An Easy Approach to Obtain Alcohol-Amines by Reduction of Alcohol Functionalized Imines †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
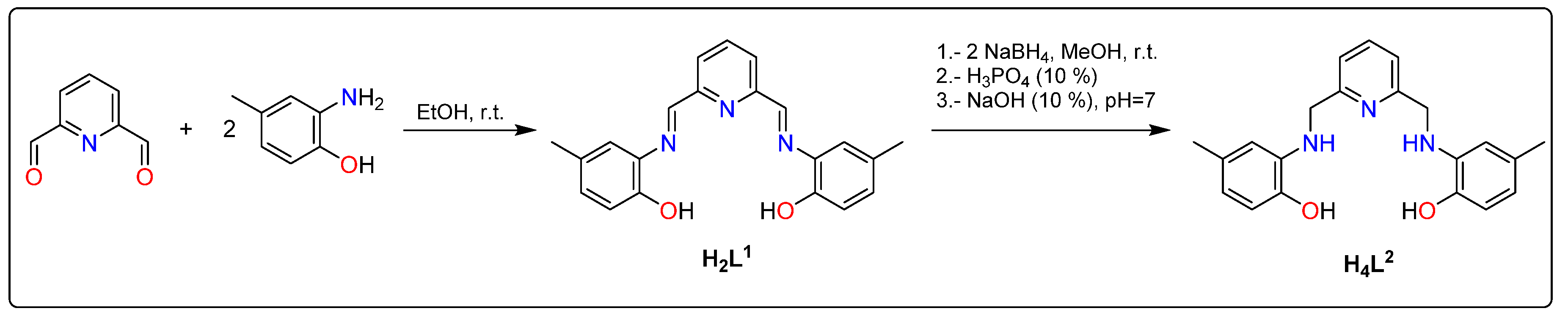
2.2. Syntheses of the Alcohol-Imine and Its Reduction to Alcohol-Amine
3. Results and Discussion
3.1. Synthesis
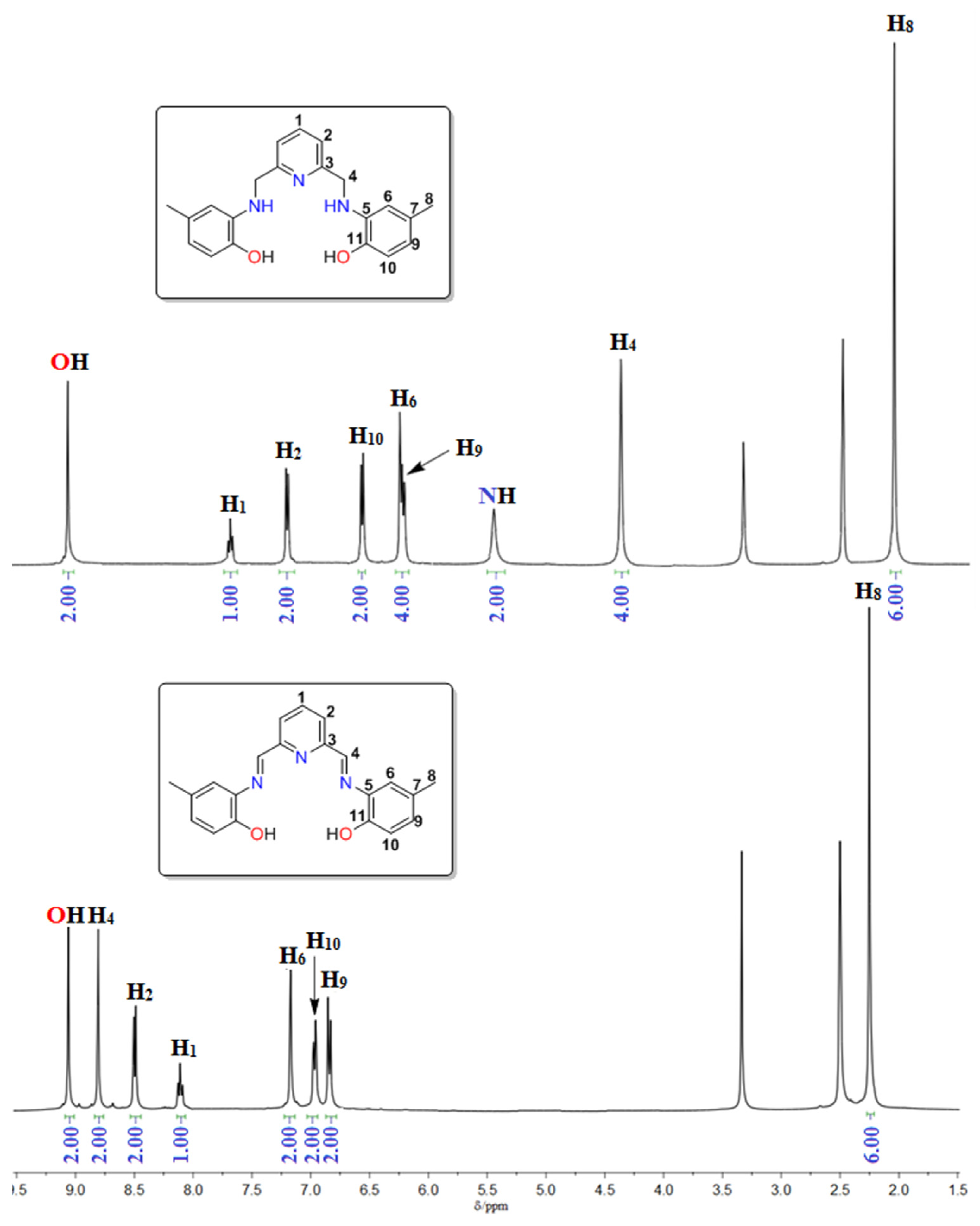
3.2. Spectroscopic Characterization
- The ν(C=Nimine) band, present in the spectrum of H2L1 at 1623 cm−1, is absent in the spectrum of H4L2.
- The spectrum of H4L2 shows a sharp band at 3437 cm−1, which can be assigned to an N-H vibration, and that is absent is the spectrum of H2L1.
- The singlet at 8.79 (2H) ppm, assigned to the imine nitrogen atoms H4 in the spectrum of H2L1, is absent in the spectrum of H4L2.
- All the aromatic hydrogen atoms are displaced to a higher field in the spectrum of H4L2 with respect to that of H2L1, in agreement with less delocalization of the charge.
- The spectrum of H4L2 shows two new singlets with respect to that of H2L1. These singlets are located at 5.45 (2H) and 4.37 (4H) ppm, and can be assigned to the protons of NH and CH2 groups, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Averill, D.F.; Broman, R.F. Substituted salen and baen tetradentate Schiff-base ligands. Synthesis, characterization, and electrochemistry of cobalt(III) complexes. Inorg. Chem. 1978, 17, 3389–3394. [Google Scholar] [CrossRef]
- Mandewale, M.C.; Thorat, B.; Patil, U.; Yamgar, R. Review: Synthesis and applications of Schiff bases. Int. J. Chem. Pharm. Sci. 2015, 3, 1919–1928. [Google Scholar]
- Riley, D.L.; Neyt, N.C. Approaches for performing reductions under continuous-flow conditions. Synthesis 2018, 50, 2707–2720. [Google Scholar] [CrossRef]
