Abstract
Hand-arm vibration syndrome (HAVS) is characterized by cold-induced vasospasms of peripheral vasculature, by changes in sensorineural function and by pain. Vibration frequency and amplitude along with pressure applied at the fingertips while gripping a tool may also affect vascular and sensorineural function. Little is known about how these two exposure factors interact to affect the risk of developing HAVS. This study uses a newly developed rat tail model to examine the effects of vibration on vascular and sensorineural function. Exposure to 2N of pressure for 10 consecutive days resulted in an increase in blood flow in the tail, which may have been the result of an increased sensitivity of the arteries to acetylcholine. There was also an increased sensitivity of the small myelinated fibbers to electrical stimulation and of the sensory receptors to a pressure stimulus. Based on these findings, pressure has its own effects on vascular and sensorineural physiology, and these effects can be different from those of vibration.
1. Introduction
Hand-arm vibration syndrome (HAVS) is characterized by changes in sensorineural and peripheral vascular function. The primary symptom of HAVS is cold-induced finger blanching due to spasms of the peripheral vasculature, which is referred to as vibration white finger (VWF). Other symptoms include a loss of sensory function and pain. Epidemiological, field and laboratory studies have examined the effects of vibration frequency and amplitude on the risk of developing the symptoms of HAVS [1,2,3,4]. However, grip force, or pressure applied at the finger tips while gripping a tool, may also affect vascular and sensorineural function [5,6].
Researchers at NIOSH have developed a new rat tail model for examining the effects of vibration and applied pressure on vascular and sensorineural function. A diagram of the new model can be seen in Figure 1, and a detailed description of the model is presented in [6]. This model was used to study the effects of applied pressure on physiological measures of vascular and sensorineural function.
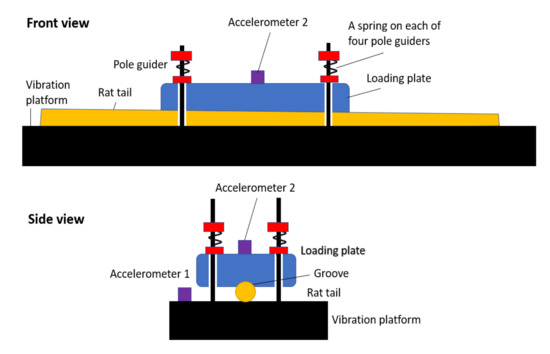
Figure 1.
Modified rat tail model (Adapted from [7]).
2. Methods
2.1. Animals
Male Sprague Dawley rats (6 weeks of age; approximately 250 g) were obtained from Hilltop Breeders (Scottsdale, PA, USA) and acclimated to mild physical restraint in a Broome style restrainer. After acclimation, animals were assigned to two groups. One group was restrained and had their tails exposed to 2 newtons (N) of pressure (n = 6) for 4 h/day for 10 consecutive days. A second group (controls = 3) was maintained in restrainers without pressure applied to their tails. All procedures were approved by the Institutional Animal Care and Use Committee and were in compliance with both the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Physiological Measures
2.2.1. Laser Doppler
On days 1, 5 and 10 of the study, blood flow was measured by laser Doppler (Perimed: Järfälla, Sweden) both before and after exposure. Blood flow measurements were collected in perfusion units at 0.2 Hz for 5 min and average blood flow was calculated over for the 5 min recording period.
2.2.2. Sensory Measures
On days 2 and 9 of this study, sensory nerve function was measured using the current perception threshold test (CPT); moreover, on days 1, 5 and 10, sensitivity of receptors that respond to pressure was measured using the Randall–Selitto (R–S) test.
2.3. Microvessels
Animals were humanely euthanized 1 d following the last exposure by using an AVMA approved procedure, and ventral tail arteries were assessed for responsiveness to the α1-adrenoreceptor agonist, to phenylephrine (PE) and to the vasodilating agent, acetylcholine (ACh). Responsiveness to changes in internal vascular pressure was also measured.
2.4. Analyses
Average blood flow and average responses to the CPT or Randall-Sellitto test were calculated and used for analyses. Data were analysed using repeated measures with ANOVA and post-hoc t-tests. Pre-post measures were analysed using t-tests. p < 0.05 was considered significant.
3. Results
Post-exposure laser Doppler measurements were increased as compared to pre-exposure measurements in the animals exposed to pressure on day 5 of this study (Figure 2).
