1. Introduction
Liposomes are spherical vesicles that have been increasingly used for biomedical and biotechnological purposes over the past years as a result of their drug delivery capacity. They consist of natural or artificial lipids that form at least one bilayer, which surrounds an aqueous solution core. Liposomes are used as drug carriers due to their numerous advantages. They are biocompatible and biodegradable; they can carry hydrophilic, lipophilic and amphiphilic molecules; and their production is simple. However, they do not exhibit specific drug delivery unless their surface is modified with the appropriate ligands [
1].
Cell-derived vesicles (CVs) are bioinspired drug carriers, as they are derived from whole cells by physical means, such as extrusion. Their most striking characteristic is their ability to retain the composition of the plasma membrane of their parental cells, leading to in vivo organotropism and targeting of specific tissues. As they have a physical origin, there are no concerns regarding their biocompatibility and biodegradability [
2]. CVs have been used as carriers for anticancer drugs in order to deliver the substance directly to the cancer cells, reinforcing their action and limiting their side effects [
3].
The purpose of this study is the investigation of the mechanisms by which liposomes and autologous CVs are internalized by three different breast cancer cell lines. Two of the selected cell lines, 4T1 (mouse) and MDA-MB-231 (human), are triple negative breast cancer cell lines, and the third one, MCF-7 (human), is negative only for the protein HER2. It is essential to understand the mechanisms of endocytosis of drug carriers in order to improve their efficacy and determine their sub-cellular fate and localization [
4].
Liposomes and CVs were produced, physicochemically characterized, and were loaded with the fluorescent dye FITC-dextran. The uptake of the particles was studied using inhibitors of the clathrin-dependent and caveolin-dependent endocytic pathways, chlorpromazine and filipin, respectively. The cells were also incubated at 4 °C to inactivate the active processes and examine whether the particles are internalized passively. The results of the uptake experiments show that the endocytosis of the autologous CVs is an active process, mainly via the caveolin pathway. Liposomes are internalized actively by both pathways but also passively.
2. Experiments
2.1. Cell Lines and Culture Conditions
4T1, MDA-MB-231 and MCF-7 cell lines were routinely grown in RPMI 1640 Medium (Gibco, ThermoFischer Scientific, Greece), supplemented with 10% fetal bovine serum and 1% antibiotic penicillin/streptomycin at 37 °C in 5% CO2. When confluent, cells were washed with PBS and treated with Trypsin 0.25% (Biowest) for seeding in new culture flasks.
2.2. Liposome Preparation
1,2-distearoyl-sn-glycerol-3-phosphatidylcholine (PC), 1,2-distearoyl-sn-glycero-3- phospho-(1
’-rac-glycerol) (sodium salt) (PG) and cholesterol (CHOL), in a proportion of PC/PG/CHOL 9:1:5 (molar) formed liposomes by the thin film hydration method in 10% PBS. Next, samples were probe sonicated (Sonics Vibra Cell, Sonics & Materials, Leics, UK), centrifuged, and annealed at 40 °C in a water bath for 1 h. Lipid concentration was measured by Stewart assay [
5].
2.3. Cellular Vesicles Isolation
For CV isolation, cells were cultured until full confluency, detached with trypsin, centrifuged, and washed ×3 with PBS and were resuspended in water. CVs were obtained by ultracentrifugation (Thermo Sorvall WX90 Ultra, Thermo Scientific, Waltham, MA, USA) for 2 h at 60,000 rpm and 4 °C, and were resuspended in 10% PBS. Finally, CVs were extruded through polycarbonate membranes of 400 and 200 nm pore size (Whatman, GE Healthcare Life Sciences, Life Science Chemilab, Greece). Protein concentration was measured by Bradford assay (Biorad, Hercules, CA, USA), and lipid concentration by the Stewart method [
5].
2.4. FITC Loading
FITC-dextran 36 mM was mixed in the liposome or CV dispersion. The mixture was freeze-dried; rehydrated; and, after ultracentrifugation for 2 h at 60.000 rpm and 4 °C, the pellet was resuspended in PBS. Finally, the samples were extruded through polycarbonate membranes of 400 and 200 nm pore size.
