A New Approach to 5-Functionalized 1,2-Dihydropyrimidin-2-ones/imines via Base-Induced Chloroform Elimination from 4-Trichloromethyl-1,2,3,4-tetrahydropyrimidin-2-ones/imines †
Abstract
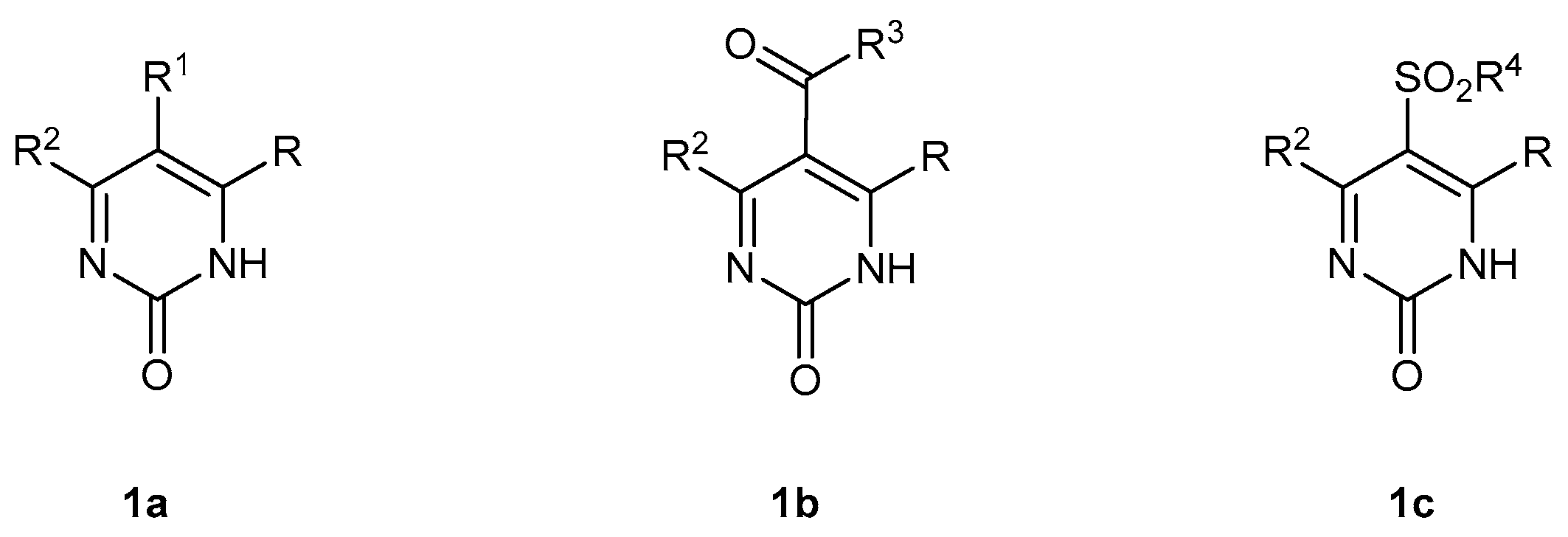
:1. Introduction
2. Results and Discussion
3. Conclusions
Acknowledgments
References
- Perrine, J.W.; Houlihan, W.J.; Takesue, E.I. Anti-inflammatory and other pharmacodynamic properties of five members of the 4-aryl-1-isopropyl-2(1H)-quinazolinone series. Arzneim. Forsch. 1984, 34, 879–885. [Google Scholar]
- Voronina, T.A.; Gordiichuk, G.N.; Andronati, S.A.; Garibova, T.L.; Zhilina, Z.I. Synthesis and pharmacological properties of some 4-phenyl-quinazoline-2-ones. Pharm. Chem. J. 1981, 15, 495–497. [Google Scholar] [CrossRef]
- Kandeel, M.M.; Abbady, M.S.; Youssef, M.S.K. Synthesis of 4-Substituted 3-Methy1-1-pheny1-2-pyrazo1ine-5-thione by Heterocycles. Bull. Soc. Chim. Fr. 1988, 6, 1005–1008. [Google Scholar]
- Hamdy, N.A. Synthesis of new pyridine, pyrazole and pyrimidine derivatives of potential antimicrobial effect. Egypt. J. Chem. 2005, 48, 749–758. [Google Scholar]
- Nagaraj, A.; Reddy, C.S. Synthesis and biological study of novel methylene-bis-chalcones and substituted methylene-bis-pyrimidines/pyrimidinones. J. Heterocycl. Chem. 2007, 44, 1181–1185. [Google Scholar] [CrossRef]
- Brown, D.J. Pyrimidines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 3, pp. 57–155. [Google Scholar]
- Undheim, K.; Benneche, T. Pyrimidines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; Volume 6, pp. 93–231. [Google Scholar]
- Rewcastle, G.W. Pyrimidines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C., Scriven, E.F.V., Taylor, R., Eds.; Elsevier: Oxford, UK, 2008; Volume 8, pp. 117–272. [Google Scholar]
- Bergmann, W.; Johnson, T.B. Die Synthese des 5-Acetyl-uracils (Untersuchungen über Pyrimidine, CXXXVII. Mitteil.). Chem. Ber. 1933, 66, 1492–1496. [Google Scholar] [CrossRef]
- Jones, W.D.; Huber, E.W.; Grisar, J.M.; Schnettler, R.A. The synthesis of 5- and 6-acyl-2(1H)-pyrimidinones. J. Heterocycl. Chem. 1987, 24, 1221–1227. [Google Scholar] [CrossRef]
- Mulwad, V.V.; Shirodkar, J.M. Synthesis of some of the antibacterial compounds from 4-hydroxycoumarins: Part II. Indian J. Chem. Sect. B 2002, 41, 1263–1267. [Google Scholar]
