Abstract
Instant Cascara (IC) is a beverage made from dried coffee cherries, enriched in nutrients and bioactive compounds such as caffeine and other phytochemicals with a positive impact on the brain–gut axis health. The use of dried coffee cherries as novel foods in drinks was authorized by the EU in 2023. The process for obtaining IC involves the concentration of the regular drink by spray-drying. Colorectal cancer, chronic gut disease, is the third most common cancer type causing 1 million deaths/year. In high-income countries, colon and rectum cancers were one of the top ten causes of death in 2019. The present research aimed to obtain novel and preliminary information about the potential prophylactic or therapeutic effect of IC on colon cancer. In vitro cell models were used to analyze its genotoxicity and effects on key physiological cell events such as intracellular ROS production, proliferation and apoptosis associated with the pathogenesis of cancer. IC was determined non-genotoxic using the comet assay, reduced ROS production in normal and cancer colon cells and selectively affected the proliferation and apoptosis of colon cancer cells by labeled annexin incorporation assay. In conclusion, our preliminary data supported the safety and potential use of IC as a sustainable promoter of gastrointestinal health. Therefore, the upcycling of dried coffee cherries into IC may contribute to the sustainability of the coffee industry and to achieving Global Sustainable Development Goals (3: “Good health and well-being” and 12: “Responsible consumption and production”).
1. Introduction
Instant Cascara (IC) is a beverage made from dried coffee cherries, enriched in nutrients and bioactive compounds such as caffeine and other phytochemicals. These compounds could have a positive impact on promoting the brain–gut axis health. The European Union (EU) authorized the use of dried coffee cherries as a novel food in beverages in 2023. Food security exists when all people, at all times, have physical and economic access to sufficient, safe and nutritious food that meets their dietary needs and food preferences for an active and healthy life [1]. The use of this by-product as IC may contribute to the sustainability of the coffee industry and to achieve Global Sustainable Development Goals (3: “Good health and well-being” and 12: “Responsible consumption and production”). In addition, this product may support the European Green Deal, which aims to provide healthy and affordable food.
Colorectal cancer, chronic gut disease, is the third most common cancer type causing 1 million deaths/year [2]. In high-income countries, colon and rectum cancers were one of the top ten causes of death in 2019. According to the CDC (Centers for Disease Control and Prevention) data, the risk of colorectal cancer increases with age and other factors may contribute, such as inflammatory bowel disease or Crohn’s disease, family history or lifestyle (lack of regular physical activity, diet poor in fruit and vegetables, alcohol and tobacco consumption...) [3]. Therefore, the present research aimed to obtain novel and preliminary information about the potential prophylactic or therapeutic effect of IC on colon cancer.
2. Material and Methods
2.1. Sample Preparation
Coffee fruit cascara of Arabica species and Tabi variety was from Colombia and kindly provided by Supracafé S.A. (Móstoles, Madrid, Spain). Coffee fruit cascara was obtained from wet coffee berry processing, with a 21-day-sun-drying period. Subsequently, it underwent a sanitization procedure utilizing a carbon dioxide atmosphere (Martin Bauer MABA-PEX process). The IC powder was obtained through spray drying the aqueous extract of the coffee fruit cascara at an inlet air temperature of 170 °C. Outlet air temperatures varied from 90 to 110 °C. As described by Iriondo-DeHond et al. (2020), the IC beverage was formulated at 10 mg/mL [4]. The preparation consisted of diluting the powder obtained after the spray-drying process with water at room temperature.
2.2. Cell Culture
HepG2, Caco-2 and CCD-18 cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 50 U/mL penicillin, 50 mg/mL streptomycin and 1% L-glutamine. Cell cultures were incubated at 37 °C in a 5% CO2 atmosphere. HepG2 and CCD-18 cells were obtained from the Centro de Instrumentación Científica (CIC) of the Universidad de Granada, Spain, and Caco-2 cells were provided by the Bioanalytical Techniques Unit (Instituto de Investigación en Ciencias de la Alimentación, Consejo Superior de Investigaciones Científicas (CSIC)-Universidad Autónoma de Madrid (UAM), Madrid, Spain).
