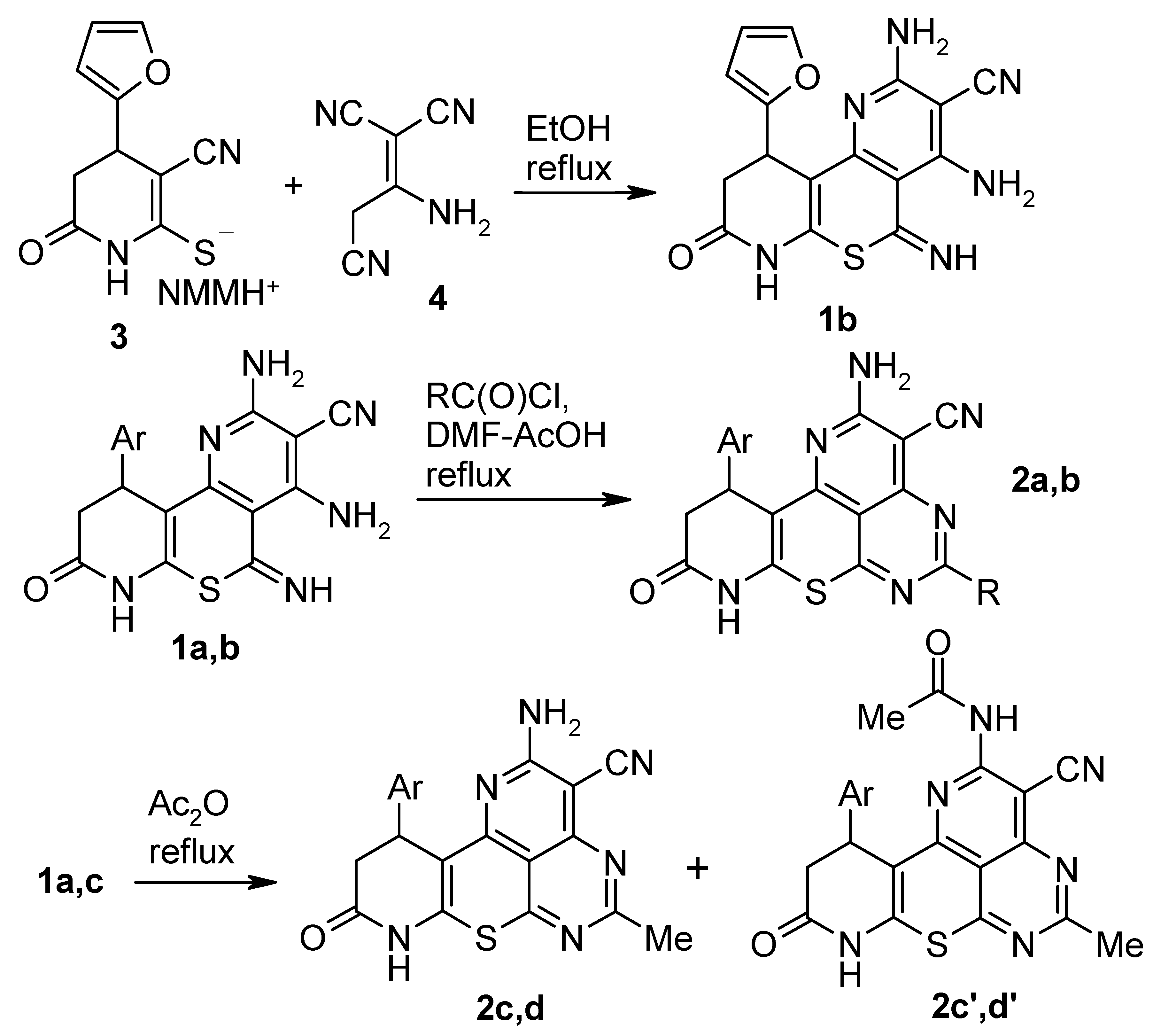
In our earlier work we have shown [
1,
2] that 3-cyanopyridine-2-thiolates react with malononitrile dimer (2-amino-1,1,3-tricyanopropene) in hot EtOH to afford 2,4-diamino-5-imino-5H-pyrido[2’,3’:2,3]thiopyrano[4,5-b]pyridines
1. The compounds
1a,
b,
c are promising reagents to prepare polyheterocyclic ensembles. We found that compounds
1a,
b easily react with acid chlorides with the closure of the pyrimidine ring and the formation of new polyheterocyclic ensembles, 8,9,10,11-tetrahydro-7-thia-1,4,6,8-tetraazabenzo[de]anthracenes
2a,
b in modest (34–57%) yields (
Scheme 1). The relatively low yields of the polycycles
2 are due to non-optimal reaction conditions, the presence of several nucleophilic centers in the molecule of
1 and associated ambiguity of acylation. Confirmation of this assumption resulted from the fact that acylation of compounds
1a,
c with Ac
2O gives mixtures. Thus, when
1a,
c were heated under reflux in Ac
2O, products of acylation at the 2-NH
2 group,
2c’,
d’ were obtained along with the expected compounds
2c,
d (the molar ratio of compounds
2d:
2d’ was ~1:2 (~24% and ~47%, respectively), whereas the molar ratio of compounds
2c:
2c’ was ~3:1 (~59% and ~20%, respectively). The starting compound
1b was prepared by analogy with known procedure [
1] from pyridine-2-thiolate
3b and malononitrile dimer
4.
Presumably, the formation of the tetracyclic system of
2 proceeds as a cascade process starting with acylation of the 4-NH
2 group of compound
1 followed by the intramolecular cyclization involving the imino group at the
peri position. Thiatetraazabenzo[
de]anthracenes
2 are yellow-brown or green-brown powders that are insoluble in EtOH, sparingly soluble in acetone, AcOH, DMF, and moderately soluble in hot DMSO. As we have shown in prior work [
3,
4,
5], such polycyclic assemblies can be used as chemical protection agents for plants, corrosion inhibitors, antitumor agents, DNA intercalators, nd so on. The structure of polyheterocyclic ensembles