- Dorokhov, V.A.; Komkov, A.V.; Vasil’ev, L.S.; Azarevich, O.G.; Gordeev, M.F. Synthesis of functional derivatives of trifluoromethylpyrimidines from acetylacetone, trifluoroacetonitrile, and aryl isocyanates. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1991, 40, 2311–2313. [Google Scholar] [CrossRef]
- Altural, B.; Akcamur, Yu.; Saripinar, E.; Yildirim, I.; Kollenz, G. Reactions of cyclic oxalyl compounds, part 29: A simple synthesis of functionalized 1H-pyrimidines. Monatsh. Chem. 1989, 120, 1015–1020. [Google Scholar] [CrossRef]
- Palanki, M.S.S.; Erdman, P.E.; Gayo-Fung, L.M.; Shevlin, G.I.; Sullivan, R.W.; Suto, M.J.; Goldman, M.E.; Ransone, L.J.; Bennett, B.L.; Manning, A.M. Inhibitors of NF-κB and AP-1 Gene Expression: SAR Studies on the Pyrimidine Portion of 2-Chloro-4-trifluoromethylpyrimidine- 5-[N-(3‘,5‘-bis-(trifluoromethyl)phenyl)carboxamide]. J. Med. Chem. 2000, 43, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Roschger, P. Synthesis and reactions of “Biginelli-compounds”. Part I. J. Heterocycl. Chem. 1989, 26, 55–64. [Google Scholar] [CrossRef]
- Kadysh, V.P; Stradyn’, Y.P.; Khanina, E.L.; Dubur, G.Y.; Mutsenietse, D.K. Electrochemical reduction of hydrogenated 2-pyrimidones on a graphite electrode. Chem. Heterocycl. Compd. 1985, 21, 95–99. [Google Scholar] [CrossRef]
- Slavinskaya, V.A.; Dubur, G.Y.; Sile, D.A; Kreile, D.R.; Khanina, E.L. Method of obtaining 2-oxo-4-phenyl-5-carbethoxy-6-methylpyrimidine. USSR Patent 632695, 1978; Chem. Abstr. 1979, 90, 121631y. [Google Scholar]
- Khanina, E.L.; Dubur, G.Y. Oxidation of some derivatives of tetrahydropyrimidine-5-carboxylic acid with selenium dioxide. Chem. Heterocycl. Compd. 1982, 18, 412–414. [Google Scholar] [CrossRef]
- Kestenansky, J.L.; Khmelnitsky, Y. Biocatalytic combinatorial synthesis. Bioorg. Med. Chem. 1999, 7, 2157–2162. [Google Scholar] [CrossRef]
- Puchala, A.; Belaj, F.; Bergman, J.; Kappe, C.O. On the reaction of 3,4-dihydropyrimidones with nitric acid. Preparation and X-ray structure analysis of a stable nitrolic acid. J. Heterocycl. Chem. 2001, 38, 1345–1352. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A.; Minghini, E.; Sabbatini, S.; Bertolasi, V. Model Studies toward the Synthesis of Dihydropyrimidinyl and Pyridyl α-Amino Acids via Three-Component Biginelli and Hantzsch Cyclocondensations. J. Org. Chem. 2003, 68, 6172–6183. [Google Scholar] [CrossRef]