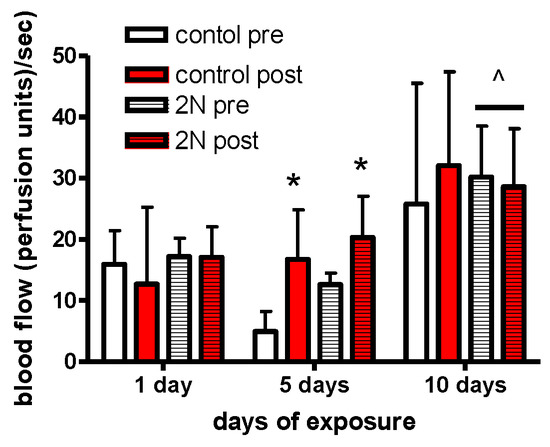
Figure 2.
Exposure to control conditions or 2N of applied pressure resulted in acute increases in blood flow in both groups post exposure on day 5 of this experiment (* p < 0.05, different than pre-exposure measurements). There were no pre-post-exposure differences on day 10 of the experiment; however, in the pressure-exposed group, both pre- and post-exposure blood flow was increased in comparison with blood flow on days 1 and 5 (^ different than day 1, p < 0.05).
Pre-exposure laser Doppler measurements in the animals exposed to pressure were higher on day 10 of exposure (Figure 3).
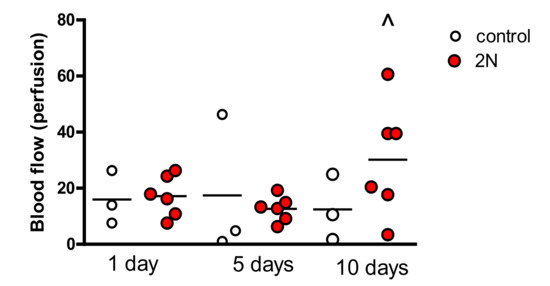
Figure 3.
Pre-exposure blood flow was significantly increased after 10 days of exposure to 2N of pressure in comparison with blood flow in animals after 1 and 5 days of exposure (^ different than day 1, p < 0.05).
After 10 days of exposure, the responsiveness of the ventral tail artery to the vasoconstricting agent, PE (Figure 4A), and the vasodilation in response to the increasing vascular pressure (Figure 4C) were not affected in the arteries of the animals exposed to applied pressure. However, the arteries of the exposed animals were more sensitive to vasodilation in response to ACh (Figure 4B).
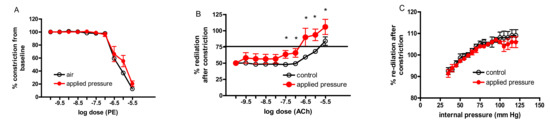
Figure 4.
Sensitivity to PE-induced vasoconstriction in the ventral tail artery was not affected by exposure to pressure (A), but the artery was more sensitive to acetylcholine (ACh)-induced vasodilation (B). There were no exposure-related differences in vasodilation induced by increasing the internal pressure within the artery (C). (* different than controls, p < 0.05).
The 250 Hz pre-exposure CPT was reduced after 9 days of exposure to applied pressure (Figure 5).
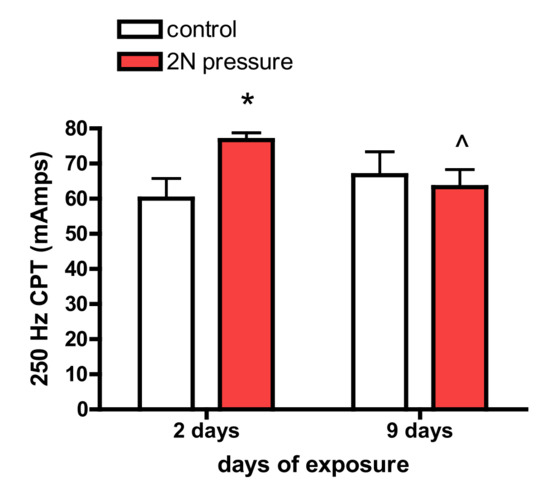
Figure 5.
The (CPT) at 2000 and 5 Hz was not affected by exposure to pressure; however, after 9 days of exposure to pressure, animals were more sensitive to electrical stimulation at 250 Hz (reduction in threshold). (* different than same day control; ^ less than 2 days of exposure, p < 0.05).
The tails of the animals exposed to pressure for 10 d were more sensitive to the pressure applied with an R–S pressure meter (Figure 6).