2 was confirmed by the results of spectral studies (IR spectroscopy,
1H NMR spectroscopy, HPLC-MS) and elemental analysis.
1H NMR spectra of tetraazabenzo[
de]anthracenes
2 showed the
ABX pattern of protons 10-CH
2 and 11-CH
2: the signals of protons 10-CH
cis are observed upfield (δ 2.60–2.81 ppm) as a pseudo doublet (unresolved doublet of doublets) with coupling constants in the range
J 16.3–16.4 Hz, while signals of proton 10-CH
trans appeared as doublet of doublets with
2J = 16.3–16.4 Hz and
3J = 7.0–7.4 Hz at δ 3.17–3.28 ppm. The signals of protons 11-CH were observed as a pseudo doublet (unresolved doublet of doublets) in the region of δ 4.91–5.22 ppm. In addition,
1H NMR spectra of compounds
2 showed the signals of 8-NH protons at δ 11.21–11.40 ppm as well as the characteristic signals of substituents at C-5 and C-11. The signals of the 2-NH
2 group appeared as a broadened peak at 7.42–7.76 ppm, whereas in the case of NHAc derivatives
2c’,
d’ this signal disappeared and singlets at δ 10.51 and δ 10.68 ppm were observed, respectively.
In summary, we have demonstrated that polyheterocyclic ensembles of 1,4,6,8-tetraaza-7-thiabenzo[de]anthracene may be prepared in moderate yields by acylation of the available 2,4-diamino-5-imino-5H-pyrido[2’,3’:2,3]thiopyrano[4,5-b]pyridines. In the presence of a large excess of the acylating agent, acylation may take place concurrently at the 2-NH2 group.
Experimental
IR spectra were recorded on a Thermo Nicolet Magna-IR 750 spectrometer in KBr pellets. 1Н NMR spectra were recorded on a Bruker DPX-400 spectrometer (400 MHz) in DMSO-d6 using TMS as an internal standard. HPLC-MS analysis was performed on a Shimadzu LC-10AD LC with a Shimadzu SP D-10A UV–Vis (254 nm) detector and Sedex 75 ELSD, combined with a PE SCIEX API 150EX mass spectrometer, atmospheric pressure electrospray ionization. Selected experimental procedures are given.