- Shanmugam, P.; Perumal, P.T. Regioselective dehydrogenation of 3,4-dihydropyrimidin-2(1H)-ones mediated by ceric ammonium nitrate. Tetrahedron 2006, 62, 9726–9734. [Google Scholar] [CrossRef]
- Shanmugam, P.; Perumal, P.T. An unusual oxidation–dealkylation of 3,4-dihydropyrimidin-2(1H)-ones mediated by Co(NO3)2·6H2O/K2S2O8 in aqueous acetonitrile. Tetrahedron 2007, 63, 666–672. [Google Scholar] [CrossRef]
- Arukwe, J.; Benneche, T.; Undheim, K. Synthesis of 5-stannylpyrimidines and their use in Pd-catalysed ketone formation. J. Chem. Soc. Perkin Trans. 1 1989, 0, 255–259. [Google Scholar] [CrossRef]
- Dyer, E.; Johnson, T.B. Researches on Pyrimidines. CXL. Pyrimidines Derived from Carbethoxymalonic Aldehyde. J. Am. Chem. Soc. 1934, 56, 222–225. [Google Scholar] [CrossRef]
- Benneche, T.; Undheim, K. Syntheses and Reactions of Some 5-Vinyl- and 5-Ethynylpyrimidines. Acta Chem. Scand. Ser. B 1983, 37, 235–239. [Google Scholar] [CrossRef]
- Arukwe, J.; Undheim, K. Lithiation in the Synthesis of 5-Pyrimidinyl Ketones. Acta Chem. Scand. Ser. B 1986, 40, 588–592. [Google Scholar] [CrossRef]
- Arukwe, J.; Undheim, K. Organomanganese(II) Reagents in the Synthesis of 5-Pyrimidinyl Ketones. Acta Chem. Scand. Ser. B 1986, 40, 764–767. [Google Scholar] [CrossRef]
- Gaare, K.; Repstad, T.; Benneche, T.; Undheim, K. Preparation of 5-(Pyrrolylcarbonyl)- and 5-(Imidazolylcarbonyl)pyrimidines. Acta Chem. Scand. 1993, 47, 57–62. [Google Scholar] [CrossRef]
- Eynde, J.J.V.; Audiart, N.; Canonne, V.; Michel, S.; Haverbeke, Y.V.; Kappe, C.O. Synthesis and Aromatization of Dihydropyrimidines Structurally Related to Calcium Channel Modulators of the Nifedipine-Type. Heterocycles 1997, 45, 1967–1978. [Google Scholar]
- Caldwell, W.T.; Sayin, A.N. The Preparation of a Pyrimidine Analog (Isostere) of Promizole and Other Substituted Pyrimidines. J. Am. Chem. Soc. 1952, 74, 4314–4317. [Google Scholar] [CrossRef]
- Hafez, A.A.A. Synthesis of Some Heterocyclic Sulfones Related to Quinolinol. Collect. Czech. Chem. Commun. 1993, 58, 2222–2226. [Google Scholar] [CrossRef]
