Enhancement of the Solubility of Rosuvastatin Calcium by Nanovesicular Formulation: A Systematic Study Based on a Quality by Design Approach †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Nanovesicular Carrier
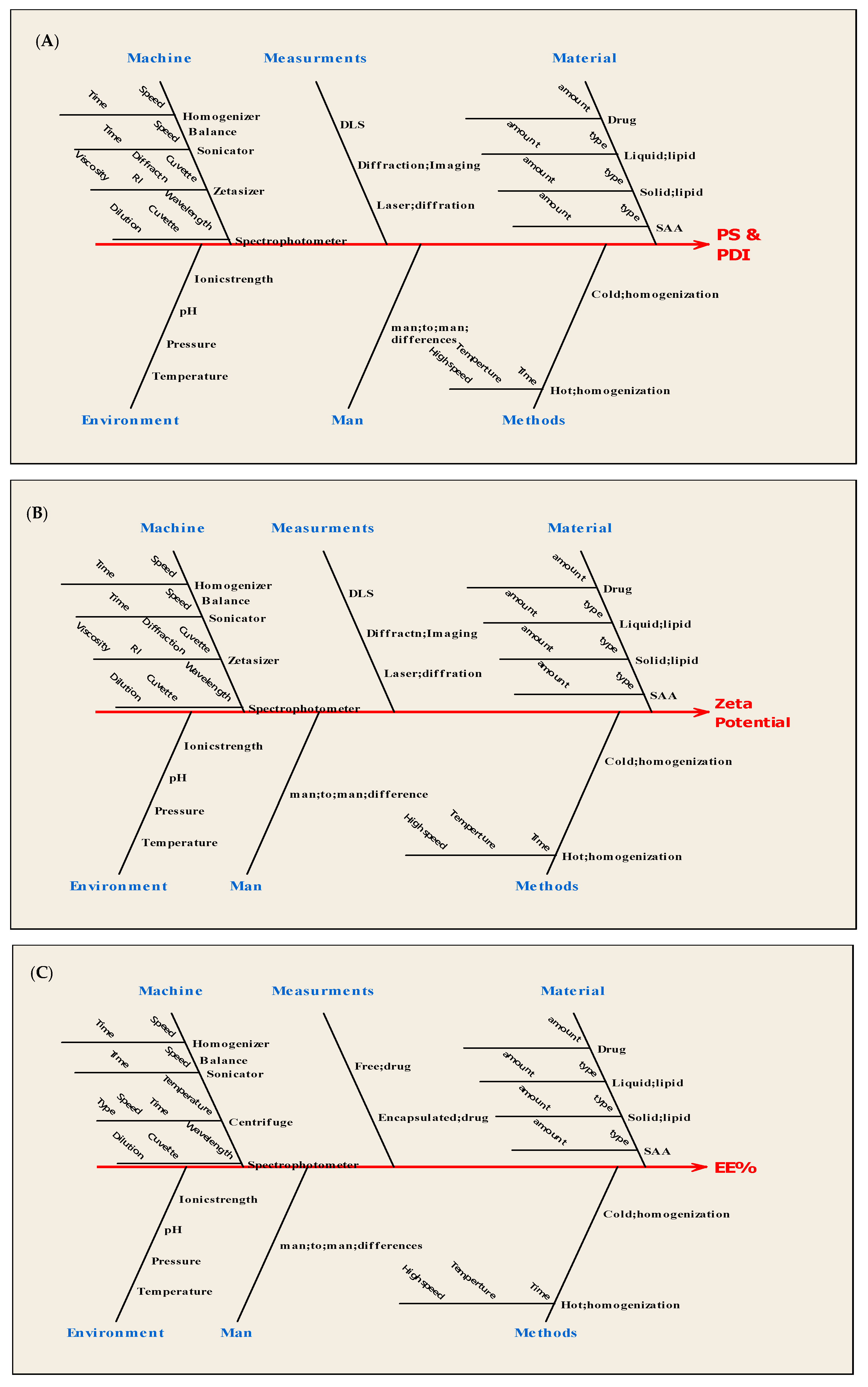
2.2.2. Characterization of Nanoparticles
2.2.3. Quality by Design Paradigm
2.2.4. In-Vitro Drug Release
2.2.5. In-Vivo Pharmacodynamics Study
3. Results and Discussion
3.1. Quality Target Product Profile and Risk Analysis
3.2. Screening of Different Solid Lipids, Liquid Lipids, and Surfactants (SAA) for Nanovesicular Formulation
3.3. Response Surface Design Analysis
3.3.1. Particle Size Analysis
3.3.2. PDI Analysis
3.3.3. Zeta Potential Analysis
3.3.4. EE% Analysis
3.4. Model Validation, Data Optimization, and Control Strategy Establishment
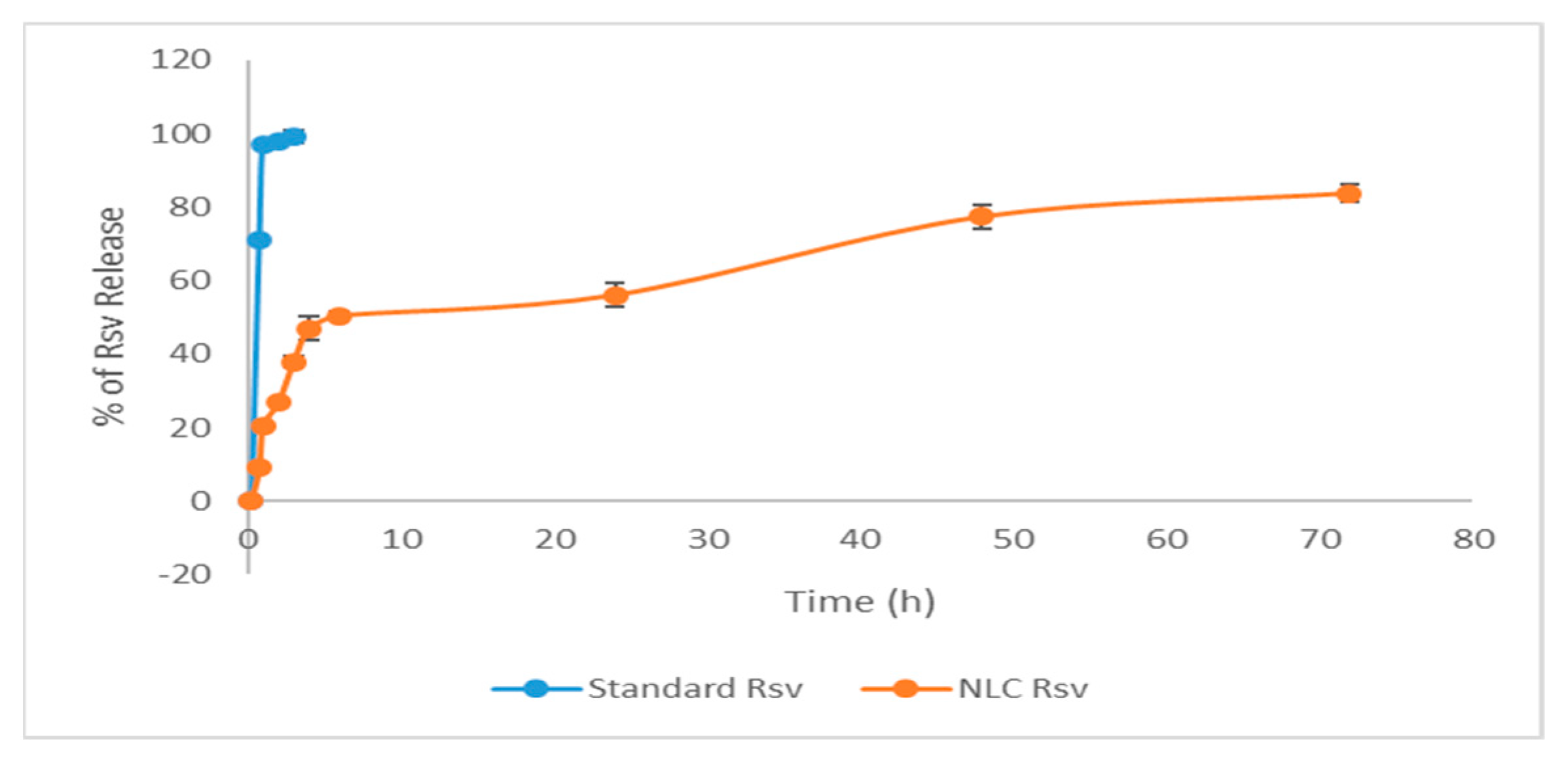
3.5. In-Vitro Drug Release Analysis
3.6. In-Vivo Pharmacodynamics Study
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Khan, M.A.; Burgess, D.J. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. Int. J. Pharm. 2011, 419, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Ranpise, N.; Mohan, G. Development and Evaluation of Solid Self Nano-Emulsifying Formulation of Rosuvastatin Calcium for Improved Bioavailability. Trop. J. Pharm. Res. 2015, 14, 575. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; A comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Patil-Gadhe, A.; Pokharkar, V. Pulmonary targeting potential of rosuvastatin loaded nanostructured lipid carrier: Optimization by factorial design. Int. J. Pharm. 2016, 501, 199–210. [Google Scholar] [CrossRef]
- Xu, X.; Costa, A.P.; Khan, M.A.; Burgess, D.J. Application of quality by design to formulation and processing of protein liposomes. Int. J. Pharm. 2012, 434, 349–359. [Google Scholar] [CrossRef]
- Amasya, G.; Badilli, U.; Aksu, B.; Tarimci, N. Quality by design case study 1: Design of 5-fluorouracil loaded lipid nanoparticles by the W/O/W double emulsion—Solvent evaporation method. Eur. J. Pharm. Sci. 2016, 84, 92–102. [Google Scholar] [CrossRef]