2.5. Physicochemical Characterization
The particle size distribution (mean hydrodynamic diameter and polydispersity index) of liposomes and CVs in PBS, pH 7.4 (10 mM), was measured by dynamic light scattering (DLS) (Malvern Nano-Zs, Malvern Instruments, Malvern, UK) at 25 °C. ζ-potential was measured with the same dispersions and conditions.
2.6. Uptake Experiments
Cells (1 × 105 cells/mL) were plated in 6-well-plates. The next day, 2 of the wells were used as a control, 2 of them were pre-incubated for 30 min with chlorpromazine 10 μg/mL, and 2 of them were pre-incubated for 30 min with filipin 5 μg/mL. Another 6-well plate was pre-incubated at 4 °C for 30 min. After this pre-incubation time, an aliquot of the FITC-dextran loaded samples was added to each well, and the plates were incubated for 2 h either in the presence of the liposomes or the CVs at 37 °C or at 4 °C, respectively. Then, wells were washed three times with PBS and the cells were lysed with Triton 20% and collected with a cell scraper. FITC uptake was measured by spectrofluorophotometry (Shimadzu RF-5301 PC, Tokyo, Japan), with excitation at 490 nm and emission at 520 nm, and protein concentration was measured by Bradford assay (Biorad, Hercules, CA, USA).
3. Results
3.1. Physicochemical Characterization
The liposomes and CVs were characterized as described above using DLS.
Table 1 shows the results of the size distribution, polydispersity index (PDI), and ζ-potential. The protein to lipid ratio of the CVs is also reported.
3.2. Uptake Experiments
3.2.1. Uptake of Liposomes
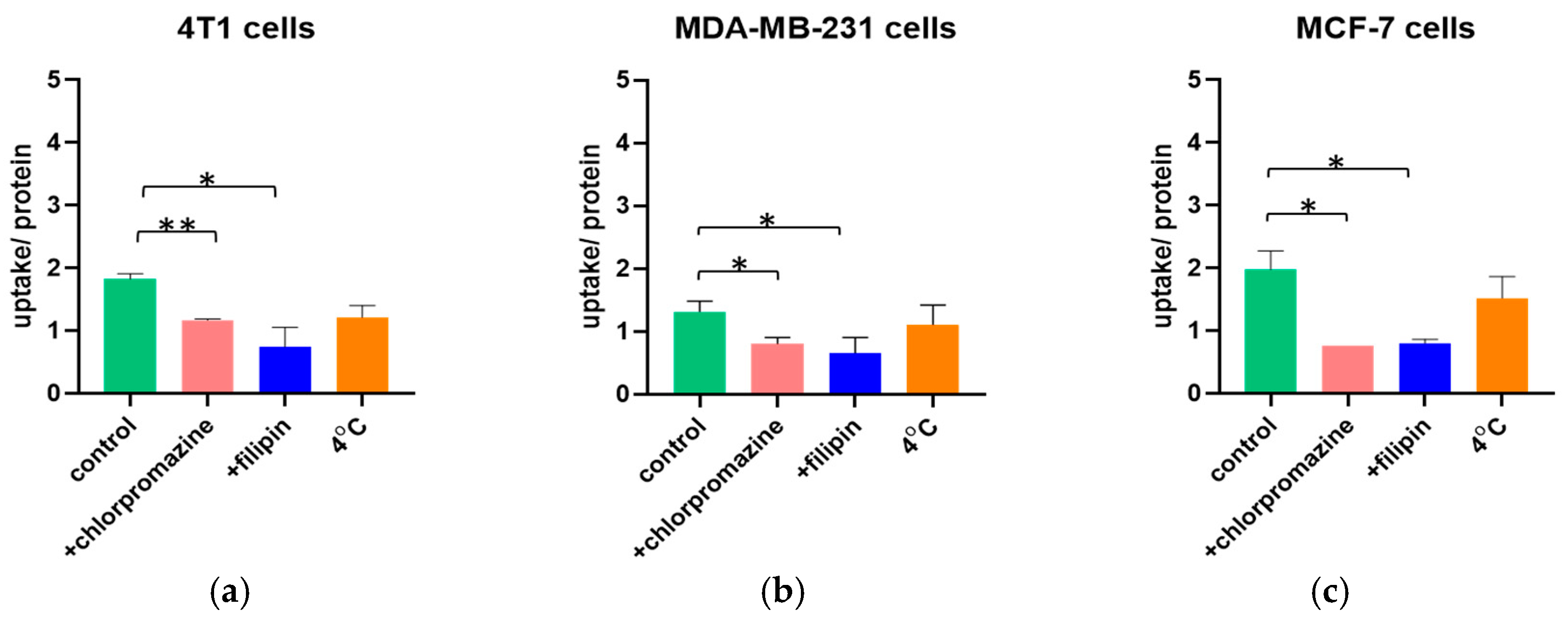
The mechanism of endocytosis of negatively charged liposomes in the breast cancer cell lines was investigated by incubating the cells either at 37 °C in the presence of the transport inhibitors chlorpromazine and filipin, or at 4 °C.
