Functionalized BODIPY Derivatives as Potential Fluorescent Labels †
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of BODIPY Derivatives
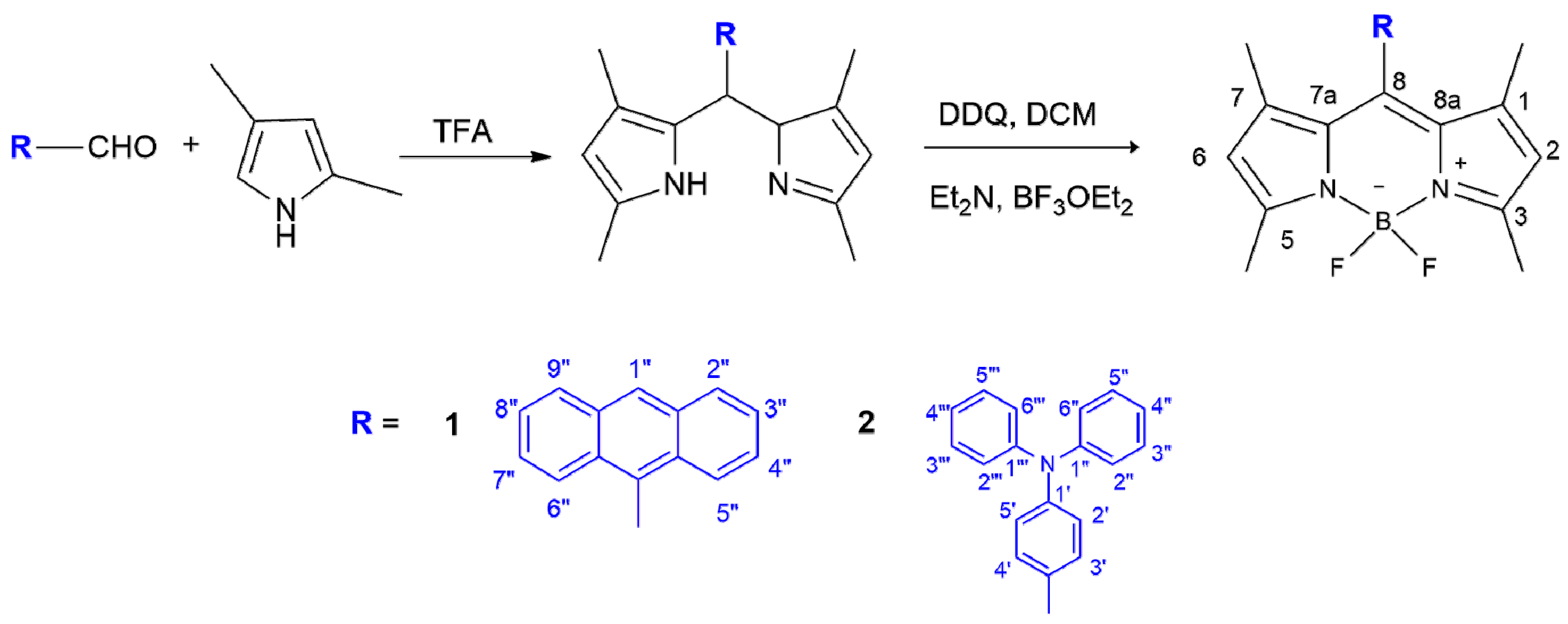
2.2.1. BODIPY Derivative 1
2.2.2. BODIPY Derivative 2
3. Results and Discussion
3.1. Synthesis
3.2. Optical Properties Study
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Freidus, L.G.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Alternative fluorophores designed for advanced molecular imaging. Drug Discov. Today 2018, 23, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nguyen, B.; Burgess, K. Functionalization of the 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) core. Bioorg. Med. Chem. Lett. 2008, 18, 3112–3116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, H.-M.; Zhang, X.-T.; Wang, S.; Xing, G.-W. 8-(4-aminophenyl) BODIPYs as fluorescent pH probes: Facile synthesis, computational study and lysosome imaging. ChemistrySelect 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Deng, M.; Gong, D.; Han, S.-C.; Zhu, X.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. BODIPY based phenylthiourea derivatives as highly selective MeHg+ and Hg2+ ions fluorescent chemodosimeter and its application to bioimaging. Sens. Actuators B Chem. 2017, 243, 195–202. [Google Scholar] [CrossRef]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A dual channel sulphur-containing macrocycle functionalised BODIPY probe for the detection of Hg(II) in mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Hazem, E.O.; Sayed, S.E.; Ferreira, R.C.M.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. 4-(4,5-Diphenyl-1H-imidazole-2-yl)-N,N-dimethylaniline-Cu(II) complex, a highly selective probe for glutathione sensing in water-acetonitrile mixtures. Dyes Pigments 2018, 159, 45–48. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Demas, J.N.; Crosby, G.A. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
| Cpd | UV-vis | Fluorescence | |||
|---|---|---|---|---|---|
| λmax (nm) | log ε | λem (nm) | ΦF | Stokes’ Shift (nm) | |
| 1 | 502 | 3.99 | 518 | 0.011 | 16 |
| 2 | 497 | 3.96 | 519 | 0.005 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.C.R.; Nogueira, M.B.; Costa, S.P.G.; Raposo, M.M.M. Functionalized BODIPY Derivatives as Potential Fluorescent Labels. Proceedings 2019, 9, 36. https://doi.org/10.3390/ecsoc-22-05701
Gonçalves RCR, Nogueira MB, Costa SPG, Raposo MMM. Functionalized BODIPY Derivatives as Potential Fluorescent Labels. Proceedings. 2019; 9(1):36. https://doi.org/10.3390/ecsoc-22-05701
Chicago/Turabian StyleGonçalves, Raquel C. R., Mariana B. Nogueira, Susana P. G. Costa, and M. Manuela M. Raposo. 2019. "Functionalized BODIPY Derivatives as Potential Fluorescent Labels" Proceedings 9, no. 1: 36. https://doi.org/10.3390/ecsoc-22-05701
APA StyleGonçalves, R. C. R., Nogueira, M. B., Costa, S. P. G., & Raposo, M. M. M. (2019). Functionalized BODIPY Derivatives as Potential Fluorescent Labels. Proceedings, 9(1), 36. https://doi.org/10.3390/ecsoc-22-05701






