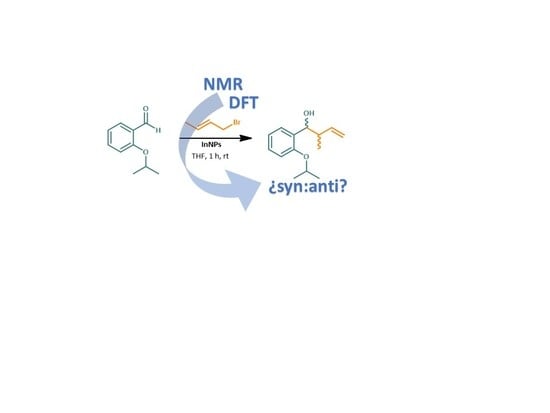
Applications of Computational and NMR Methodologies to the Study of Homoallylic Alcohols Diastereomers †
Abstract
Share and Cite
Dorn, V.; Martínez, E.L.; Radivoy, G. Applications of Computational and NMR Methodologies to the Study of Homoallylic Alcohols Diastereomers. Proceedings 2019, 9, 12. https://doi.org/10.3390/ecsoc-22-05782
Dorn V, Martínez EL, Radivoy G. Applications of Computational and NMR Methodologies to the Study of Homoallylic Alcohols Diastereomers. Proceedings. 2019; 9(1):12. https://doi.org/10.3390/ecsoc-22-05782
Chicago/Turabian StyleDorn, Viviana, Emilio Lorenzo Martínez, and Gabriel Radivoy. 2019. "Applications of Computational and NMR Methodologies to the Study of Homoallylic Alcohols Diastereomers" Proceedings 9, no. 1: 12. https://doi.org/10.3390/ecsoc-22-05782
APA StyleDorn, V., Martínez, E. L., & Radivoy, G. (2019). Applications of Computational and NMR Methodologies to the Study of Homoallylic Alcohols Diastereomers. Proceedings, 9(1), 12. https://doi.org/10.3390/ecsoc-22-05782