- Bal’on, Ya. G.; Smirnov, V.A. 1,2,2,2-Tetrachloro-1-arylethylisocyanates. J. Org. Chem. USSR 1980, 16, 648–653. [Google Scholar]
- Takamatsu, M.; Sekiya, M. Reactions of 1-Trichloromethyl-substituted Amines with Potassium tert-Butoxide. Chem. Pharm. Bull. 1980, 28, 3098–3105. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yamamoto, H. Synthetic Studies on Quinazoline Derivatives. II. The Reactions of 2-Trichloro- and 2-Trifluoroacetamidobenzophenones with Primary Amines. Chem. Pharm. Bull. 1981, 29, 2135–2156. [Google Scholar] [CrossRef]
- Vovk, M.V.; Bal’on, Ya. G.; Krainikova, I.G.; Samaray, L.I. Structure of cyclocondensation products of the reaction of 1-chloroalkyl isocyanates with 2-aminopyridine and 2-aminothiazole. Ukr. Khim. Zh. 1995, 61, 63–68, Chem. Abstr. 1996, 125, 328670. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Kuksa, V.A. Utilization of the amidoalkylation reaction in the synthesis of hydrogenated pyrimidine-2-thiones. Chem. Heterocycl. Compd. 1997, 33, 91–95. [Google Scholar] [CrossRef]
- Shutalev, A.D. Synthesis of 5-arylsulfonyl-1,2,3,4-tetrahydropyrimidine-2-thiones. Chem. Heterocycl. Compd. 1997, 33, 1469–1470. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Kishko, E.A.; Sivova, N.V.; Kuznetsov, A.Y. A New Convenient Synthesis of 5-Acyl-1,2,3,4-tetrahydropyrimidine-2-thiones/ones. Molecules 1998, 3, 100–106. [Google Scholar] [CrossRef]
- Fesenko, A.A.; Shutalev, A.D. Diastereoselective synthesis of 5-benzylthio- and 5-mercaptohexahydropyrimidin-2-ones. Tetrahedron Lett. 2007, 48, 8420–8423. [Google Scholar] [CrossRef]
- Fesenko, A.A.; Cheshkov, D.A.; Shutalev, A.D. Synthesis of diethyl 2-thioxo-1,2,3,4-tetrahydro- and hexahydropyrimidine-5-phosphonates. Mendeleev Commun. 2008, 18, 51–53. [Google Scholar] [CrossRef]
- Chattaway, F.D.; James, E.J.F. The Condensation of Chloral with Urea and Phenyl Urea. Proc. R. Soc. Lond. 1931, 134, 372–384. [Google Scholar]
- Coppin, N.G. S; Titherley, A.W. The Condensation of Chloral Hydrate and Carbamide. J. Chem. Soc. 1914, 105, 32–36. [Google Scholar] [CrossRef]
- Zaugg, H.E. Recent Synthetic Methods Involving Intermolecular α-Amidoalkylation at Carbon. Synthesis 1970, 2, 49–73. [Google Scholar] [CrossRef]
- Zaugg, H.E. α-Amidoalkylation at Carbon: Recent Advances - Part I. Synthesis 1984, 02, 85–110. [Google Scholar] [CrossRef]
