Thermosensitive Nasal In Situ Gels of Lipid-Based Nanosystems to Improve the Treatment of Alzheimer’s Disease †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of RVG-Loaded NLC and RVG-Loaded Nanoemulsions
2.3. Screening of Excipients
2.4. Preparation of Thermosensitive In Situ Gels
2.5. Particle Size, Polydispersity Index (PDI), and Zeta Potential (ZP)
2.6. Organoleptic Analysis
2.7. pH and Osmolarity
2.8. Rheological and Texture Analysis
3. Results
3.1. Screening of Excipients
3.2. Particle Size, PDI and ZP
3.3. Organoleptic Analysis
3.4. pH and Osmolarity Measurements
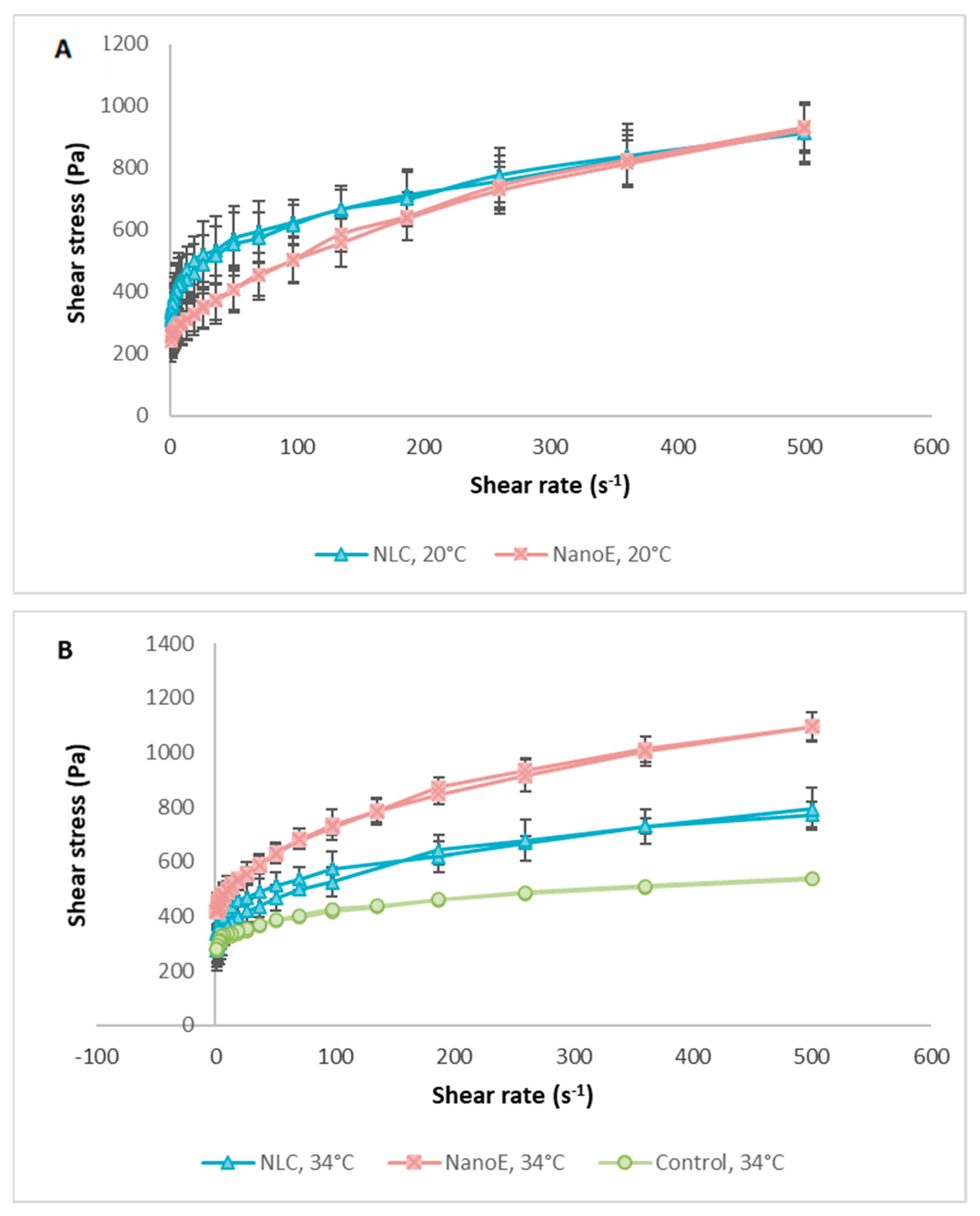
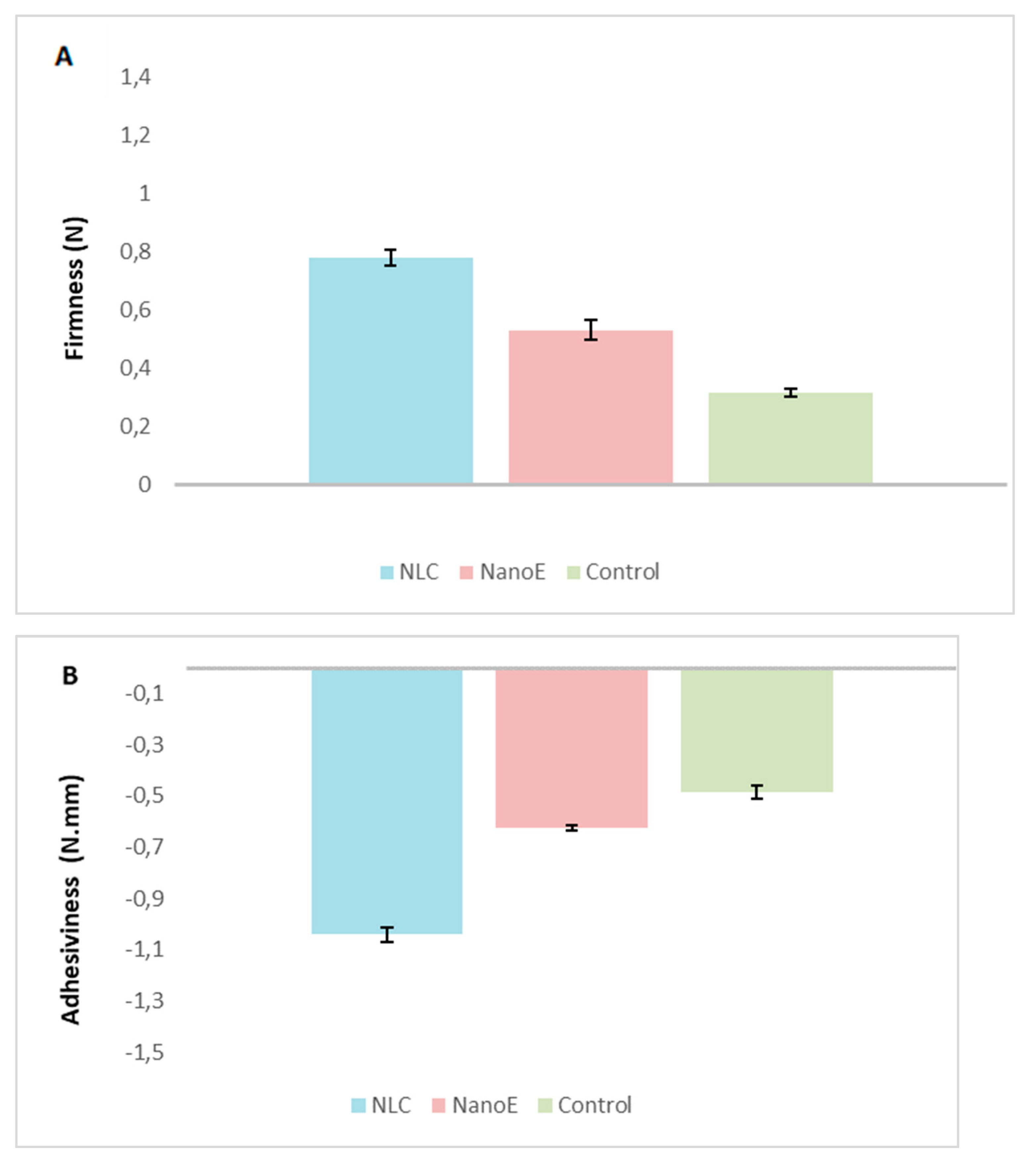
3.5. Rheological and Texture Analysis
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| CNS | Central nervous system |
| BBB | Blood-brain barrier |
| NLC | Nanostructured lipid carriers |
| NanoE | Nanoemulsions |
| RVG | Anticholinesterase drug |
| PDI | Polydispersity index |
| ZP | Zeta potential |
References
- Kales, H.C.; Lyketsos, C.G.; Miller, E.M.; Ballard, C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. Int. Psychogeriatr. 2019, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, M.K.; Shrivastava, S.K. Cholinesterase as a Target for Drug Development in Alzheimer’s Disease. In Targeting Enzymes for Pharmaceutical Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 257–286. [Google Scholar]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Amaral, M.; Lobo, J.S.; Silva, A.C. Lipid nanoparticles for nasal/intranasal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Almeida, H.; Amaral, M.; Lobo, J.S.; Silva, A. Intranasal lipid nanoparticles for the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2017, 23, 6553–6562. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Moreira, J.; Amaral, M.; Lobo, J.S.; Silva, A. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.S.H.; Abu-Thabit, N.Y. Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sabale, A.S.; Kulkarni, A.D.; Sabale, A.S. Nasal In Situ Gel: Novel Approach for Nasal Drug Delivery. J. Drug Deliv. Ther. 2020, 10, 183–197. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Kokare, C.R. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: Optimization and in vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Sousa Lobo, J.M.; Silva, A.C. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Agomelatine-based in situ gels for brain targeting via the nasal route: Statistical optimization, in vitro, and in vivo evaluation. Drug Deliv. 2017, 24, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Helena Amaral, M.; Lobão, P.; Manuel Sousa Lobo, J. Influence of drug incorporation, temperature and storage time on the pH, textural and rheological properties of different poloxamer hydrogels. Curr. Drug Deliv. 2013, 10, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Peixoto, D.; Teixeira, I.; Rodrigues, M.; Alves, G.; Santos, A.O. Nanoemulsions and thermosensitive nanoemulgels of phenytoin and fosphenytoin for intranasal administration: Formulation development and in vitro characterization. Eur. J. Pharm. Sci. 2020, 141, 105099. [Google Scholar] [CrossRef] [PubMed]
- Scherließ, R. Nasal formulations for drug administration and characterization of nasal preparations in drug delivery. Ther. Deliv. 2020, 11, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, H.; Cheng, S.; Pozzoli, M.; Messerotti, E.; Traini, D.; Young, P.; Kourmatzis, A.; Ong, H.X. Smart thermosensitive chitosan hydrogel for nasal delivery of ibuprofen to treat neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Adnet, T.; Groo, A.-C.; Picard, C.; Davis, A.; Corvaisier, S.; Since, M.; Bounoure, F.; Rochais, C.; Pluart, L.L.; Dallemagne, P. Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer’s Disease Treatment. Pharmaceutics 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.E.; Elmowafy, E.; Casettari, L.; Alexander, C. Star-shaped poly (oligoethylene glycol) copolymer-based gels: Thermo-responsive behaviour and bioapplicability for risedronate intranasal delivery. Int. J. Pharm. 2018, 543, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Bandyopadhyay, A.K. Development and characterization of mucoadhesive in situ nasal gel of midazolam prepared with Ficus carica mucilage. AAPS Pharmscitech 2010, 11, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
| Size (nm) | PDI | ZP (mV) | |
|---|---|---|---|
| NLC 1 | 114.00 ± 1.91 | 0.22 ± 0.00 | −30.63 ± 0.29 |
| NanoE 2 | 135.80 ± 0.50 | 0.14 ± 0.00 | −20.87 ± 0.21 |
| In situ NLC gel 3 | 141.70 ± 0.40 | 0.45 ± 0.00 | −4.06 ± 1.03 |
| In situ NanoE gel 4 | 146.10 ± 1.73 | 0.43 ± 0.02 | −4.09 ± 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, S.; Forbes, B.; Lobo, J.M.S.; Silva, A.C. Thermosensitive Nasal In Situ Gels of Lipid-Based Nanosystems to Improve the Treatment of Alzheimer’s Disease. Proceedings 2021, 78, 37. https://doi.org/10.3390/IECP2020-08648
Cunha S, Forbes B, Lobo JMS, Silva AC. Thermosensitive Nasal In Situ Gels of Lipid-Based Nanosystems to Improve the Treatment of Alzheimer’s Disease. Proceedings. 2021; 78(1):37. https://doi.org/10.3390/IECP2020-08648
Chicago/Turabian StyleCunha, Sara, Ben Forbes, José Manuel Sousa Lobo, and Ana Catarina Silva. 2021. "Thermosensitive Nasal In Situ Gels of Lipid-Based Nanosystems to Improve the Treatment of Alzheimer’s Disease" Proceedings 78, no. 1: 37. https://doi.org/10.3390/IECP2020-08648
APA StyleCunha, S., Forbes, B., Lobo, J. M. S., & Silva, A. C. (2021). Thermosensitive Nasal In Situ Gels of Lipid-Based Nanosystems to Improve the Treatment of Alzheimer’s Disease. Proceedings, 78(1), 37. https://doi.org/10.3390/IECP2020-08648







