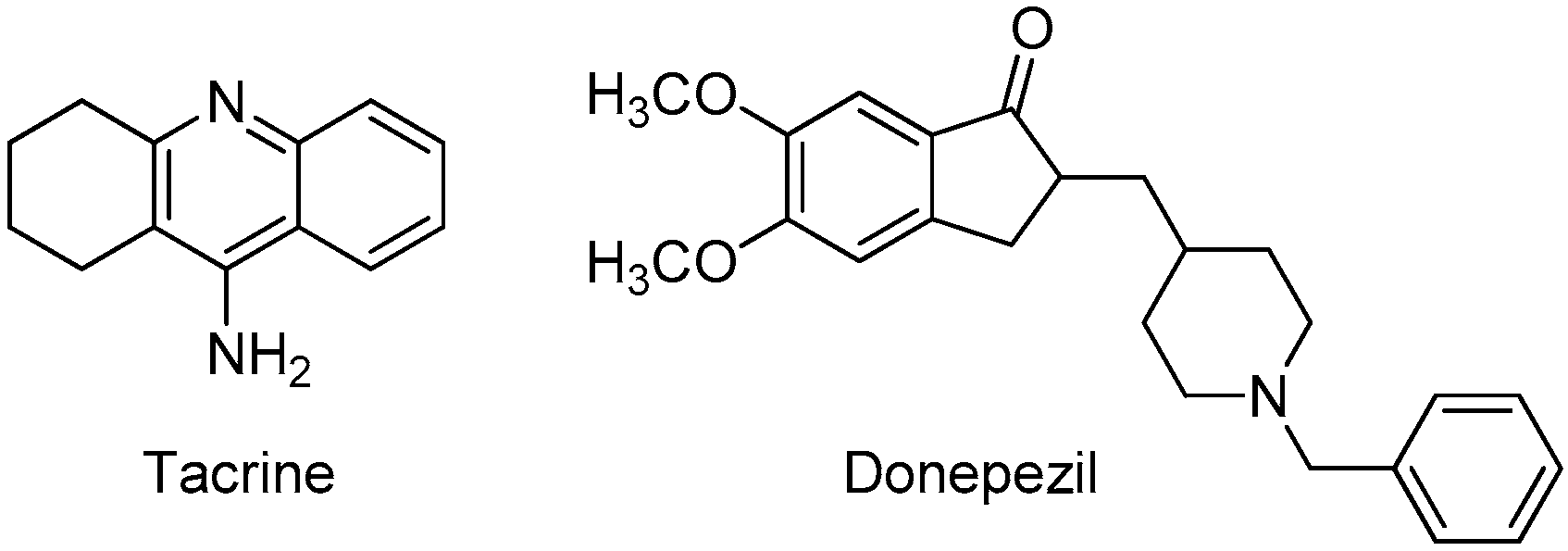
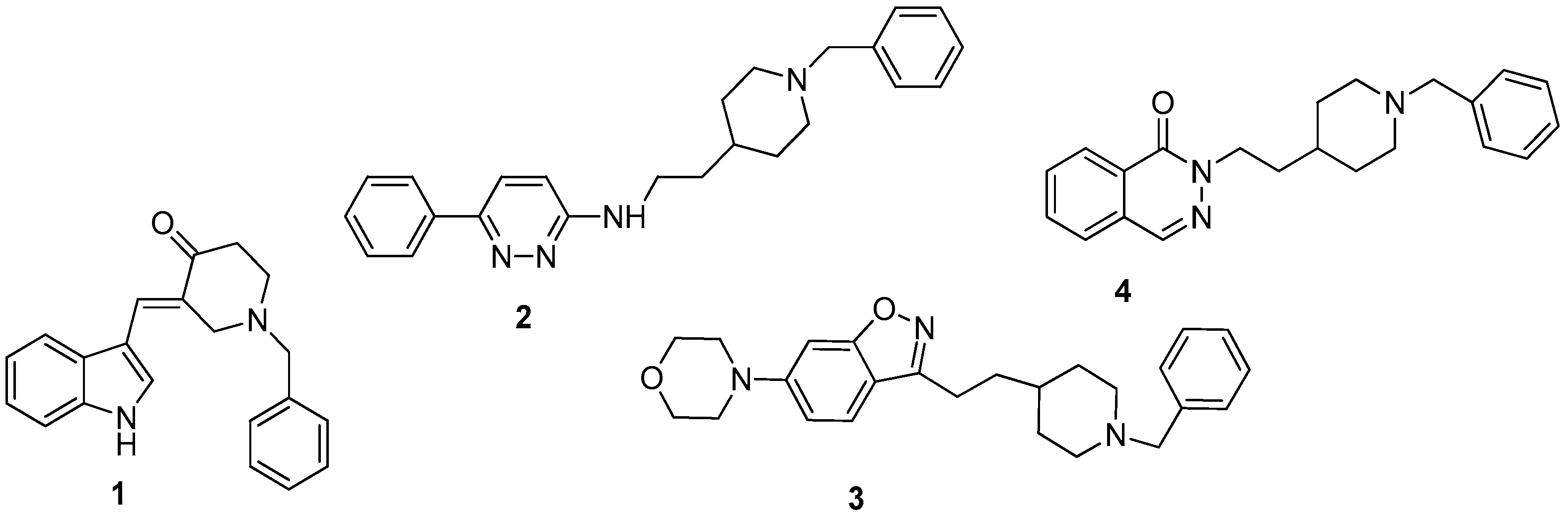
:1. Introduction
2. Materials and Methods
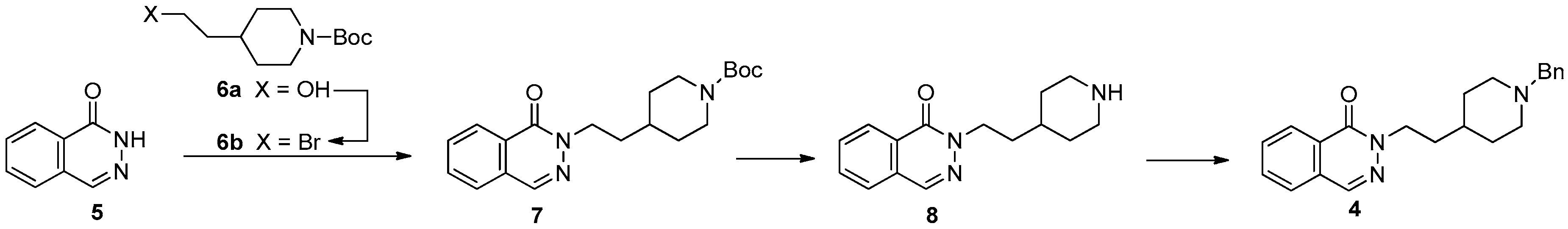
3. Results and Discussion
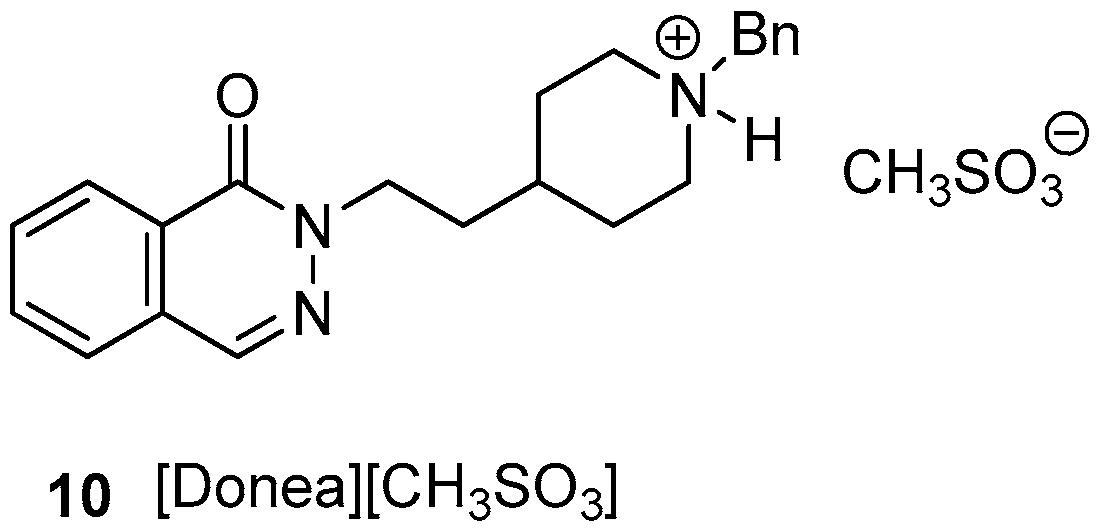
3.1. Chemistry
3.2. Solubility Test
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dementia 2018, 14, 367–429. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.M.; Kivipelto, M.; Greig, N.H. Acetylcholinesterase and its inhibition in Alzheimer disease. Clin. Neuropharmacol. 2004, 27, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Simões, M.C.; Pereira Dias Villegas, F.; Soares Moreira, M.; De Freitas Silva, M.; Máximo Riquel, M.; Mattos da Rosa, P.; Rosa Castelli, M.; Henrique dos Santos, M.; Gomes Soares, M.; Villegas, C. Donepezil: An important prototype to the design of new drug candidates for Alzheimer’s disease. Mini