Effect of Gamma Sterilization on CBD-Loaded PLGA Microparticles †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Elaboration of CBD-Loaded-PLGA Microparticles
2.3. Sterilization of CBD-Loaded-PLGA-Microparticles
2.4. Characterization of CBD-Loaded-PLGA-Microparticles
2.4.1. Particle Size and Morphology
2.4.2. DSC Analysis
2.4.3. Drug Content and In Vitro Drug Release
2.4.4. Statistical Analysis
3. Results
3.1. Effect on the Physicochemical Properties of CBD-Loaded Microparticles
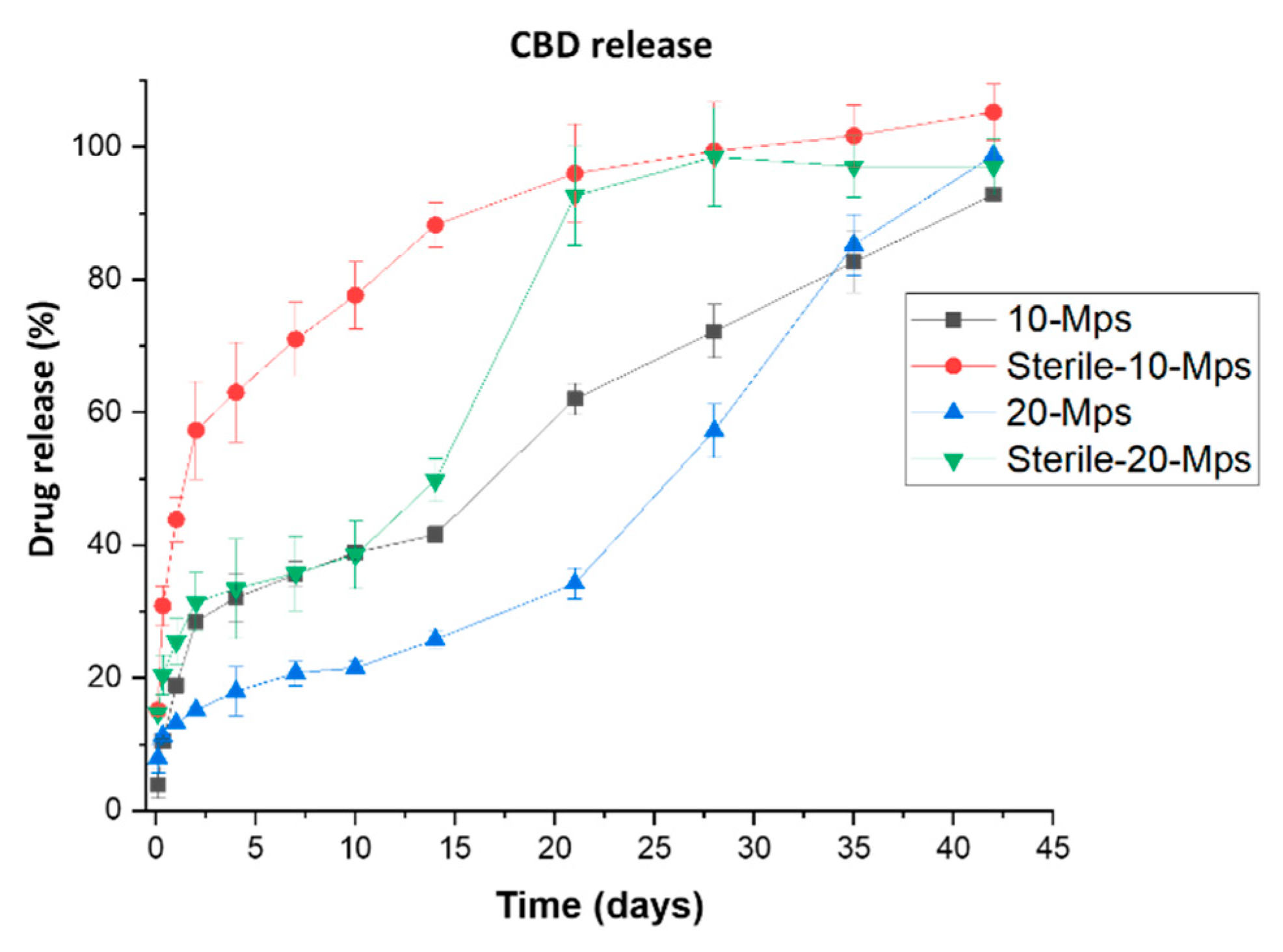
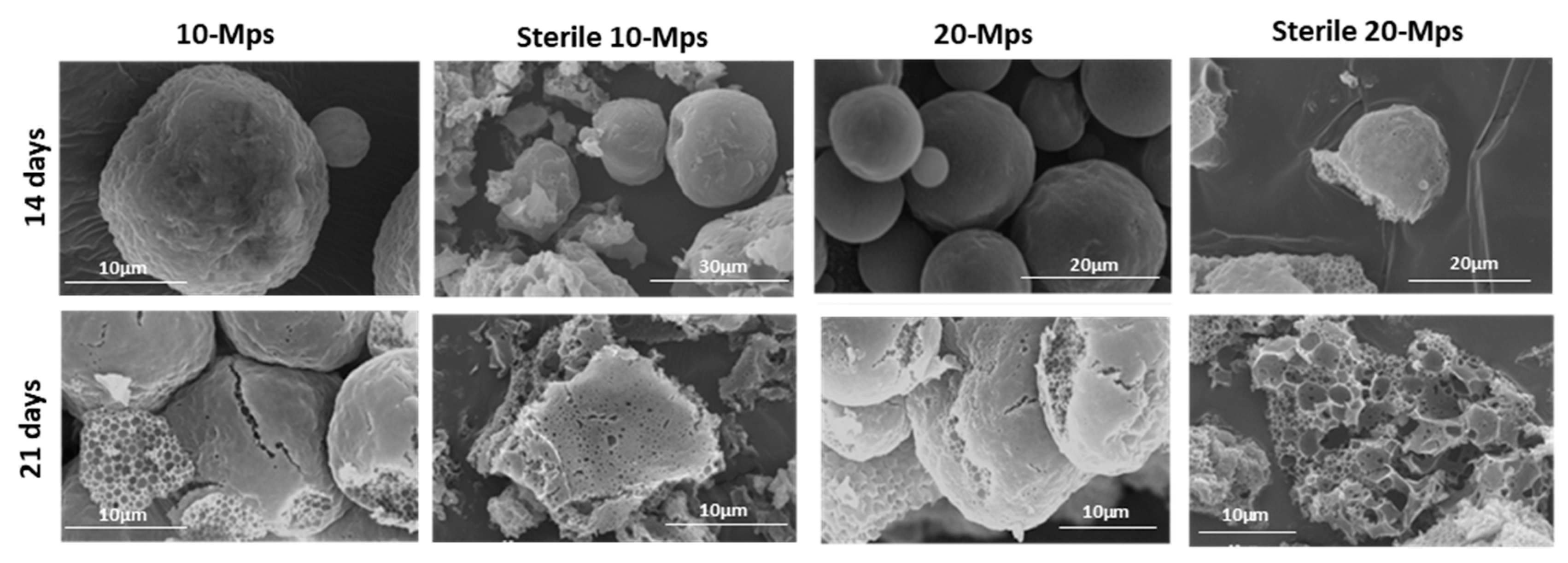
3.2. Effect on the Drug Content and Drug Release
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharm. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A.; Sofware, C.M.-S. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1150, 122188. [Google Scholar] [CrossRef] [PubMed]
- Viudez-Martínez, A.; García-Gutiérrez, M.S.; Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Manzanares, J. Effects of cannabidiol plus naltrexone on motivation and ethanol consumption. Br. J. Pharm. 2018, 175, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef] [PubMed]
- De La Ossa, D.H.P.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; García-Taboada, E.; Aberturas, M.D.R.; Molpeceres, J.; Velasco, G.; Torres-Suárez, A.I. Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS ONE 2013, 8, e54795. [Google Scholar]
