In-Vitro Antidiabetic Propensities, Phytochemical Analysis, and Mechanism of Action of Commercial Antidiabetic Polyherbal Formulation “Mehon” †
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Extract
2.3. Estimation of Total Phenol Content (TPC)
2.4. Estimation of Total Flavonoid Content (TFC)
2.5. α-Amylase Inhibition Assay
2.6. α-Glucosidase Inhibition Activity
2.7. Effect of Tested Extracts on Glucose Adsorption Capacity
2.8. Effect of Tested Extracts on In-Vitro Glucose Diffusion
2.9. Effect of Tested Extracts on In-Vitro Amylolysis Kinetics
2.10. Effect of Tested Extracts on Glucose Uptake by Yeast Cells
2.11. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Bioactivity Assays
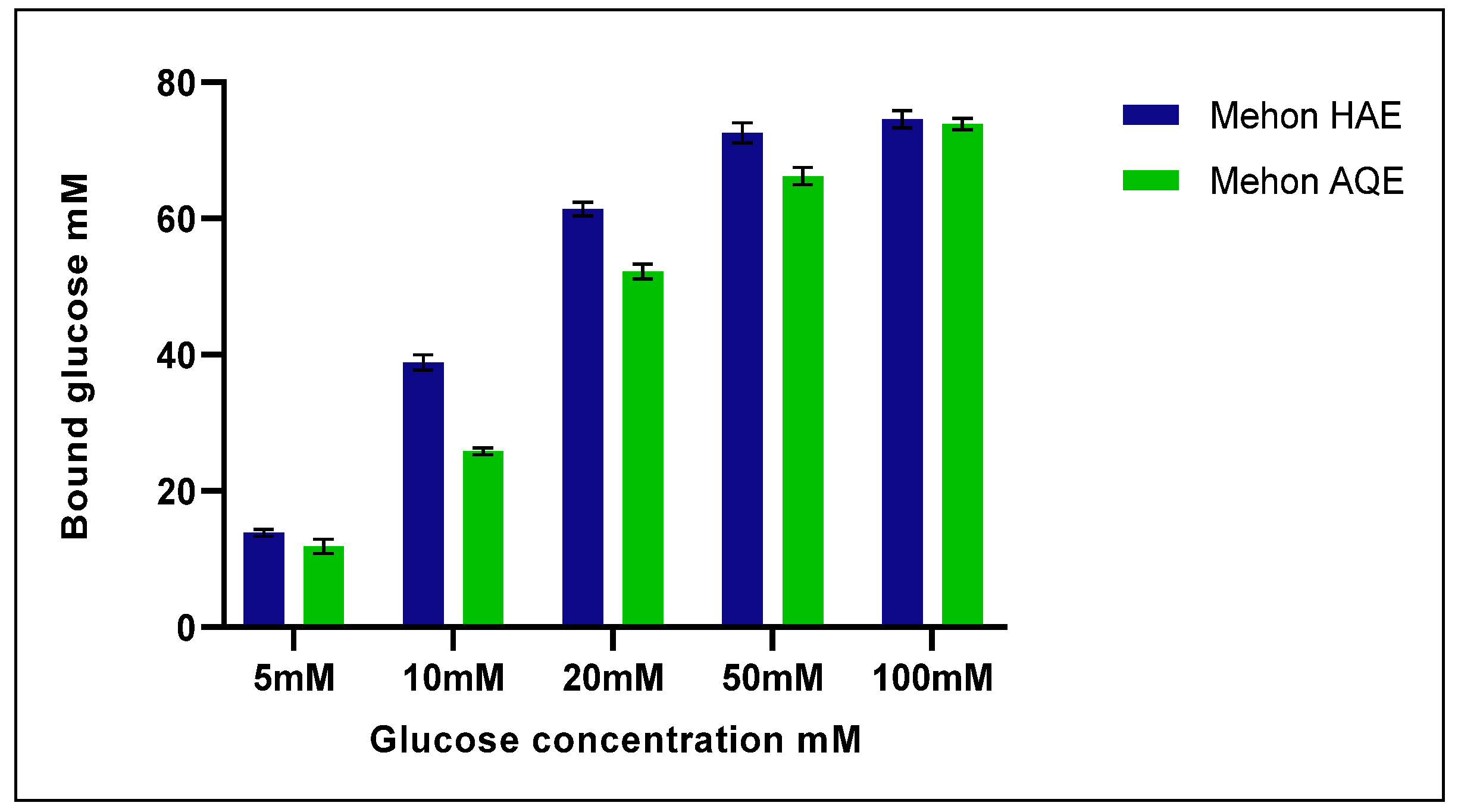
3.3. Effect of Tested Extracts on Glucose Adsorption Capacity
3.4. Effect of Tested Extracts on In-Vitro Glucose Diffusion
3.5. Effect of Tested Extracts on In-Vitro Amylolysis Kinetics
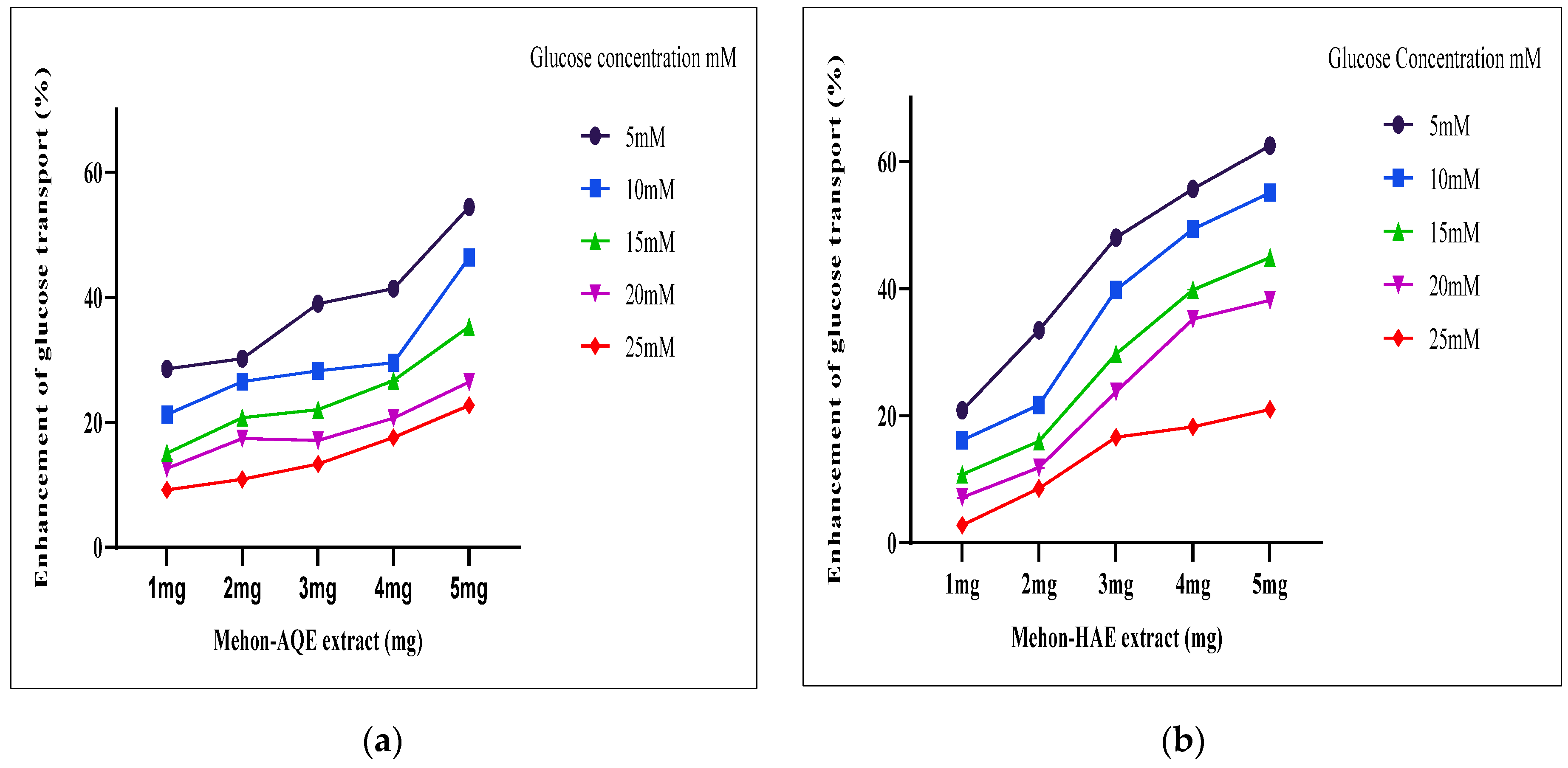
3.6. Effect of Tested Extracts on Glucose Uptake by Yeast Cells
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Dey, L.; Anoja, S.; Attele, Y.C.S. Type two diabetes; Alternative medicine review. Thorne Res. 2002, 7, 45–58. [Google Scholar]
- Srivastava, A.K.; Maurya, R. Antihyperglycemic activity of compounds isolated from Indian medicinal plants. Indian J. Exp. Biol. 2010, 48, 294–298. [Google Scholar]
- Roglic, G.; Wild, S.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004, 27, 1047–1053. [Google Scholar]
- Chattopadhyay, R.R. A comparative evaluation of some blood sugar lowering agents of plant origin. J. Ethnopharmacol. 1999, 67, 367–372. [Google Scholar] [CrossRef]
