How Could QbD Address the R&D Challenges of ‘Nose-To-Brain’ Liposomal Resveratrol Formulations? †
Abstract
:1. Introduction
2. Experiments
3. Results
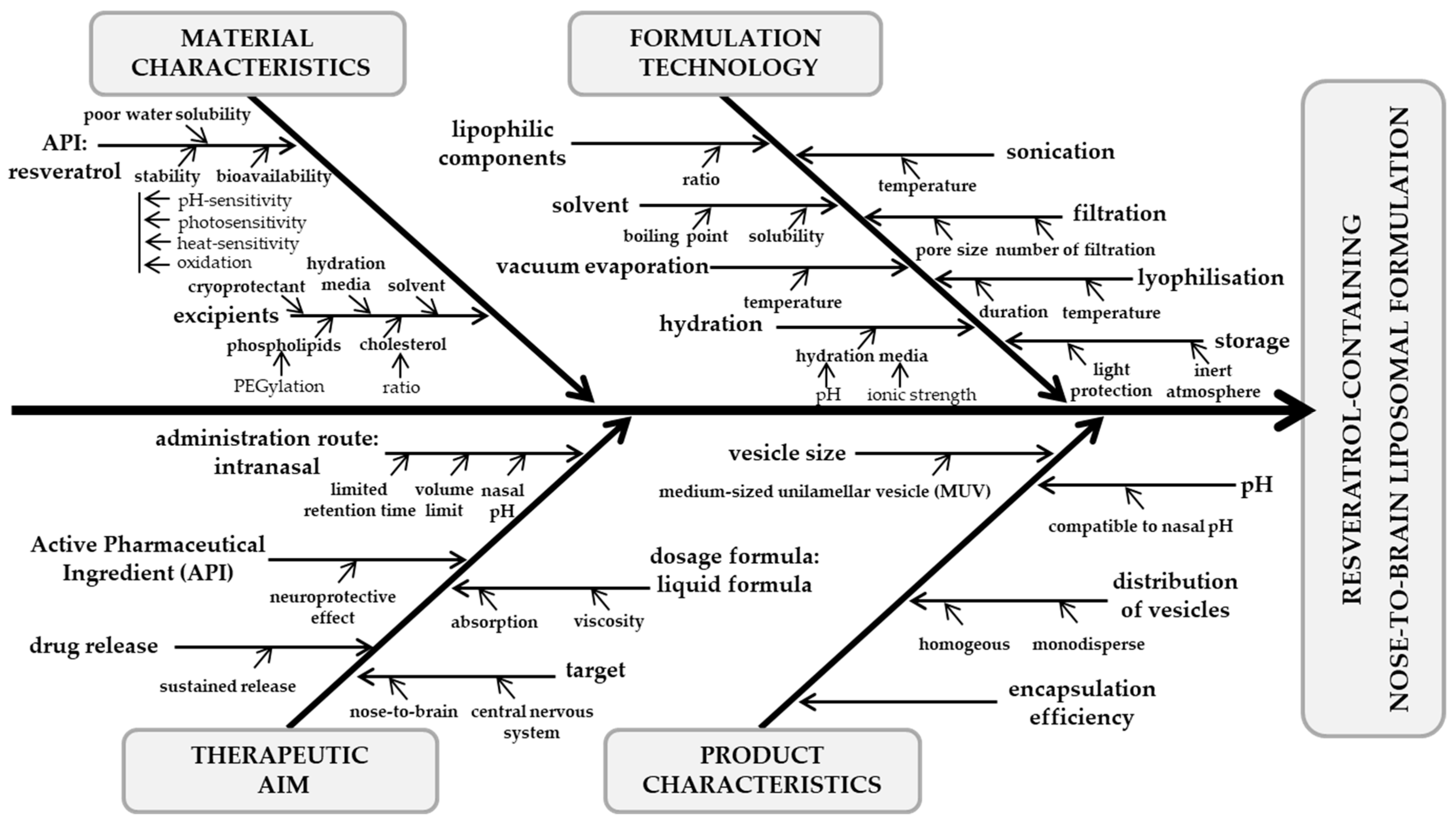
3.1. Ishikawa Diagram
3.2. Quality Target Product Profile and Critical Quality Attributes
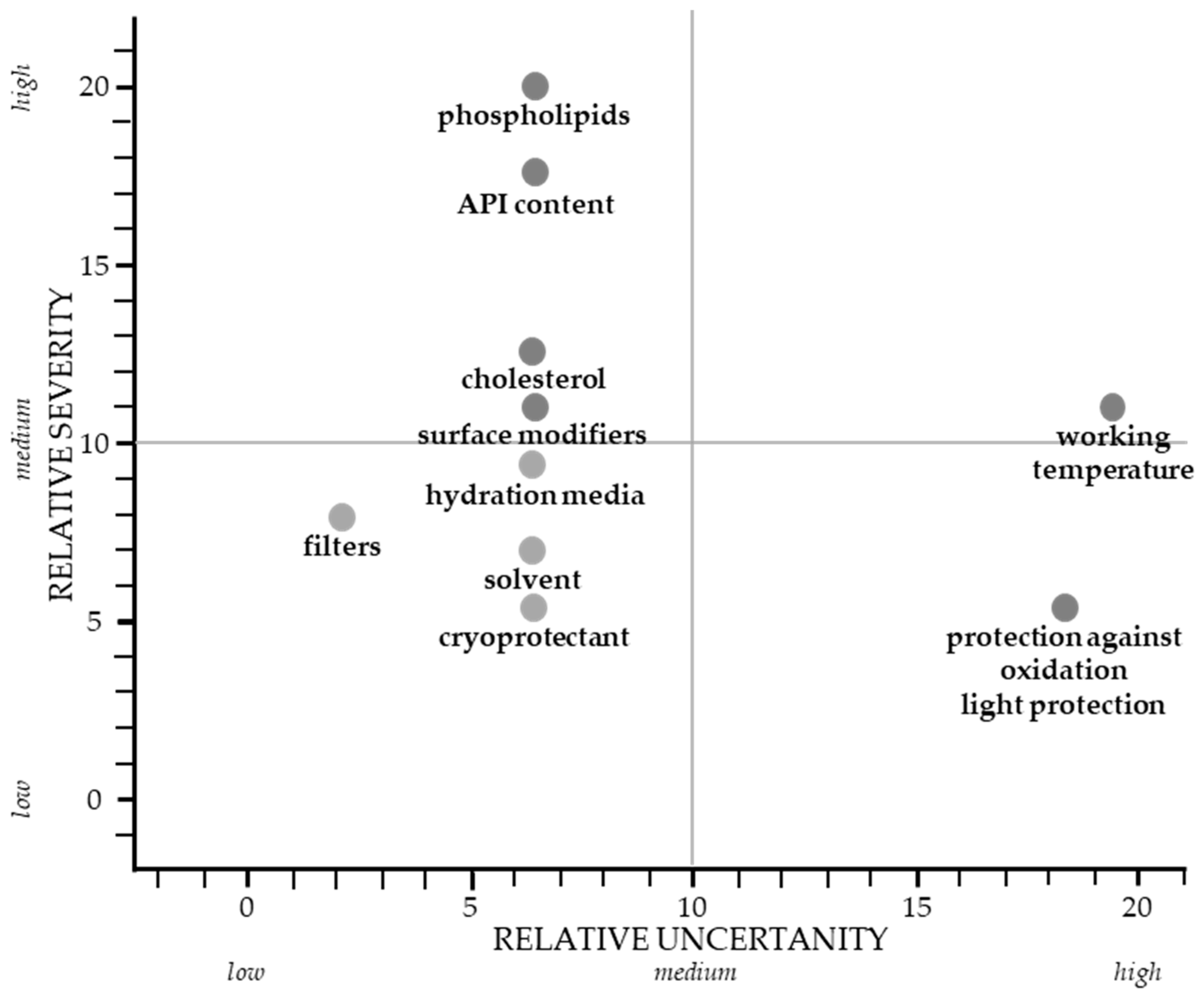
3.3. Critical Material Attributes and Process Parameters
4. Discussion
4.1. Ishikawa Diagram
4.2. Quality Target Product Profile and Critical Quality Attributes
4.3. Critical Material Attributes and Process Parameters
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| API | active pharmaceutical ingredient |
| BBB | blood-brain barrier |
| CMAs | Critical Material Attributes |
| CPPs | Critical Process Parameters |
| CQAs | Critical Quality Attributes |
| DOE | Design of Experiments |
| DS | Design Space |
| GI | gastrointestinal |
| MUV | medium-sized unilamellar vesicle |
| NF-κB | nuclear factor-kappa B |
| PEG | polyethilene glycol |
| QbD | Quality by Design |
| QTPP | Quality Target Product Profile |
| R&D | research and development |
| RA | Risk Assessment |
| ROS | reactive oxygen species |
| RSV | resveratrol |
References
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Dordević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol brain delivery for neurological disorders prevention and treatment. Front. Pharmacol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Matté, A.; Battastini, A.M.O.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Neuroprotective effects of resveratrol against Aβ administration in rats are improved by lipid-core nanocapsules. Mol. Neurobiol. 2013, 47, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Fu, Q.; Ma, R.; Xiang, J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in Parkinsonian rats. J. Neurol. Sci. 2011, 304, 29–34. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; D’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Kamble, M.S.; Bhalerao, K.K.; Bhosale, A.V.; Chaudhari, P.D. A Review on Nose-to-Brain Drug Delivery. Agrociencia 2013, 2, 516–525. [Google Scholar]