- Schwendeman, S.P.; Shah, R.B.; Bailey, B.A.; Schwendeman, A.S. Injectable controlled release depots for large molecules. J. Control. Release 2014, 190, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Keles, H.; Naylor, A.; Clegg, F.; Sammon, C. Studying the release of hGH from gamma-irradiated PLGA microparticles using ATR-FTIR imaging. Vib. Spectrosc. 2014, 71, 76–84. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Delie, F.; Cohen, M.; Martin-Sabroso, C.; Mezzanzanica, D.; Figini, M.; Satta, A.; Torres-Suárez, A. Enhancing ovarian cancer conventional chemotherapy through the combination with cannabidiol loaded microparticles. Eur. J. Pharm. Biopharm. 2020, 154, 246–258. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.R.; Mesquita, P.C.; Machado, P.R.; Farias, K.J.; de Almeida, Y.M.; Fernandes-Pedrosa, M.F.; Cornelio, A.M.; Socrates, T.; da Silva-Junior, A.A. Monitoring structural features, biocompatibility and biological efficacy of gamma-irradiated methotrexate-loaded spray-dried microparticles. Mater. Sci. Eng. C 2017, 80, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Montanari, L.; Cilurzo, F.; Valvo, L.; Faucitano, A.; Buttafava, A.; Groppo, A.; Genta, I.; Conti, B. Gamma