- Facchetti, G.; Bucci, R.; Fuse, M.; Rimoldi, I. Asymmetric hydrogenation vs transfer hydrogenation in the reduction of cyclic imines. ChemistrySelect 2018, 3, 8797–8800. [Google Scholar] [CrossRef]
- Elsen, H.; Faerber, C.; Ballmann, G.; Harder, S. LiAlH4: From stoichiometric reduction to imine hydrogenation catalysis. Angew. Chem. Int. Ed. 2018, 57, 7156–7160. [Google Scholar] [CrossRef] [PubMed]
- Saaby, S.; Winckelmann, I.; Sondergaard, K.; Liang, X.; Ke, Y.; Wang, X.; Ye, J. Process for the hydrogenation of imines. U.S. Patent Appl. 20110077418A1, 31 March 2011. [Google Scholar]
- Kocovsky, P.; Malkov, A.V. Lewis. Bases as Catalysts in the Reduction of Imines and Ketones with Silanes (n → σ*). In From Lewis Base Catalysis in Organic Synthesis; Vedejs, E., Denmark, S.E., Eds.; Weily Online Library: Hoboken, New Jersey, USA, 2016; Volumes 1–3, pp. 1077–1112. ISBN 978-3-52-767514-2. [Google Scholar]
- Chen, B.-C.; Sundeen, J.E.; Guo, P.; Bednarz, M.S.; Zhao, R. Novel triethylsilane mediated reductive N-alkylation of amines:improved synthesis of 1-(4-imidazolyl)methyl-4-sulfonylbenzodiazepines, new farnesyltransferase inhibitors. Tetrahedron Lett. 2001, 42, 1245–1246. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Bhongle, N.; Su, X.; Ribe, S.; Senanayake, C.H. Novel diacid accelerated borane reducing agent for imines. Tetrahedron Lett. 2002, 43, 8617–8620. [Google Scholar] [CrossRef]
- Itsuno, S. Boron hydride reduction. ACS Symp. Ser. 2016, 1236, 241–274. [Google Scholar] [CrossRef]
- Arnáiz, A.; Cuevas, J.V.; García-Herbosa, G.; Carbayo, A.; Casares, J.A.; Gutierrez-Puebla, E. Revealing the diastereomeric nature of pincer terdentate nitrogen ligands 2,6-bis(arylaminomethyl)pyridine through coordination to palladium. J. Chem. Soc. Dalton Trans. 2002, 2581–2586. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.C.; Bastida, R.; Macías, A.; Pérez-Lourido, P.; Valencia, L. Zn(II) complexes with pyridine derived N6 and N8 donor azamacrocyclic ligands. Polyhedron 2007, 26, 5317–5323. [Google Scholar] [CrossRef]