2.3. Comet Assay (Analysis of DNA Damage)
This assay was carried out according to Olive et al. (1992) [5]. Briefly, HepG2 cells (1.5 × 105 cells/mL) were seeded onto a 24-well plate. After 24 h, cells were treated with IC (10, 100 and 1000 µg/mL). After incubation for 24 h, cells were mixed with LMP agarose type VII and distributed on slides that had been pre-coated with LMP agarose type VII and left to set on ice. Cells were lysed in darkness for 1 h in a high salt alkaline buffer (2.5 M NaCl, 0.1 M EDTA, 0.01 M Tris, 1% Triton X-100, pH 10). Slides were placed in electrophoresis buffer (0.3 M NaOH, 1 mM EDTA, pH 13, cooled in a refrigerator) for 40 min in darkness. Electrophoresis was performed for 30 min at 25 V. After electrophoresis, the slides were neutralized using 0.4 M Tris pH 7.5 and fixed in methanol. Then, DNA was stained with 1X GelRed® in Tris-acetate EDTA (1X TAE) for 5 min. Benzo(a)pyrene (BaP, 100 µM) was used as a positive control. Finally, cells were examined under a fluorescence microscope connected to a computerized image analysis system (Komet 5.1). Results were expressed as percentage of Tail DNA. Images of 50 randomly selected cells per concentration were evaluated, and the test was carried out three times.
2.4. Analysis of ROS Production
Caco-2 and CCD-18 cells were plated in 96-well plates (5 × 104 and 1 × 104 cells/well, respectively) and treated with IC (100 µg/mL) for 24 h. Intracellular Reactive Oxygen Species (ROS) production was determined using an oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Afterward, 2 µL of DCFH-DA (5 mg/mL in DMSO) was added to each well and incubated for 30 min at 37 °C. Then, cells were washed with 1X PBS and IC was added for 45 min. Fluorescence was measured in a fluorimeter microplate reader (excitation = 485 nm/emission = 528 nm). Tert-butyl hydroperoxide 1 mM was used as a positive oxidation control and vitamin C (10 µg/mL) as an antioxidant agent. MTT assay for cell viability was performed in the same plate to correct ROS values [6].
2.5. Colony Formation Assay
This assay was performed to evaluate cell proliferation. Caco-2 and CCD-18 cells were seeded in 24-well plates (104 cells and 25 × 104 cells, respectively). After 24 h, cells were treated with IC (100 µg/mL) and cultured for 7 days. Cell colonies were washed once with 1X PBS, fixed with 3% paraformaldehyde for 10 min and permeabilized with 2% methanol for 2 min. Finally, cells were stained with 0.5% crystal violet in 20% methanol for 10 min, washed twice with water and the wells were left to dry and photographs were taken with an optical microscope [7]. The wells were destained with 0.1 M sodium citrate in 50% ethanol (pH 4.2) and the absorbance was measured at 550 nm.
2.6. Labeled Annexin-V FITC Incorporation Assay
eBioscience™ Annexin V-FITC Apoptosis Detection Kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was used to detect apoptotic cells. Caco-2 cells were seeded in 6-well plates (0.5 × 106 cells/well) and after treatment with IC (0.5–2 mg/mL) for 18 and 24 h. Cells were resuspended in 100 µL 1X annexin binding buffer and incubated with 5 µL of annexin V-FITC for 10 min. Cells were washed with 1X annexin binding buffer, and after adding 5 µL of PI (propidium iodide), the samples were kept on ice. The cells were analyzed using a flow cytometer (FACSCalibur, Beckton Dickinson, Franklin Lakes, NJ, USA) and the Flowing Software 2.5.1 (Perttu Terho, Turku Centre for Biotechnology, University of Turku, Turku, Finland). Etoposide (300 µM) was used as a positive control.
2.7. Statistical Analysis
Results were expressed as the mean ± standard deviation (SD) (n = 3). One-way analysis of variance (ANOVA) was performed and statistical comparisons were conducted using Tukey’s test. Values of p ≤ 0.05 were considered statistically significant. Data were analyzed using the Statgraphics Centurion 19 software (Statgraphics Technologies, Inc., The Plains, VA, USA).