- Horvát, S.; Fehér, A.; Wolburg, H.; Sipos, P.; Veszelka, S.; Tóth, A.; Kis, L.; Kurunczi, A.; Balogh, G.; Kürti, L.; et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur. J. Pharm. Biopharm. 2009, 72, 252–259. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Csóka, I.; Pallagi, E.; Paál, T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today 2018, 7, 1340–1343. [Google Scholar] [CrossRef]
- ICH. Pharmaceutical Development Q8. ICH Harmon. Tripart. Guidel. 2009, 8, 1–28. [Google Scholar]
- Jain, S. Quality by Design (QbD): A Comprehensive Understanding of Implementation and Challenges in Pharmaceutical Development. Int. J. Pharm. Pharm. Sci. 2014, 6, 29–35. [Google Scholar]
- Pallagi, E.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. Adaptation of the quality by design concept in early pharmaceutical development of an intranasal nano-sized formulation. Int. J. Pharm. 2015, 491, 384–392. [Google Scholar] [CrossRef]
- Pallagi, E.; Karimi, K.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. New aspects of developing a dry powder inhalation formulation applying the quality-by-design approach. Int. J. Pharm. 2016, 511, 151–160. [Google Scholar] [CrossRef]
- Karimi, K.; Pallagi, E.; Szabó-Révész, P.; Csóka, I.; Ambrus, R. Development of a microparticle-based dry powder inhalation formulation of ciprofloxacin hydrochloride applying the quality by design approach. Drug Des. Dev. Ther. 2016, 10, 3331–3343. [Google Scholar] [CrossRef]
- Gieszinger, P.; Csóka, I.; Pallagi, E.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Ambrus, R. Preliminary study of nanonized lamotrigine containing products for nasal powder formulation. Drug Des. Dev. Ther. 2017, 11, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef]
- Pallagi, E.; Ismail, R.; Paál, T.L.; Csóka, I. Initial Risk Assessment as part of the Quality by Design in peptide drug containing formulation development. Eur. J. Pharm. Sci. 2018, 122, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation; D’Souza, G., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1522, pp. 17–22. [Google Scholar] [CrossRef]