Rev. Med. Chem. 2014, 14, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Vila, N.; Besada, P.; Viña, D.; Sturlese, M.; Moro, S.; Terán, C. Synthesis, biological evaluation and molecular modeling studies of phthalazin-1(2H)-one derivatives as novel cholinesterase inhibitors. RSC Adv. 2016, 6, 46170–46185. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.; Gordeev, E.; Ananikov, V. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
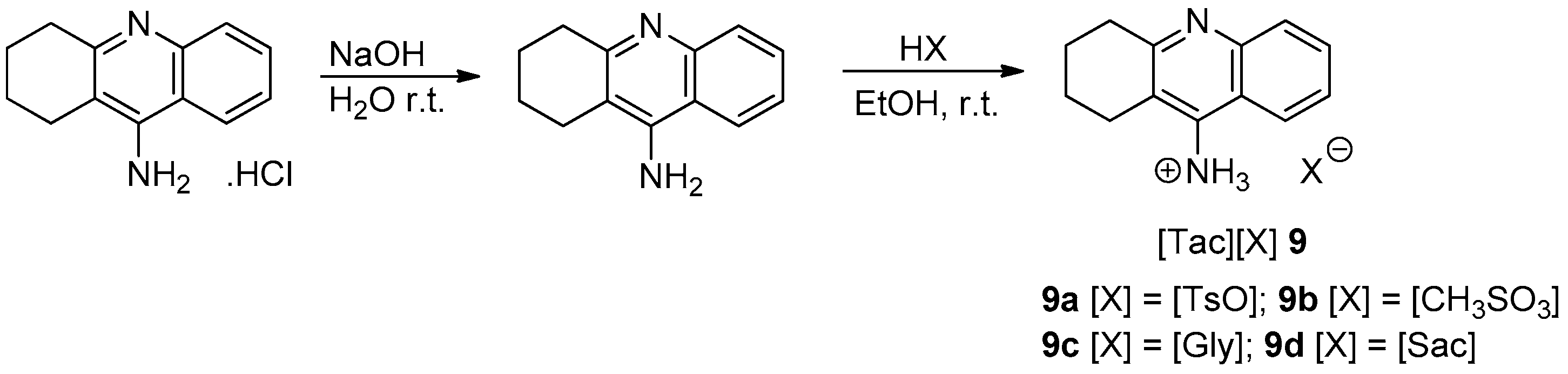

| API-IL | State at r.t. | Water Solubility of API-IL 1 | Water Solubility of API 1 |
|---|---|---|---|
| [Tac][TsO] | Solid | 4 | |
| [Tac][CH3SO3] | liquid | 700 | 100 |
| [Tac][Gly] | Solid | 800 | Tacrine HCl |
| [Tac][Sac] | Solid | 2 | |
| [Donea][CH3SO3] | Liquid | 23 | Insoluble Compound 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tornero, B.; Fernández-Stefanuto, V.; Tojo, E.; Besada, P.; Terán, C. Development of Novel API-ILs for the Optimization of Anti-Alzheimer Drugs. Proceedings 2019, 9, 47. https://doi.org/10.3390/ecsoc-22-05669
Tornero B, Fernández-Stefanuto V, Tojo E, Besada P, Terán C. Development of Novel API-ILs for the Optimization of Anti-Alzheimer Drugs. Proceedings. 2019; 9(1):47. https://doi.org/10.3390/ecsoc-22-05669
Chicago/Turabian StyleTornero, Belén, Verónica Fernández-Stefanuto, Emilia Tojo, Pedro Besada, and Carmen Terán. 2019. "Development of Novel API-ILs for the Optimization of Anti-Alzheimer Drugs" Proceedings 9, no. 1: 47. https://doi.org/10.3390/ecsoc-22-05669
APA StyleTornero, B., Fernández-Stefanuto, V., Tojo, E., Besada, P., & Terán, C. (2019). Development of Novel API-ILs for the Optimization of Anti-Alzheimer Drugs. Proceedings, 9(1), 47. https://doi.org/10.3390/ecsoc-22-05669