- Basalious, E.B.; El-Sebaie, W.; El-Gazayerly, O. Application of Pharmaceutical QbD for Enhancement of the Solubility and Dissolution of a Class II BCS Drug using Polymeric Surfactants and Crystallization Inhibitors: Development of Controlled-Release Tablets. AAPS PharmSciTech 2011, 12, 799–810. [Google Scholar] [CrossRef]
- Singh, H.; Gupta, R.D.; Gautam, G. Formulation Development, Characterization, and in Vitro-in Vivo Study of Antihyperlipidemic Drug Rosuvastatin Calcium—Solid Lipid Nanoparticles. Asian J. Pharm. Clin. Res. 2018, 11, 436. [Google Scholar] [CrossRef]
- Patil-Gadhe, A.; Pokharkar, V. Montelukast-loaded nanostructured lipid carriers: Part i Oral bioavailability improvement. Eur. J. Pharm. Biopharm. 2014, 88, 160–168. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Galal, O.; Mohammed, A.R.; El-Abhar, H.S. Tropisetron modulates peripheral and central serotonin/insulin levels via insulin and nuclear factor kappa B/receptor for advanced glycation end products signalling to regulate type-2 diabetes in rats. RSC Adv. 2018, 8, 11908–11920. [Google Scholar] [CrossRef]
- Schaalan, M.; El-Abhar, H.S.; Barakat, M.; El-Denshary, E.S. Westernized-like-diet-fed rats: Effect on glucose homeostasis, lipid profile, and adipocyte hormones and their modulation by rosiglitazone and glimepiride. J. Diabetes Complic. 2009, 23, 199–208. [Google Scholar] [CrossRef]
- Sarfraz, R.M.; Ahmad, M.; Mahmood, A.; Minhas, M.U.; Yaqoob, A. Development and Evaluation of Rosuvastatin Calcium Based Microparticles for Solubility Enhancement: An In Vitro Study. Adv. Polym. Technol. 2017, 36, 433–441. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Yang, J.; Wan, H. Influence of liquid lipid content on the properties of puerarin-loaded lipid nanoparticles. J. Chin. Adv. Mater. Soc. 2014, 2, 9–19. [Google Scholar] [CrossRef]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841. [Google Scholar] [CrossRef]
- Khames, A.; Khaleel, M.A.; El-Badawy, M.F.; El-Nezhawy, A.O.H. Natamycin solid lipid nanoparticles—sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomed. 2019, 14, 2515–2531. [Google Scholar] [CrossRef]