3. Results and Discussion
The genotoxic effect of IC was studied in HepG2 cells using the comet assay. As shown in Figure 1, IC caused no DNA damage per se at all concentrations tested (10–1000 µg/mL), because no significant differences were observed (p > 0.05) in the strand breaks compared with the untreated cells (C0). However, as expected, treatment with BaP showed a significant increase in these breaks (C1), as described in previous work [8]. These data supported the food safety of the beverage.
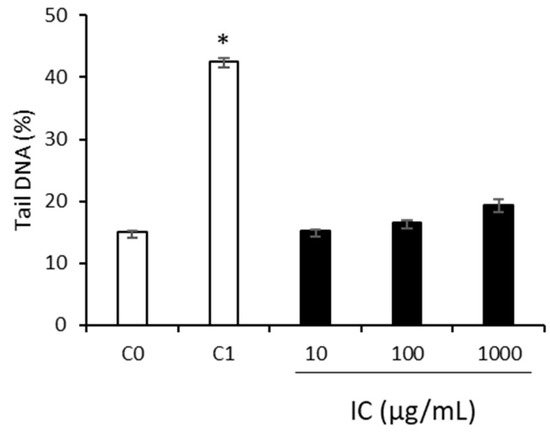
Figure 1.
Induction of DNA strand breaks due to IC on human HepG2 cells. C0, untreated cells. C1, cells treated with BaP (100 µM). Asterisks indicate a significant difference from the control (C0). * p < 0.05.
On the other hand, intracellular Reactive Oxygen Species (ROS) production was examined in cancer and normal colon cells (Caco-2 and CCD-18 cell lines, respectively). Oxidative damage from ROS contributes to carcinogenesis by causing mutations in DNA and higher ROS levels in colon cancer, among others [9]. ROS production was reduced in CCD-18 cells after treatment with IC (95.63% of reduction). In tumor colon cells (Caco-2), a reduction in intracellular ROS production (34%) was also observed.
Cancer is defined as a disease of excessive cell proliferation subject to regulation by factors both intrinsic and extrinsic to the cell [10]. The effect of IC on CCD-18 and Caco-2 cell proliferation was also studied by clonogenic assay. Figure 2 shows that IC at 100 µg/mL did not affect the proliferation of normal colon cells, although IC did reduce the proliferation of cancer cells (p < 0.05), exhibiting a selective effect.
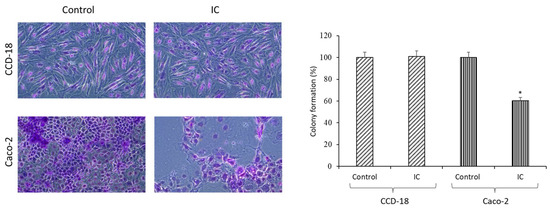
Figure 2.
Effect of IC (100 µg/mL) on cell proliferation. Representative images of crystal violet-stained cells are shown on the (left panel). The bar graph (right panel) shows the mean of the colony formation percentage (absorbance). Asterisks indicate a significant difference from the control. * p < 0.05.
In addition to cell proliferation, evasion of apoptosis is a hallmark of cancer. Apoptosis is a programmed cell death that promotes the elimination of cells that present severe DNA damage [11]. Therefore, the effect of IC on apoptosis induction in Caco-2 cancer cells was evaluated via flow cytometry using labeled annexin-V FITC assay. IC significantly (p < 0.05) induced apoptosis in colon tumor cells in a dose- and time-dependent manner, up to 36% of apoptotic cells at 2 mg/mL at 24 h (Figure 3), although without reaching the result of the positive control (etoposide 300 µM: 47%).
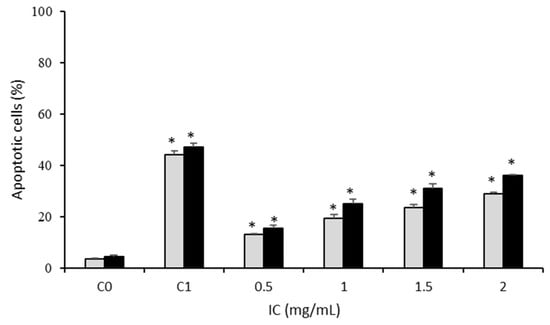
Figure 3.