irradiation effects on stability of poly(lactide-co-glycolide) microspheres containing clonazepam. J. Control. Release 2001, 75, 317–330. [Google Scholar] [CrossRef]
- Çalış, S.; Bozdağ, S.; Kaş, H.; Tunçay, M.; Hıncal, A. Influence of irradiation sterilization on poly(lactide-co-glycolide) microspheres containing anti-inflammatory drugs. IL Farm. 2002, 57, 55–62. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Sui, H.; Liu, Y.; Park, K.; Wang, W. Comparative studies on the properties of glycyrrhetinic acid-loaded PLGA microparticles prepared by emulsion and template methods. Int. J. Pharm. 2015, 496, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, A.; D’Oca, M.; Campisi, M.; De Caro, V.; Giandalia, G.; Giannola, L.I.; Brai, M.; Calderaro, E. Effects of gamma-irradiation on trehalose-hydroxyethylcellulose microspheres loaded with vancomycin. Eur. J. Pharm. Biopharm. 2005, 59, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Malyala, P.; Pallaoro, M.; Giuliani, M.; Petersen, H.; O’Hagan, D.T.; Singh, M. A two-stage strategy for sterilization of poly(lactide-co-glycolide) particles by γ-irradiation does not impair their potency for vaccine delivery. J. Pharm. Sci. 2011, 100, 646–654. [Google Scholar] [CrossRef] [PubMed]