- Ojo, C.M.; Osunsanmi, O.F.; Zaharare, E.G.; Mosa, A.R.; Cele, D.N.; Oboh, M.O.; Opoku, A.R. In-vitro Anti-diabetic and Antioxidant Efficacy of Methanolic Extract of Encephalartos ferox leaves. Pharm. J. 2019, 11, 455–460. [Google Scholar] [CrossRef]
- Thengya, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R.Br. stem bark. J. Herb. Med. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Mayur, B.; Sandesh, S.; Shruti, S.; Sung-Yum, S. Antioxidant and 𝛼-glucosidase inhibitory properties of Carpesium abrotanoides L. J. Med. Plants Res. 2010, 4, 1547–1553. [Google Scholar]
- Pennathur, S.; Heinecke, W.J. Mechanisms of oxidative stress in diabetes: Implications for the pathogenesis of vascular disease and antioxidant therapy. Front. Biosci. 2004, 9, 565–574. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Alpha glucosidase inhibitory effect and enzyme kinetics of coastal medicinal plants. Bangladesh J. Pharmacol. 2012, 7, 186–191. [Google Scholar] [CrossRef]
- Thilagam, E.; Parimaladevi, B.; Kumarappan, C.; Mandal, C.S. 𝛼-glucosidase and 𝛼-amylase inhibitory activity of Senna surattensis. J. Acupunct. 2013, 6, 24–30. [Google Scholar]
- Edwards, C.A.; Blackburn, A.N.; Craigen, L. Viscosity of food gums determined in vitro related to their hypoglycemic actions. Am. J. Clin. Nutr 1987, 46, 72–77. [Google Scholar] [CrossRef]
- Harish, M.; Ahmed, F.; Urooj, A. In vitro hypoglycemic effects of Butea monosperma Lam. leaves and bark M. J. Food Sci. Technol 2011, 51, 308–314. [Google Scholar] [CrossRef]
- Chang, CH.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Sembiring, N.E.; Elya, B.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharm. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism; Cengage Learning: Hampshire, UK, 2012. [Google Scholar]