- Kose, M.; McKee, V. Bis{2,6-bis[(2-hydroxy-5-methylphenyl)-iminomethyl]pyridine} monohydrate. Acta Cryst. 2011, E67, o3193. [Google Scholar] [CrossRef] [PubMed]
- Aubert, P.-H.; Audebert, P.; Capdevielle, P.; Maumy, M.; Rochea, M. Electrochemical oxidative polymerization of binuclear ”anil” and”salen”-type complexes and tetrahydro derivatives. New J. Chem. 1999, 23, 297–301. [Google Scholar] [CrossRef]
- Bastida, R.; de Blas, A.; Fenton, D. E.; Rial, C.; Rodriguez, T.; Sousa, A. Electrochemical synthesis of neutral complexes with N2SO tetradentate ligands. J. Chem. Soc. Dalton Trans. 1993, 2, 265–268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fondo, M.; Corredoira-Vázquez, J.; GarcíaDeibe, A.M.; Sanmartín-Matalobos, J. An Easy Approach to Obtain Alcohol-Amines by Reduction of Alcohol Functionalized Imines. Proceedings 2019, 9, 2. https://doi.org/10.3390/ecsoc-22-05697
Fondo M, Corredoira-Vázquez J, GarcíaDeibe AM, Sanmartín-Matalobos J. An Easy Approach to Obtain Alcohol-Amines by Reduction of Alcohol Functionalized Imines. Proceedings. 2019; 9(1):2. https://doi.org/10.3390/ecsoc-22-05697
Chicago/Turabian StyleFondo, Matilde, Julio Corredoira-Vázquez, Ana M. GarcíaDeibe, and Jesús Sanmartín-Matalobos. 2019. "An Easy Approach to Obtain Alcohol-Amines by Reduction of Alcohol Functionalized Imines" Proceedings 9, no. 1: 2. https://doi.org/10.3390/ecsoc-22-05697
APA StyleFondo, M., Corredoira-Vázquez, J., GarcíaDeibe, A. M., & Sanmartín-Matalobos, J. (2019). An Easy Approach to Obtain Alcohol-Amines by Reduction of Alcohol Functionalized Imines. Proceedings, 9(1), 2. https://doi.org/10.3390/ecsoc-22-05697