- Wang, X. Development of antibody arrays for measuring biosimilar conformational comparability at molecular level. J. Bioequiv. Availab. 2012, 1, 24–26. [Google Scholar] [CrossRef]
- Sahu, A.K.; Kumar, T.; Jain, V. Formulation optimization of erythromycin solid lipid nanocarrier using response surface methodology. Biomed Res. Int. 2014, 2014, 689391. [Google Scholar] [CrossRef]
- Carmena, R. Type 2 diabetes, dyslipidemia, and vascular risk: Rationale and evidence for correcting the lipid imbalance. Am. Heart J. 2005, 150, 859–870. [Google Scholar] [CrossRef]
- Martin, P.D.; Warwick, M.J.; Dane, A.L.; Brindley, C.; Short, T. Absolute Oral Bioavailability of Rosuvastatin in Healthy White Adult Male Volunteers. Clin. Ther. 2003, 25, 2553–2563. [Google Scholar] [CrossRef]
- Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R.S.; Narang, J.K. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015, 5, 182–191. [Google Scholar]
| Factors | Low Level (−1) | High Level (+1) | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 Lipid (%) | 1 | 3 | ||||||
| X2 Surfactant (%) | 0.1 | 0.3 | ||||||
| X3 Solid lipid (SL): Liquid lipid (LL) | 7:3 | 10:0 | ||||||
| X4 Drug amount (mg) | 5 | 10 | ||||||
| Code | X1 | X2 | X3 | X4 | Y1 = Particle Size (nm) | Y23 = Polydispersity index | Y3 =Zeta potential(mV) | Y4 = Entrapment efficiency (%) |
| F1 | −1 | −1 | 1 | −1 | 279.2 ± 1.56 | 0.452 ± 0.05 | −14.3 ± 2.07 | 45.43 ± 5.98 |
| F2 | 1 | −1 | −1 | −1 | 232.8 ± 3.57 | 0.175 ± 0.09 | −19.2 ± 1.87 | 76.71 ± 8.83 |
| F3 | 1 | 1 | 1 | −1 | 308.9 ± 3.54 | 0.589 ± 0.13 | −14.1 ± 4.08 | 81.37 ± 3.78 |
| F4 | −1 | 1 | 1 | −1 | 400.4 ± 1.65 | 0.643 ± 0.08 | −12.9 ± 2.98 | 85.57 ± 2.98 |
| F5 | 1 | −1 | 1 | 1 | 400.3 ± 2.56 | 0.384 ± 0.04 | −14.7 ± 0.98 | 67.12 ± 4.14 |
| F6 | 1 | −1 | −1 | 1 | 300.0 ± 2.73 | 0.228 ± 0.05 | −21.4 ± 3.09 | 94.40 ± 9.06 |
| F7 | 1 | 1 | −1 | 1 | 255.0 ± 2.90 | 0.292 ± 0.09 | −16.6 ± 4.09 | 89.20 ± 3.87 |
| F8 | −1 | 1 | −1 | 1 | 256.5 ± 1.65 | 0.400 ± 0.04 | −16.3 ± 1.87 | 77.39 ± 5.76 |
| F9 | −1 | −1 | −1 | 1 | 806.1 ± 2.63 | 0.538 ± 0.06 | −18.1 ± 3.04 | 78.67 ± 4.31 |