- Rolfes, S.R.; Pinna, K.; Whitney, E. Understanding Normal and Clinical Nutrition; Cengage Learning Inc.: Belmont, CA, USA, 2008. [Google Scholar]
- Carene, M.N.; Picot, A.; Subratty, H.; Mahomoodally, F.M. Inhibitory Potential of Five Traditionally Used Native Antidiabetic Medicinal Plants on 𝛼-Amylase, 𝛼-Glucosidase, Glucose Entrapment, and Amylolysis Kinetics In Vitro. Adv. Pharmacol. Sci. 2014, 2014, 739834. [Google Scholar]
- Bhutkar, M.A.; Bhise, SB. In vitro assay of alpha amylase inhibitory activity of some indigenous plants. Int. J. Chem. Sci. 2012, 10, 457–462. [Google Scholar]
- Lopez, G.; Ros, G.; Rincon, F.; Periago, M.J.; Martinez, M.C.; Ortuno, J. Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agr. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Illiano, G.; Cuatrecasas, P. Glucose transport in fat cell membranes. J. Biochem. 1971, 246, 2472–2479. [Google Scholar] [CrossRef]
- Teusink, B.; Diderich, J.A.; Westerhoff, H.V.; Van Dam, K.; Walsh, MC. Intracellular glucose concentration in derepressed yeast cells consuming glucose is high enough to reduce the glucose transport rate by 50%. J. Bacteriol. 1998, 180, 556–562. [Google Scholar] [CrossRef]
- Eichler, H.G.; Korn, A.; Gasic, S.; Pirson, W.; Businger, J. The effect of a new specific α-amylase inhibitor on post prandial glucose and insulin excursions in normal subjects and type 2 (non-insulin-dependent) diabetic patient. Diabetologia 1984, 26, 278–281. [Google Scholar] [CrossRef]
- Chiba, S. Molecular mechanism in alpha glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
| Mehon Extracts | TPC GAE/mg | TFC QE/mg | α-Amylase Inhibition IC50 μg/mL | α-Glucosidase Inhibition IC50 μg/mL |
|---|---|---|---|---|
| HAE | 95.44 ± 0.22 | 86 ± 0.15 | 581.5 | 156.95 |
| AQE | 47.87 ± 0.29 | 38.82 ± 0.15 | 872.88 | 800.63 |