- Zaugg, H.E. α-Amidoalkylation at Carbon: Recent Advances - Part II. Synthesis 1984, 3, 181–212. [Google Scholar] [CrossRef]
- Speckamp, W.N.; Moolenaar, J.M. New Developments in the Chemistry of N-Acyliminium Ions and Related Intermediates. Tetrahedron 2000, 56, 3817–3856. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; Zhang, H.-C.; Cohen, J.H.; Turchi, I.J.; Maryanoff, C.A. Cyclizations of N-Acyliminium Ions. Chem. Rev. 2004, 104, 1431–1628. [Google Scholar] [CrossRef]
- Shutalev, A.D. Reaction of α-(thio)amidoalkylation in the synthesis of α-cyano-substituted cyclic (thio)ureas and dithiocarbamates. Chem. Heterocycl. Compd. 1993, 29, 1192–1199. [Google Scholar] [CrossRef]


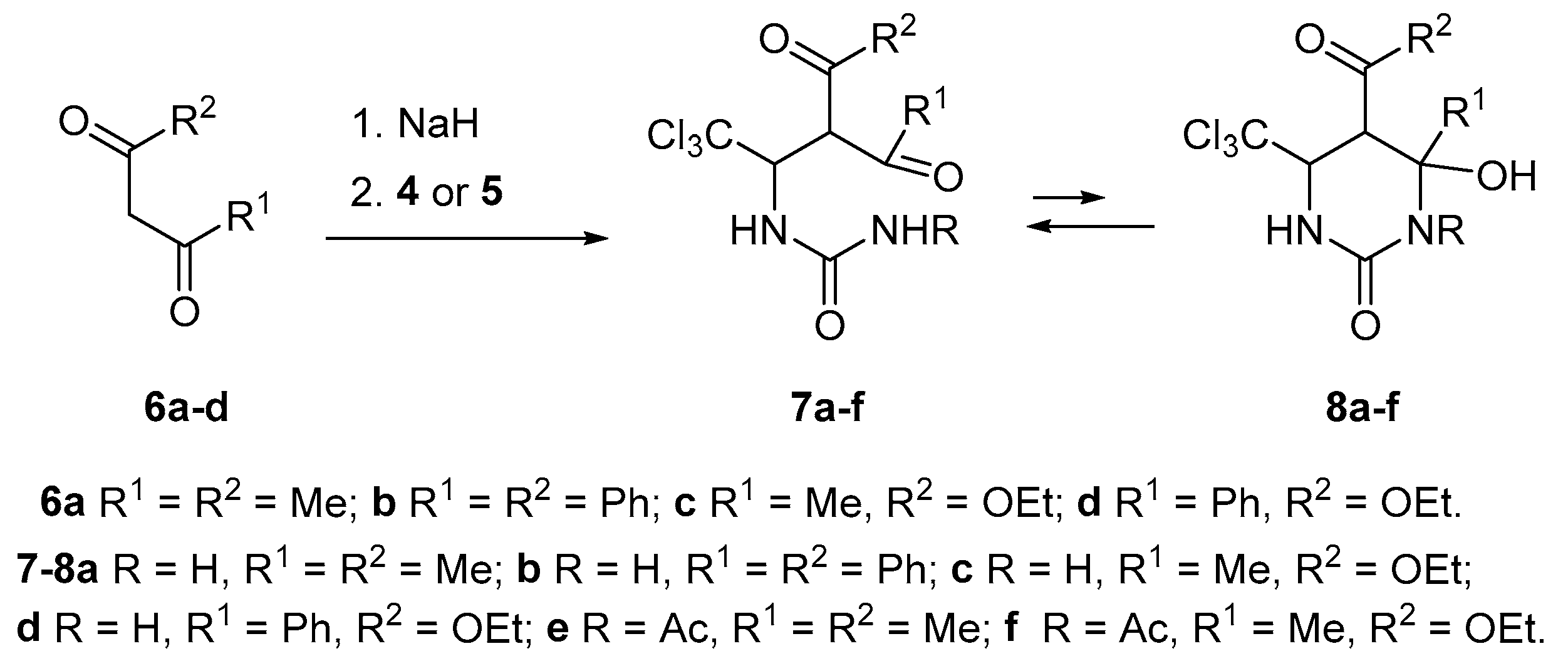






| Entry | Starting Material | Solvent | Reaction Time, h | Molar Ratio (4/6 or 5/6) | Product | Diastereomeric Ratio b | Yield, c % | |
|---|---|---|---|---|---|---|---|---|
| 1 | 6a | 4 | MeCN | 3.3 | 1:1 | 7a | - | 70 |
| 2 | 6b | 4 | THF | 4.3 | 1:1 | 7b | - | 89 |
| 3 | 6c | 4 | MeCN | 4 | 1.1:1 | 7c | 57:43 | 86 |
| 4 | 6d | 4 | MeCN | 2.7 | 1.1:1 | 7d | 72:28 | 95 |
| 5 | 6d | 4 | MeCN | 5.75 | 1:1 | 7d | 83:17 | 91 |
| 6 | 6d | 4 | MeCN | 9.3 | 1:1 | 7d | 84:16 | 90 |
| 7 | 6a | 5 | MeCN | 4.4 | 1:1 | 7e | - | 82 |
| 8 | 6c | 5 | MeCN | 4.2 | 1.1:1 | 7f | 75:25 | 69 |
| Entry | Starting Material | Solvent | Reaction Time, h | Product | Diastereomeric Ratio a (R*,S*)-10/(R*,R*)-10 | Yield, b % | |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 9a | MeCN | 4 | 10a | 95:5 | 88 |
| 2 | 4 | 9a | THF | 4.5 | 10a | 88:12 | 76 |
| 3 | 4 | 9b | MeCN | 5 | 10b | 91:9 | 85 |
| 4 | 5 | 9b | MeCN | 8 | 10c | 97:3 | 88 |
| 5 | 5 | 9c | MeCN | 4 | 10d | 85:15 | 85 |
| 6 | 5 | 9d | MeCN | 9 | 10e | 85:15 | 86 |
| 7 | 5 | 9d | THF | 6.5 | 10e | 86:14 | 90 |
| Entry | Starting Material | Solvent | Molar Ratio of 7:TsOH | Reaction Time, h | Product(s) | Molar Ratio of 12a:13 b | Yield of 12, % |
|---|---|---|---|---|---|---|---|
| 1 | 7a | MeCN | 1:0.3 | 0.6 | 12a | - | 95 |
| 2 | 7a | PhMe | 1:1.13 | 1.0 | 12a + 13 | 73:27 | - |
| 3 | 7a | EtOH | 1:1.13 | 1.0 | 12a + 13 | 94:6 | - |
| 4 | 7a | EtOH | 1:0.5 | 1.25 | 12a + 13 | 94:6 | - |
| 5 | 7a | EtOH | 1:0.3 | 0.63 | 12a + 13 | 90:10 | - |
