1. Introduction
Sustained exposure to high-level hand-transmitted vibrations may lead to angioneurotic disorders such as vibration-induced Raynaud’s syndrome, also known as vibration white finger (VWF). Many physiological, histological, and epidemiological studies [
1] have highlighted that the vibration dose assessed according to the current ISO 5349 standard may underestimate the onset predictions of such vascular injuries. In order to better tackle vibration-induced pathophysiological vascular issues, we recently set up a strategy in a two-step process [
2]. First, we hypothesized that vibrations may acutely decrease the shear stress exerted by the blood flow on the artery endothelium layer. Second, studies have shown in various fields [
3] that a chronic drop in this so-called wall shear stress (WSS) may result in arterial stenosis. Furthermore, angiographies and biopsies have emphasized this reduction in arterial lumen in patients suffering from VWF. Our approach then consisted of (i) assessing the relationship between vibration properties (frequency, amplitude) and the WSS drop and (ii) implementing a mechanobiological model which linked the vibration-reduced WSS and the resulting arterial stenosis. This current paper aims to establish how this strategy might pave the way for a new framework to prevent vibration-induced vascular risk.
2. Materials and Methods
2.1. Assessment of the Vibration-Altered Wall Shear Stress
An experimental device was set up to assess the vibration-induced WSS of the left proper volar forefinger artery at the level of the distal interphalangeal joint [
4] while subjecting the right hand to mechanical vibration. The apparatus (
Figure 1a) consisted mainly of an ultra-high-frequency ultrasound transducer connected to an ultrasound imaging system. In all, 24 volunteers in good health and who are nonsmokers, aged from 19 to 39 years old (average age 25.1), participated in a WSS measurement campaign in a room kept at a constant temperature (23 °C ± 0.5 °C). The protocol consisted of estimating the WSS for three consecutive phases of 10 s each: (i) rest, (ii) exposure to vibrations, and (iii) return to calm. Vibration was a pure harmonic acceleration at 125 Hz for six amplitudes: 1, 2, 5, 10, 20, and 40 m·s
−2 root mean square. WSS was then assessed by using a Womersley pulsatile flow model. The time averages of WSS (TAWSSs) over each of the three 10 s phases were subsequently worked out.
2.2. Modelling the Vibration-Induced Arterial Stenosis
A mechanobiological framework made up of an agent-based model (ABM) coupled with a finite elements (FE) model was set up (
Figure 1b). The ABM caught up the hemodynamics-driven and mechanoregulated cellular and molecular mechanisms involved in vibration-induced intimal hyperplasia. Actually, this latter biological vascular process was assumed to likely be in part responsible for arterial stenosis. Many biological mechanisms were taken into account to model intimal hyperplasia, such as the secretion of mediators by the endothelial cells and the smooth muscle cells (SMCs), the proliferation/apoptosis and migration of SMCs, and the synthesis/degradation of extracellular matrix [
5]. These phenomena were regulated by the WSS values, as well as the circumferential stresses (σ
θ) simulated by our FE model.
3. Result
3.1. Acute Impact of Vibrations on Arterial Hemodynamics
The drop in WSS triggered by the vibration occurred a few seconds after starting the vibratory excitation (
Figure 2a).
The TAWSSs normalized by the basal state (
Figure 2b) depended on the amplitude of the vibration in a statistically significant way. They obeyed a log
2 linear regression law of the vibration amplitude.
3.2. Chronic Response of the Artery at Tissue and Molecular Scales Due to Vibration-Induced WSS Drop
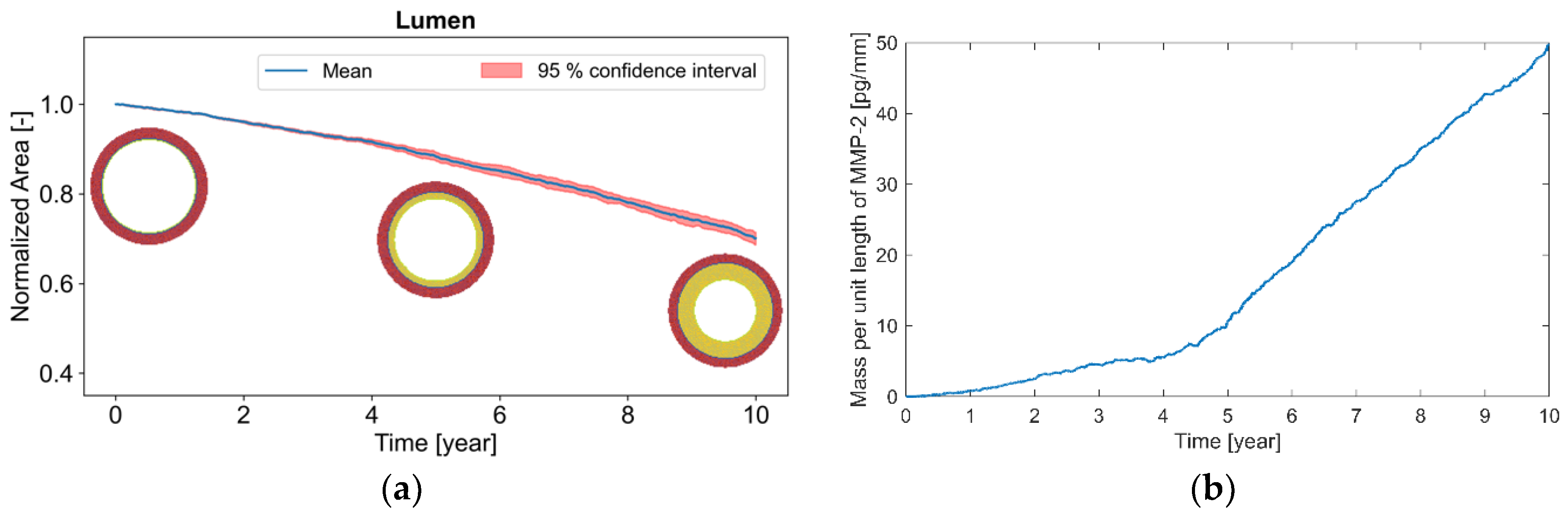
The normalized surface of the arterial lumen (
Figure 3a) continually decreased with exposure time. The reduction in this surface was 12% at 5 years and 30% at 10 years of exposure. The mass per unit length of Matrix MetalloProtease MMP-2 (
Figure 3b) accumulated continuously and considerably with the working lifetime, changing from the absence of MMP-2 initially to 50 pg/mm (picograms per mm) at the end of 10 years.