| F10 | 1 | 1 | 1 | 1 | 313.1 ± 0.95 | 0.270 ± 0.08 | −13.5 ± 1.98 | 82.96 ± 9.31 |
| F11 | −1 | −1 | −1 | −1 | 736.2 ± 1.62 | 0.479 ± 0.02 | −11.8 ± 3.50 | 62.23 ± 5.98 |
| F12 | 1 | −1 | 1 | −1 | 245.0 ± 1.16 | 0.237 ± 0.04 | −10.3 ± 2.05 | 46.44 ± 2.74 |
| F13 | 1 | 1 | −1 | −1 | 280.8 ± 3.07 | 0.262 ± 0.05 | −12.3 ± 2.08 | 93.33 ± 4.87 |
| F14 | −1 | 1 | 1 | 1 | 237.0 ± 3.60 | 0.445 ± 0.07 | −11.1 ± 1.95 | 72.60 ± 2.09 |
| F15 | −1 | 1 | −1 | −1 | 478.5 ± 2.76 | 0.815 ± 0.04 | −11.0 ± 1.08 | 64.29 ± 5.87 |
| F16 | −1 | −1 | 1 | 1 | 262.1 ± 2.84 | 0.348 ± 0.04 | −10.9 ± 1.10 | 75.91 ± 4.21 |
| CPPs/MAs | Level in Coded Value | |
|---|---|---|
| Total lipid content (X1) SAA% (X2) SL: LL ratio (X3) Drug amount (X4) | +1 −0.623 −1 +0.992 | |
| CQA | Results | |
| Expected | Observed | |
| PS (nm) | 352.345 | 310.5 |
| PDI | 0.259 | 0.243 |
| ζ-pot (mv) | −20.803 | −24.7 |
| EE (%) | 94.663 | 93.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawoud, M.H.S.H.S.; Fayez, A.M.M.; Mohamed, R.A.A.; Sweed, N.M.M. Enhancement of the Solubility of Rosuvastatin Calcium by Nanovesicular Formulation: A Systematic Study Based on a Quality by Design Approach. Proceedings 2021, 78, 34. https://doi.org/10.3390/IECP2020-08698
Dawoud MHSHS, Fayez AMM, Mohamed RAA, Sweed NMM. Enhancement of the Solubility of Rosuvastatin Calcium by Nanovesicular Formulation: A Systematic Study Based on a Quality by Design Approach. Proceedings. 2021; 78(1):34. https://doi.org/10.3390/IECP2020-08698
Chicago/Turabian StyleDawoud, Marwa H. S. H. S., Ahmed M. M. Fayez, Reem A. A. Mohamed, and Nabila M. M. Sweed. 2021. "Enhancement of the Solubility of Rosuvastatin Calcium by Nanovesicular Formulation: A Systematic Study Based on a Quality by Design Approach" Proceedings 78, no. 1: 34. https://doi.org/10.3390/IECP2020-08698
APA StyleDawoud, M. H. S. H. S., Fayez, A. M. M., Mohamed, R. A. A., & Sweed, N. M. M. (2021). Enhancement of the Solubility of Rosuvastatin Calcium by Nanovesicular Formulation: A Systematic Study Based on a Quality by Design Approach. Proceedings, 78(1), 34. https://doi.org/10.3390/IECP2020-08698