- Pramod, K.; Tahir Ma Charoo, N.; Ansari, S.; Ali, J. Pharmaceutical product development: A quality by design approach. Int. J. Pharm. Investig. 2016, 6, 129. [Google Scholar] [CrossRef]

| QTPP | Details | CQAs | Details |
|---|---|---|---|
| indication | neuroprotection | morphology of liposomes | spherical conventional vesicles |
| target patient population | ageing population | size of vesicles | medium-sized unilamellar vesicles (MUV) |
| route of administration | ‘nose-to-brain’ | surface modification | polyethylene glycol (PEG) chains |
| dosage form | aqueous solution; lyophilised plaques | polydispersity | monodisperse formulation |
| drug target | dopaminergic neurons; β-amyloid plaques | zeta potential | highly charged |
| drug release | sustained release | phase transition temperature | formulation-suitable, trans-resveratrol decreases the value |
| viscosity | enough but not too viscous | API content | trans-resveratrol-containing vesicles |
| osmolarity | tolerable | position of the API | in the lipophilic double membrane |
| pH | suitable for intranasal administration and trans-resveratrol stability | encapsulation efficiency | high value |
| stability | stable formulation | ||
| homogeneity | homogenous formulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, Z.; Pallagi, E.; Dobó, D.G.; Csóka, I. How Could QbD Address the R&D Challenges of ‘Nose-To-Brain’ Liposomal Resveratrol Formulations? Proceedings 2021, 78, 49. https://doi.org/10.3390/IECP2020-08661
Németh Z, Pallagi E, Dobó DG, Csóka I. How Could QbD Address the R&D Challenges of ‘Nose-To-Brain’ Liposomal Resveratrol Formulations? Proceedings. 2021; 78(1):49. https://doi.org/10.3390/IECP2020-08661
Chicago/Turabian StyleNémeth, Zsófia, Edina Pallagi, Dorina Gabriella Dobó, and Ildikó Csóka. 2021. "How Could QbD Address the R&D Challenges of ‘Nose-To-Brain’ Liposomal Resveratrol Formulations?" Proceedings 78, no. 1: 49. https://doi.org/10.3390/IECP2020-08661
APA StyleNémeth, Z., Pallagi, E., Dobó, D. G., & Csóka, I. (2021). How Could QbD Address the R&D Challenges of ‘Nose-To-Brain’ Liposomal Resveratrol Formulations? Proceedings, 78(1), 49. https://doi.org/10.3390/IECP2020-08661