3.3. Chart for Forecasting the Arterial Stenosis
The degree of arterial stenosis (expressed in %) is defined as the reduction in the arterial lumen normalized by its basal value. The abacus for forecasting the arterial stenosis (
Figure 4) predicted a 20% arterial stenosis degree for an employee exposed to vibration for 2.5 h per day for 10 years. This same level of stenosis was also reached after 15 years of work for a daily exposure to vibrations of about 1 h 10 min.
4. Discussion
The development stage of VWF can be classified according to the degree of arterial stenosis: (i) type 0: healthy, (ii) disease type I: stenosis < 50%, (iii) disease type II: stenosis > 50%, (iv) disease type III: obstruction of the proper digital artery, and (v) disease type IV: obstruction of the common artery or upstream. Our model forecasted stenosis of around 30% after 10 years of exposure to a 40 m·s−2 amplitude vibration for 4 h a day, thereby leading to type I stenosis in keeping with the aforesaid classification.
Furthermore, a relationship between vibration amplitude and the subsequent WSS was established. Therefore, the WSS drop will be assessed by measuring in the field the vibration acceleration level on the handle of a vibrating machine. Thus, knowledge of the daily exposure (the model can take into account all types of exposure cycles) and the WSS (or similarly, the acceleration on the machine), the mechanobiological model will be permitted to work out the degree of stenosis and, thus, that of the disease for chronic exposure to vibrations.
Otherwise, with regard to the molecular upshots of chronic vibration exposure, MMP-2 accumulated substantially in the artery (
Figure 3b). Thus, this enzyme could be a particularly suitable candidate biomarker for warning about and monitoring the evolution of VWF.
5. Conclusions
We succeeded in figuring out a relationship between acute vibration amplitude and the induced WSS drop. Our mechanobiological model was then able to forecast the chronic arterial stenosis elicited by that vibration-driven hemodynamic change. Linking vibration amplitude, daily exposure to vibration, working lifetime, and resulting stenosis is being used for building a new definition of vibration dose.
Author Contributions
Conceptualization, C.N. (all Sections), N.S. (
Section 2.1 and
Section 3.1), M.R. (
Section 2.2 and
Section 3.2), and E.J. (
Section 2.2 and
Section 3.2); methodology, C.N. (all Sections), N.S. (
Section 2.1 and
Section 3.1), M.R. (
Section 2.2 and
Section 3.2), and E.J. (
Section 2.2 and
Section 3.2); software, C.N. (all Sections) and M.R. (
Section 2.2 and
Section 3.2); validation, C.N. (all Sections), N.S. (
Section 2.1 and
Section 3.1), and E.J. (
Section 2.2 and
Section 3.2); formal analysis, C.N. (all Sections), N.S. (
Section 2.1 and
Section 3.1), M.R. (
Section 2.2 and
Section 3.2), and E.J. (
Section 2.2 and
Section 3.2); investigation, C.N.; resources, C.N.; data curation, C.N.; writing—original draft preparation, C.N.; writing—review and editing, C.N.; visualization, C.N.; supervision, C.N. (all Sections), N.S. (
Section 2.1 and
Section 3.1), and E.J. (
Section 2.2 and
Section 3.2); project administration, C.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the French National Agency for Medicines and Health Products (No. ID-RCB: 2018-A00614-651, 6 December 2018) and by the French National Ethical Research Committee (CPP 18 04 04, 6 December 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mechanical Vibration—Measurement and Evaluation of Human Exposure to Hand Transmitted Vibration—Supplementary Method for Assessing Risk of Vascular Disorders; ISO/TR 18570; ISO: Geneva, Switzerland, 2017.
- Noël, C.; Settembre, N.; Reda, M.; Jacquet, E. A Multiscale Approach for Predicting Certain Effects of Hand-Transmitted Vibration on Finger Arteries. Vibration 2022, 5, 213–237. [Google Scholar] [CrossRef]
- Humphrey, J.D. Vascular Adaptation and Mechanical Homeostasis at Tissue, Cellular, and Sub-Cellular Levels. Cell Biochem. Biophys. 2008, 50, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Noël, C.; Settembre, N. Assessing Mechanical Vibration-Altered Wall Shear Stress in Digital Arteries. J. Biomech. 2022, 131, 110893. [Google Scholar] [CrossRef]
- Reda, M.; Noël, C.; Settembre, N.; Chambert, J.; Lejeune, A.; Rolin, G.; Jacquet, E. Agent-Based Model of the Vibration-Induced Intimal Hyperplasia. Biomech. Model. Mechanobiol. 2022, 21, 1457–1481. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).