Effect of IC treatment (0.5–2 mg/mL) on apoptosis induction in Caco-2 cells, for 18 (  ) and 24 (
) and 24 (  ) h. C0, untreated cells. C1, cells exposed to etoposide (300 µM). Asterisks indicate significant differences between control (C0) and treated cells. * p < 0.05.
) h. C0, untreated cells. C1, cells exposed to etoposide (300 µM). Asterisks indicate significant differences between control (C0) and treated cells. * p < 0.05.
Taking into account all these results, IC may have the potential to be a gastrointestinal health promoter, reducing intracellular ROS levels, cancer cell proliferation and induction of apoptosis in tumor cells, showing a selective effect without affecting the proliferation of normal cells. To our knowledge, no powdered beverages have been developed from dried coffee cherries, which could result in the concentration of nutrients and bioactive compounds, increase shelf life and facilitate better storage conditions.
4. Conclusions
The Instant Cascara (IC) beverage obtained through spray drying resulted in non-genotoxic, reduced ROS production in normal and cancer colon cells and selectively affected the proliferation and apoptosis of colon cancer cells. These preliminary data supported the safety and potential use of IC as a sustainable promoter of gastrointestinal health. Therefore, the upcycling of dried coffee cherries into IC may contribute to the sustainability of the coffee industry and to achieving the Global Sustainable Development Goals. In addition, this powdered beverage could increase shelf life and facilitate better storage conditions.
Author Contributions
Conceptualization, A.I.H., P.M. and M.D.d.C.; methodology, V.S.-M. and A.I-D.; formal analysis, V.S.-M. and M.B.L.-P.; data curation, V.S.-M. and M.B.L.-P.; writing—review and editing, V.S.-M., M.B.L.-P., A.I.-D., A.I.H., P.M. and M.D.d.C.; visualization, V.S.-M.; supervision, A.I.H., P.M. and M.D.d.C.; project administration, A.I.H., P.M. and M.D.d.C.; funding acquisition, A.I.H., P.M. and M.D.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación, grant number PID2019-111510RB-I00 and RTI2018-097549-B-I00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perez-Escamilla, R. Food Security and the 2015–2030 Sustainable Development Goals: From Human to Planetary Health: Perspectives and Opinions. Curr. Dev. Nutr. 2017, 1, e000513. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/Cancer/Colorectal/Basic_info/Risk_factors.htm (accessed on 10 August 2023).
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; del Castillo, M.D. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage: Melanoidins and Acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Wlodek, D.; Dufund, R.E.; Banath, J.P. Factors Influencing DNA Migration from Individual Cells Subjected to Gel Electrophoresis. Exp. Cell Res. 1992, 198, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Gámbaro, A.; Medrano-Fernandez, A.; Del Castillo, M.D. In Vitro Bioaccessibility of Bioactive Compounds from Citrus Pomaces and Orange Pomace Biscuits. Molecules 2021, 26, 3480. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, V.; Jiménez-García, L.; Herranz, S.; Luque, A.; Acebo, P.; Amesty, Á.; Estévez-Braun, A.; De Las Heras, B.; Hortelano, S. α-Hispanolol Induces Apoptosis and Suppresses Migration and Invasion of Glioblastoma Cells Likely via Downregulation of MMP-2/9 Expression and P38MAPK Attenuation. Front. Pharmacol. 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Haza, A.I.; Ávalos, A.; del Castillo, M.D.; Morales, P. Validation of Coffee Silverskin Extract as a Food Ingredient by the Analysis of Cytotoxicity and Genotoxicity. Food Res. Int. 2017, 100, 791–797. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Safe, S. Reactive Oxygen Species and Colorectal Cancer. Curr. Color. Cancer Rep. 2013, 9, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Gaglia, G.; Kabraji, S.; Rammos, D.; Dai, Y.; Verma, A.; Wang, S.; Mills, C.E.; Chung, M.; Bergholz, J.S.; Coy, S.; et al. Temporal and Spatial Topography of Cell Proliferation in Cancer. Nat. Cell Biol. 2022, 24, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Javed, Z.; Khan, K.; Khan, N.; Docea, A.O.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Apoptosis Evasion via Long Non-Coding RNAs in Colorectal Cancer. Cancer Cell Int. 2022, 22, 280. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).