| 6 | 7a | MeOH | 1:0.5 | 1.75 | 12a + 13 | 62:38 | - |
| 7 | 7b | MeCN | 1:1 | 2.2 | 12b | - | 91 |
| 8 | 7c | MeCN | 1:0.3 | 1.0 | 12c | - | 93 |
| 9 | 7c | PhMe | 1:1.1 | 1.0 | 12c | - | 84 |
| 10 | 7d | MeCN | 1:0.5 | 33 | 12d | - | 81 |
| 11 | 7d | MeCN | 1:3.0 | 14.2 | 12d | - | 75 |
| 12 | 7e | EtOH | 1:1.5 | 2.0 | 12a + 13 | 79:21 | - |
| 13 | 7f | EtOH | 1:2.0 | 3.0 | 12c | - | 77 |
| Entry | Starting Material | Solvent | Molar Ratio of 10:TsOH | Reaction Time, h | Product(s) | Molar ratio of Products, 14:10 b | Isolated Yield of 14, % |
|---|---|---|---|---|---|---|---|
| 1 | 10a | n-BuOH | 1:4.0 | 31 | 14a | - | 63 |
| 2 | 10b | n-BuOH | 1:4.0 | 25 | 14b | - | 75 |
| 3 | 10c | n-BuOH | 1:3.1 | 5 | 14b + 10b c | 28:72 | - |
| 4 | 10c | n-BuOH | 1:4.0 | 18 | 14b | - | 72 |
| 5 | 10d | n-BuOH | 1:2.0 | 2 | 14c | - | 93 |
| 6 | 10e | EtOH | 1:1.1 | 26 | 14d + 10g d | 68:32 | - |
| 7 | 10e | EtOH | 1:2.1 | 16.5 | 14d + 10g d | 80:20 | - |
| 8 | 10e | n-BuOH | 1:2.0 | 2 | 14d | - | 92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovyev, P.A.; Fesenko, A.A.; Shutalev, A.D. A New Approach to 5-Functionalized 1,2-Dihydropyrimidin-2-ones/imines via Base-Induced Chloroform Elimination from 4-Trichloromethyl-1,2,3,4-tetrahydropyrimidin-2-ones/imines. Proceedings 2019, 9, 15. https://doi.org/10.3390/ecsoc-22-05678
Solovyev PA, Fesenko AA, Shutalev AD. A New Approach to 5-Functionalized 1,2-Dihydropyrimidin-2-ones/imines via Base-Induced Chloroform Elimination from 4-Trichloromethyl-1,2,3,4-tetrahydropyrimidin-2-ones/imines. Proceedings. 2019; 9(1):15. https://doi.org/10.3390/ecsoc-22-05678
Chicago/Turabian StyleSolovyev, Pavel A., Anastasia A. Fesenko, and Anatoly D. Shutalev. 2019. "A New Approach to 5-Functionalized 1,2-Dihydropyrimidin-2-ones/imines via Base-Induced Chloroform Elimination from 4-Trichloromethyl-1,2,3,4-tetrahydropyrimidin-2-ones/imines" Proceedings 9, no. 1: 15. https://doi.org/10.3390/ecsoc-22-05678
APA StyleSolovyev, P. A., Fesenko, A. A., & Shutalev, A. D. (2019). A New Approach to 5-Functionalized 1,2-Dihydropyrimidin-2-ones/imines via Base-Induced Chloroform Elimination from 4-Trichloromethyl-1,2,3,4-tetrahydropyrimidin-2-ones/imines. Proceedings, 9(1), 15. https://doi.org/10.3390/ecsoc-22-05678