Figure 1 describes the uptake normalized to the cell protein concentration in the three different breast cancer cell lines. When the cells were treated with the inhibitors of the clathrin-dependent or caveolin-dependent endocytic pathways, the uptake per protein was significantly lower than that of the control. Therefore, liposomes were internalized actively by both pathways. However, when active processes were inactivated (at 4 °C), the uptake was not significantly hampered, indicating that passive internalization also occurs.
3.2.2. Uptake of CVs
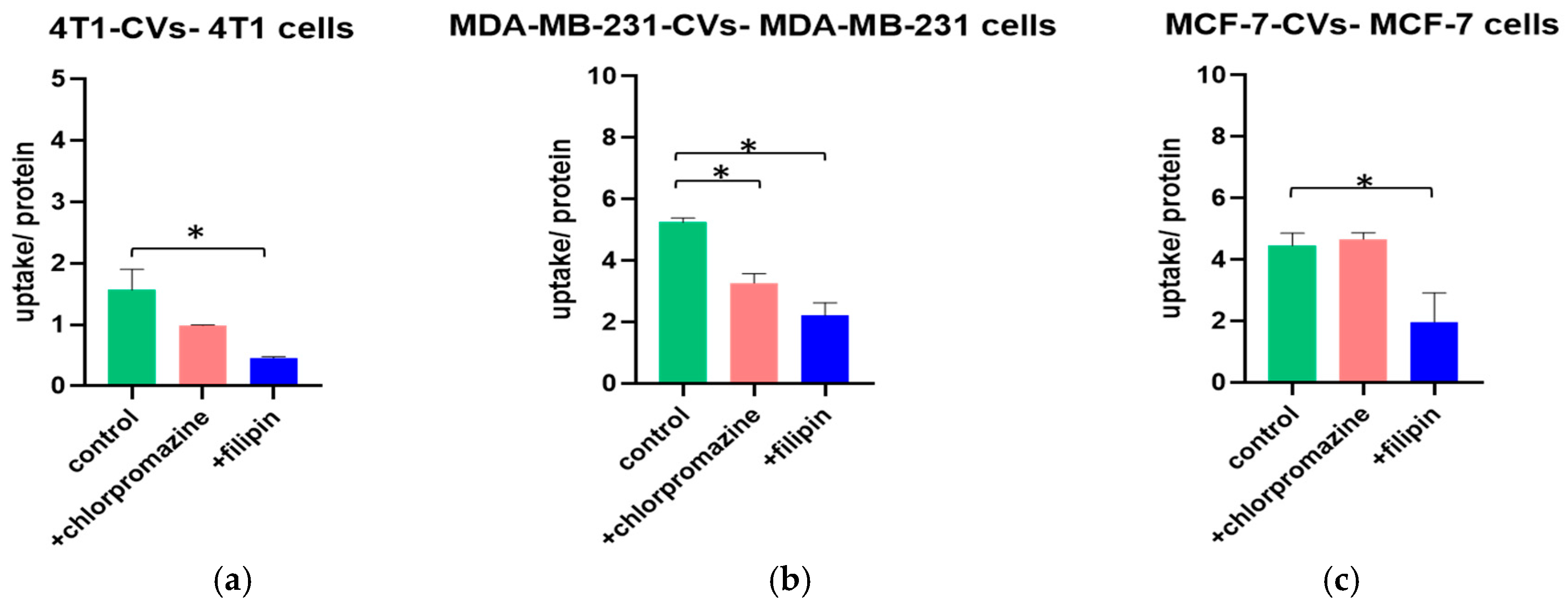
The uptake of CVs isolated from the three breast cancer cell lines mentioned above was studied under the same conditions as the liposomes.