2,4-Diamino-10-(2-furyl)-5-imino-8-oxo-7,8,9,10-tetrahydro-5
Н-pyrido[2’,3’:4,5]thiopyrano[2,3-
b]pyridine-3-carbonitrile (1b), DMF solvate (2:1). A mixture of thiolate 3b [
6,
7] (3.0 g, 9.34 mmol), malononitrile dimer 4 [
8] (1.85 g, 14.0 mmol) in 96% EtOH (30 mL) was heated under reflux for 25 h. The mixture was then kept for 48 h at 20 °С, and black precipitate was filtered and dried at 60 °С. After recrystallization from DMF, the solvate containing 0.5 molecules of DMF was obtained. Yield was 1.72 g (52%), large greenish-brown crystals, decomp. temp. >250 °С. IR spectrum, ν, cm
−1: 3464, 3320, 3223, 3171 (NH, NH
2), 2206 (C≡N), 1705 (C=O).
1H NMR spectrum (400 MHz), δ, ppm (
J, Hz): 2.61 (1Н, br. d,
2J = 16.4, 9-CH
A); 2.72 (1.5H, s) and 2.87 (1.5Н, s, 0.5N(CH
3)
2 of DMF); 2.96 (1Н, dd,
2J = 16.4,
3J = 7.0, 9-CH
B); 5.04–5.05 (1Н, m, 10-CH); 6.06–6.07 (1H, m) and 6.26–6.27 (1Н, m, Н-3,4 furyl); 6.85 (2Н, br. s, 2-NH
2); 7.16 (1Н, br. s, 4-NH
А); 7.45–7.46 (1Н, m, Н-5 furyl); 7.94 (0.5Н, br. s, 0.5 HC(O) of DMF); 10.09 (1Н, br. s, =NH); 10.48 (1H, s, C(O)NH); 10.80 (1Н, br. s, 4-NH
B). Mass spectrum,
m/
z: 647 [2M−C
4H
3O]
+, 613 [2M−2C
4H
3O+Н+MeCN]
+, 353 [М+H]
+, 285 [M−C
4H
3O]
+. Found, %: С 54.01; H 4.08; N 23.37. C
16H
12N
6O
2S · 0.5C
3H
7NO. Calculated, %: C 54.05; Н 4.02; N 23.41.
2-Amino-5-chloromethyl-9-oxo-11-phenyl-8,
9,
10,
11-tetrahydro-7-thia-1,
4,
6,
8-tetraazabenzo[de]anthracene-3-carbonitrile (2a). Chloroacetyl chloride (1 mL, 12.6 mmol) was added to a suspension of compound
1а [1] (1.0 g, 2.76 mmol) in dry DMF (5 mL), and the mixture was stirred for 20 min (the suspension turns into a solution). Then АсОН (7 mL) was added to the prepared solution (an exothermic reaction observed), and stirring continued for another 30 min, while the mixture was slowly heated to the boiling point. The mixture was heated under reflux with stirring for another 2 h for complete conversion and then cooled to room temperature. Product
2а was filtered off and washed with EtOH. Yield was 0.66 g (57%), yellow-brown fine crystalline powder, decomp. temp. >250 °С. IR spectrum, ν, cm
−1: 3455, 3319, 3214, 3154 (NH), 2214 (C≡N), 1701 (C=O).
1H NMR spectrum (400 MHz), δ, ppm (
J, Hz): 2.74 (1Н, br. d,
2J = 16.4) and 3.23 (1Н, dd,
2J = 16.4,
3J = 7.4, 10-СН
2); 4.67 (2Н, s, СН
2Сl); 4.92–4.93 (1Н, m, 11-CH); 7.19–7.27 (5Н, m, H Ph); 7.76 (2Н, br. s, NH
2); 11.31 (1H, s, NH). Mass spectrum,
m/
z: 421 [М(
35Cl)+H]
+, 423 [М(
37Cl)+H]
+.