| Sample | Glucose Content in Dialysate (mM) | |||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| Control | 0.929 ± 0.081 | 1.31 ± 0.043 | 1.503 ± 0.04 | 1.744 ± 0.13 |
| AQE | 0.767 ± 0.05(17.44) | 1.21 ± 0.053 (7.66) | 1.398 ± 0.07 (7) | 1.502 ± 0.014(13.87) |
| HAE | 0.674 ± 0.05 (27.48) | 1.062 ± 0.05(19.08) | 1.283 ± 0.043 (14.63) | 1.488 ± 0.04(14.67) |
| Glucose content in dialysate (mM) | ||||
|---|---|---|---|---|
| 30 min | 60 min | 120 min | 180 min | |
| Control | 0.0 | 0.28 ± 0.005 | 0.354 ± 0.003 | 0.446 ± 0.009 |
| AQE | 0.0 (100) | 0.134 ± 0.03 (52.5%) | 0.228 ± 0.027 (34.19%) | 0.38 ± 0.07 (14.25%) |
| HAE | 0.0 (100) | 0.152 ± 0.06 (45.7%) | 0.24 ± 0.047 (30.86%) | 0.364 ± 0.049(18.59%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, S.; Majumdar, M. In-Vitro Antidiabetic Propensities, Phytochemical Analysis, and Mechanism of Action of Commercial Antidiabetic Polyherbal Formulation “Mehon”. Proceedings 2021, 79, 7. https://doi.org/10.3390/IECBM2020-08805
Paul S, Majumdar M. In-Vitro Antidiabetic Propensities, Phytochemical Analysis, and Mechanism of Action of Commercial Antidiabetic Polyherbal Formulation “Mehon”. Proceedings. 2021; 79(1):7. https://doi.org/10.3390/IECBM2020-08805
Chicago/Turabian StylePaul, Saptadipa, and Mala Majumdar. 2021. "In-Vitro Antidiabetic Propensities, Phytochemical Analysis, and Mechanism of Action of Commercial Antidiabetic Polyherbal Formulation “Mehon”" Proceedings 79, no. 1: 7. https://doi.org/10.3390/IECBM2020-08805
APA StylePaul, S., & Majumdar, M. (2021). In-Vitro Antidiabetic Propensities, Phytochemical Analysis, and Mechanism of Action of Commercial Antidiabetic Polyherbal Formulation “Mehon”. Proceedings, 79(1), 7. https://doi.org/10.3390/IECBM2020-08805