Figure 2 shows the effect of the transport inhibitors chlorpromazine and filipin on the uptake normalized to the cell protein concentration of the autologous CVs.
The uptake per protein of 4T1-CVs was significantly lower in the presence of filipin, suggesting that the endocytosis is realized via the caveolin-dependent pathway. In the presence of chlorpromazine, the uptake was lower than that of the control, although the reduction was not statistically significant. When the MDA-MB-231 cells were treated with the transport inhibitors, the uptake of MDA-MB-231-CVs was significantly hampered. Consequently, MDA-MB-231-CVs are internalized by both the clathrin-dependent and caveolin-dependent pathways. Finally, the uptake of MCF-7-CVs was significantly lower only in the presence of filipin, indicating internalization by the caveolin-dependent pathway. In the presence of chlorpromazine, the uptake was similar to that of the control. The uptake of CVs at 4 °C is currently under evaluation.
4. Discussion
Liposomes are used widely as drug carriers, and CVs are promising carriers that can target specific organs or cells. In order to use them more effectively, it is necessary to understand the distinct endocytic mechanisms by which they enter the target cells. This first step can lead to the optimization of specific drug delivery and determine their ultimate sub-cellular fate and localization. In this study, the endocytic mechanisms of negatively charged liposomes and autologous CVs produced by three different breast cancer cell lines were investigated.
The uptake of liposomes in all selected cell lines was significantly hampered in the presence of both chlorpromazine and filipin, which means that their endocytosis is realized via the clathrin-dependent and caveolin-dependent pathways. However, at 4 °C, their uptake was not affected significantly compared to the control, suggesting that a passive endocytic mechanism is also present.
The uptake of 4T1-CVs and MCF-7-CVs seemed to be affected only in the presence of filipin, assuming that their internalization is active via the caveolin-dependent pathway. MDA-MB-231-CVs are also internalized actively, but their uptake was significantly lower in the presence of both transport inhibitors.
5. Conclusions
This research gives insight into the endocytic mechanisms used by liposomes and autologous CVs to enter 4T1, MDA-MB-231, and MCF-7 cells. It can be concluded that liposomes are internalized both actively, by the clathrin-dependent and caveolin-dependent pathways, and passively. CVs are internalized mainly actively by the caveolin-dependent pathway.
Author Contributions
S.G.A. and A.M. conceived and designed the experiments; P.S. and A.M. performed the experiments; P.S. and A.M. analyzed the data; P.S., A.M. and S.G.A. wrote/edited the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the results section of the article.
Acknowledgments
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: MIS 5031802).
![Proceedings 78 00048 i001]()
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CVs | Cellular vesicles |
| PC | 1,2-distearoyl-sn-glycerol-3-phosphatidylcholine |
| PG | 1,2-distearoyl-sn-glycero-3-phospho-(1’-rac-glycerol) |
| CHOL | Cholesterol |
| DLS | Dynamic light scattering |
| PDI | Polydispersity index |
References
- Antimisiaris, S.G.; Kallinteri, P.; Fatouros, D.G. Liposomes and Drug Delivery. Pharm. Sci. Encycl. Drug Discov. Dev. Manuf. 2010, 1–91. [Google Scholar] [CrossRef]
- Goh, W.J.; Zou, S.; Ong, W.Y.; Torta, F.; Alexandra, A.F.; Schiffelers, R.M.; Storm, G.; Wang, J.W.; Czarny, B.; Pastorin, G. Bioinspired Cell-Derived Nanovesicles versus Exosomes as Drug Delivery Systems: A Cost-Effective Alternative. Sci. Rep. 2017, 7, 14322. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.J.; Lee, C.K.; Zou, S.; Woon, E.C.; Czarny, B.; Pastorin, G. Doxorubicin-loaded cell-derived nanovesicles: An alternative targeted approach for anti-tumor therapy. Int. J. Nanomed. 2017, 12, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K. Effects of Transport Inhibitors on the Cellular Uptake of Carboxylated Polystyrene Nanoparticles in Different Cell Lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).