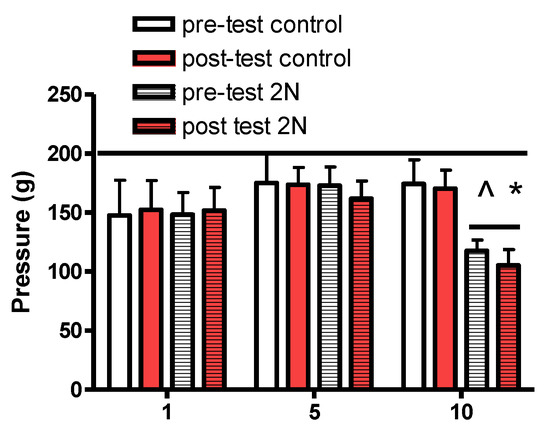
Figure 6.
The R–S pressure meter was used to measure sensitivity to pressure applied in the exposed region of the tail (similar to the von Frey filament test). The line at 200 indicates the maximum amount of pressure that was applied (to prevent injury). There were no changes in the control group over time. Animals exposed to repeated pressure became more sensitive to applied stimulus after 10 days of exposure (^ different than day 1 same exposure; * different than same day pre-test, p < 0.05).
4. Discussion and Conclusions
- Blood flow was increased in the animals exposed to 2N of pressure (within the range of pressure generable by the fingertips of a tool user) for 10 days, suggesting that the blood vessels were dilated.
- The results of the vascular responsiveness studies were consistent with the results of the laser Doppler studies: pressure resulted in an increased responsiveness to ACh-induced re-dilation.
- The failure to find an increased response to internal pressure in the micro vessels studies (mimicking an increase in blood flow) in the exposed animals suggests that ACh or nitric oxide signalling may have been affected by the exposure.
- The CPT at 250 Hz was reduced in exposed animals, indicating an increase in sensitivity. These data suggest that pressure applied at the fingertips while gripping a tool may affect Aδ (small myelinated) the pressure and temperature sensing fibbers in the fingers.
- The findings from the Randall–Selitto test are consistent with the findings of the CPT, showing an increased sensitivity to sensory stimuli after repeated exposures.
- Additional studies are warranted to examine the effects of increased pressure and to determine the mechanisms underlying the changes in vascular responsiveness.
Author Contributions
Conceptualization, K.K., C.W., X.S.X., D.E.W. and R.G.D.; methodology, K.K., C.W., X.S.X., S.W., P.C. and R.G.D.; software, C.W. and X.S.X.; formal analysis, K.K.; investigation, K.K., P.C. and S.W.; resources and funding acquisition, this work was funded by a NIOSH NORA to K.K. and R.G.D.; data curation, K.K., P.C. and S.W.; writing original draft, K.K.; writing, review and editing, K.K., C.W., X.S.X., S.W., P.C., D.E.W. and R.G.D.; supervision, K.K. and R.G.D.; project administration, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through the National Research Organization Agenda, NIOSH, grant 22037A9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available in NIOSHs study tracking and reporting system (STARS) once published.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bovenzi, M. Epidemiological evidence for new frequency weightings of hand-transmitted vibration. Ind. Health 2012, 50, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Bovenzi, M.; Pinto, I.; Picciolo, F.; Mauro, M.; Ronchese, F. Frequency weightings of hand-transmitted vibration for predicting vibration-induced white finger. Scand. J. Work. Environ. Health 2011, 37, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.G.; Welcome, D.E.; McDowell, T.W.; Wu, J.Z.; Schopper, A.W. Frequency Weighting Derived from Power Absorption of Fingers-Hand-Arm System under zh-Axis Vibration. J. Biomec. 2006, 39, 2311–2324. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, K.; Miller, G.R.; Waugh, S.; Johnson, C.; Li, S.; Kashon, M.; Wilder, D.; Rahmatalla, S.; Fathke, N. Vascular responses to vibration are frequency dependent. J. Occup. Environ. Med. 2010, 52, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Welcome, D.E.; McDowell, T.W.; Xu, X.S.; Dong, R.G. Modeling of the interaction between grip force and vibration transmissibility of a finger. Med. Eng. Phys. 2017, 45, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Starck, J.; Farkkila, M.; Aatola, S.; Pyykko, I.; Korhonen, O. Vibration Syndrome and Vibration in Pedestal Grinding. Br. J. Ind. Med. 1983, 40, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.G.; Warren, C.; Xu, X.S.; Wu, J.Z.; Welcome, D.E.; Waugh, S.; Krajnak, K. A novel rat tail model for studying human finger vibration health effects. In Proceedings of the International Conference on Hand-Arm Vibration, Nancy, France, 6–9 June 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).