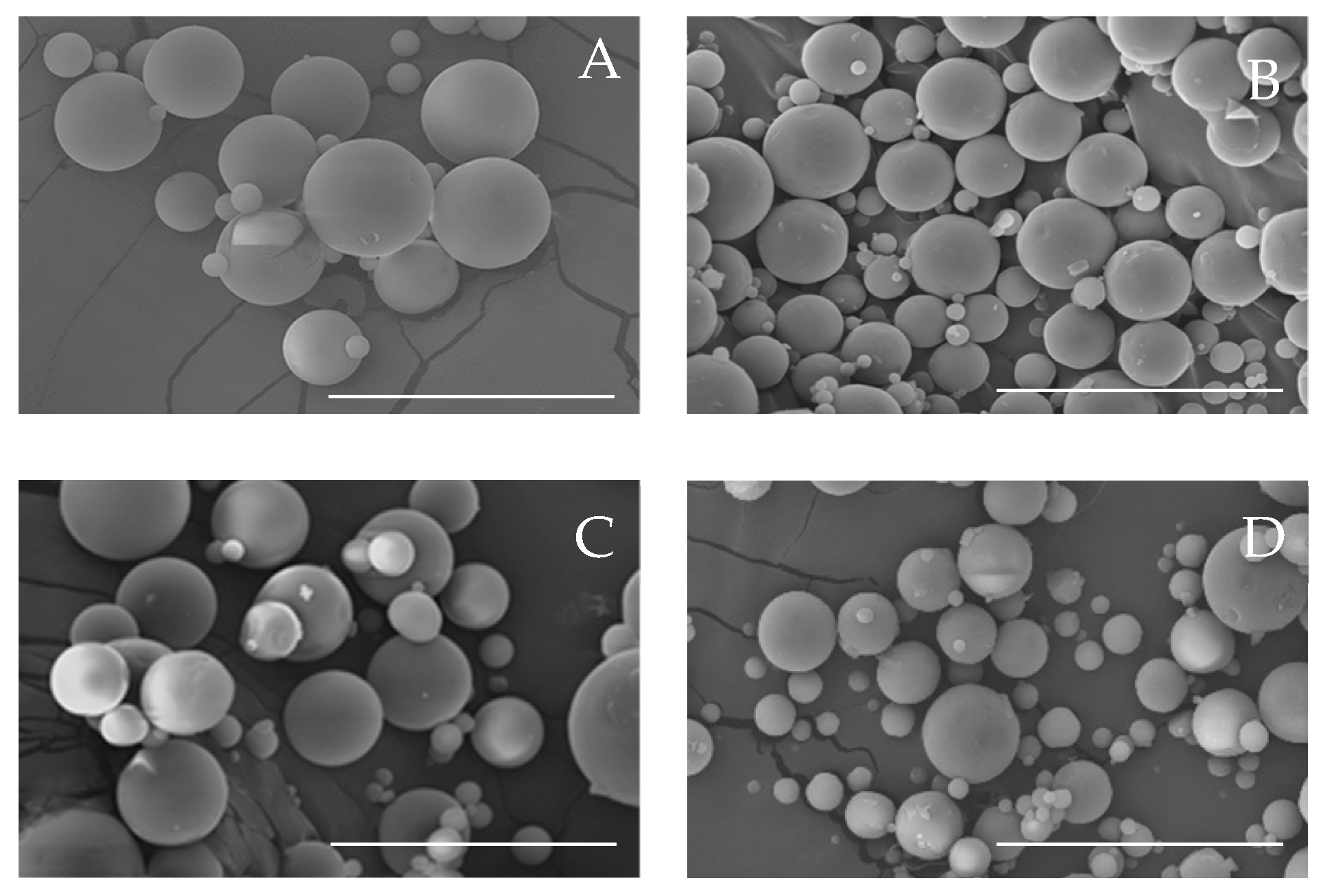
| Formulation | Process Yield (%) | Particle Size (nm) | Span | Tg (°C) | CBD Content (mg CBD/100 mg Mps) |
|---|---|---|---|---|---|
| Non-sterile 10-Mps | 89.25 ± 1.01 | 24.17 | 2.02 | 37.45 | 8.60 ± 0.42 |
| Sterile 10-Mps | 23.42 | 1.95 | 37.28 | 7.42 ± 0.13 | |
| Non-sterile 20-Mps | 81.05 ± 5.33 | 25.14 | 2.36 | 34.79 | 14.97 ± 0.20 |
| Sterile-Mps | 24.31 | 2.42 | 35.08 | 13.43 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Torres-Suárez, A.I. Effect of Gamma Sterilization on CBD-Loaded PLGA Microparticles. Proceedings 2021, 78, 31. https://doi.org/10.3390/IECP2020-08801
Fraguas-Sánchez AI, Fernández-Carballido A, Torres-Suárez AI. Effect of Gamma Sterilization on CBD-Loaded PLGA Microparticles. Proceedings. 2021; 78(1):31. https://doi.org/10.3390/IECP2020-08801
Chicago/Turabian StyleFraguas-Sánchez, Ana Isabel, Ana Fernández-Carballido, and Ana Isabel Torres-Suárez. 2021. "Effect of Gamma Sterilization on CBD-Loaded PLGA Microparticles" Proceedings 78, no. 1: 31. https://doi.org/10.3390/IECP2020-08801
APA StyleFraguas-Sánchez, A. I., Fernández-Carballido, A., & Torres-Suárez, A. I. (2021). Effect of Gamma Sterilization on CBD-Loaded PLGA Microparticles. Proceedings, 78(1), 31. https://doi.org/10.3390/IECP2020-08801





