Nanotechnological Strategies for Administration of Poorly Soluble Neuroactive Drugs †
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Nanoparticle Preparation
2.3. Nanoparticle Characterization
2.4. In Vitro URB597 Release Studies
2.5. In Vivo Behavioral Study
3. Results
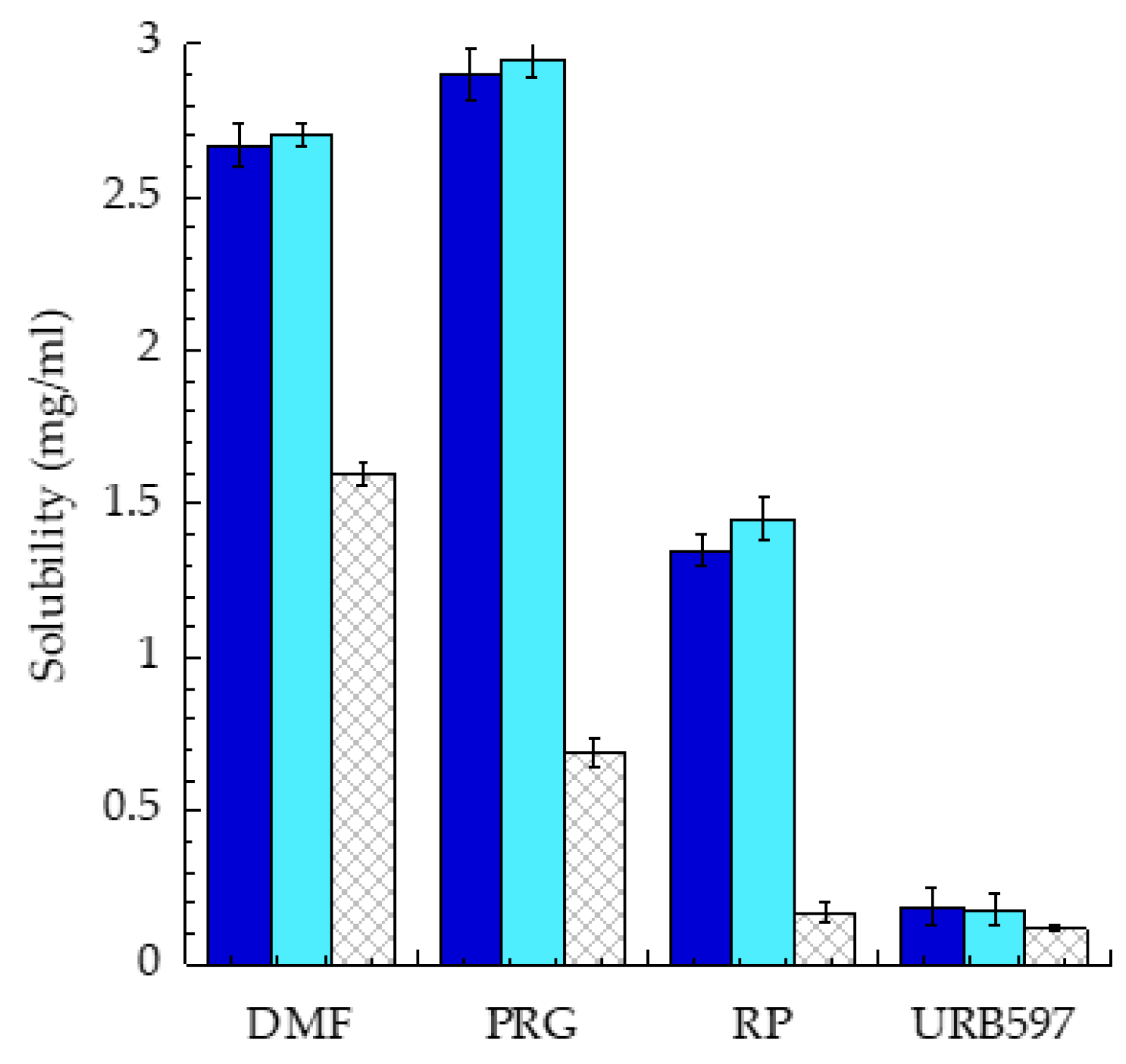
3.1. Nanoparticle Preparation
3.2. Nanoparticle Characterization
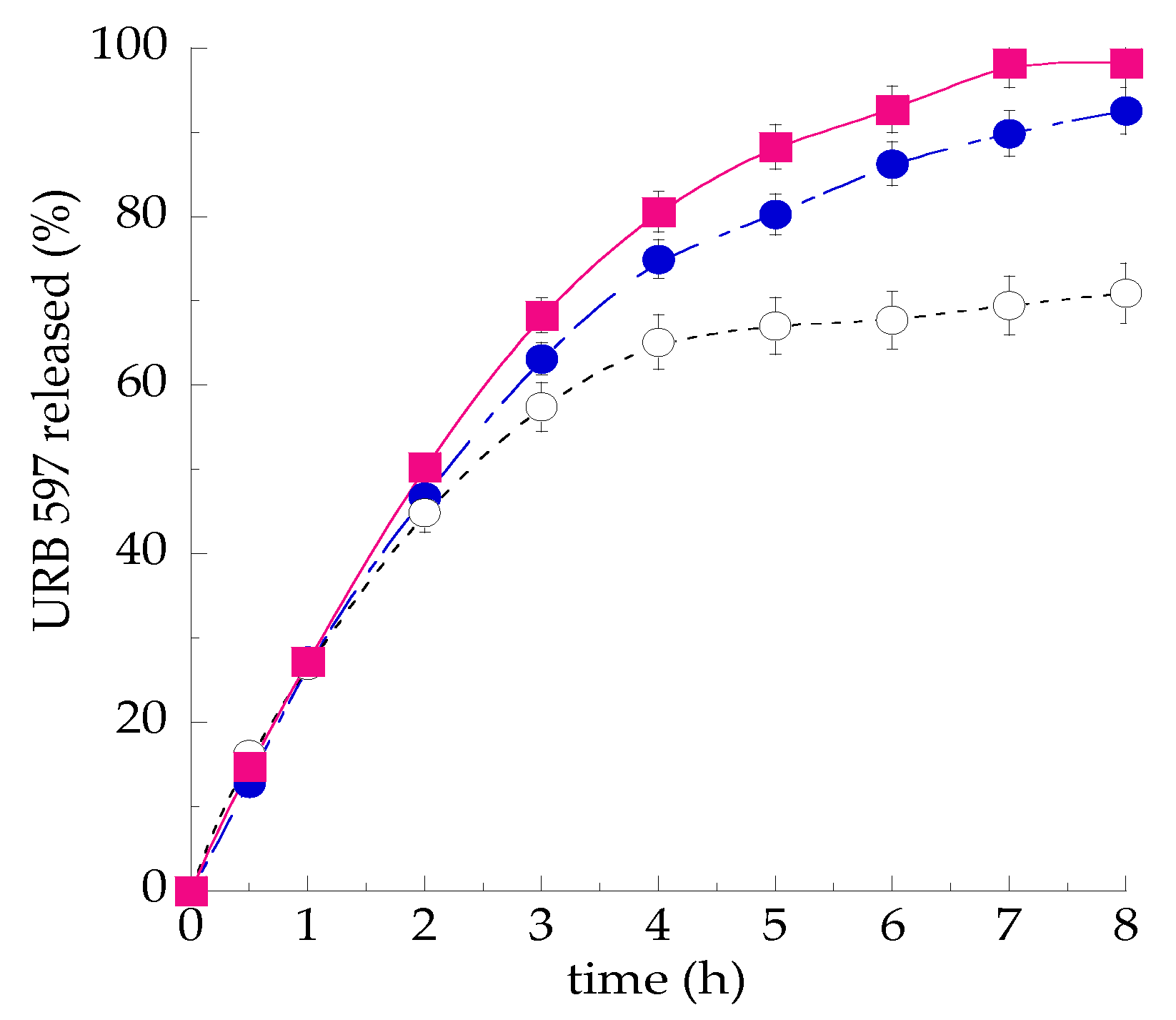
3.3. In Vitro Release Kinetics of URB597 from SLN
3.4. In Vivo Behavioral Test
4. Conclusions
Author Contributions
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLN | solid lipid nanoparticles |
| NLC | nanostructured lipid carriers |
| P80 | polysorbate 80 |
| DMF | dimethyl fumarate |
| RP | retynil palmitate |
| PRG | progesterone |
References
- Esposito, E.; Drechsler, M.; Mariani, P.; Carducci, F.; Servadio, M.; Melancia, F.; Ratano, P.; Campolongo, P.; Trezza, V.; Cortesi, R.; et al. Lipid nanoparticles for administration of poorly water soluble neuroactive drugs. Biomed. Microdevices 2017, 19, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, L.T.; Rodrigues, M.; Alves, G.; Santos, A.O. Intranasal fosphenytoin: The promise of phosphate esters in nose-to-brain delivery of poorly soluble drugs. Int. J. Pharm. 2020, 120040. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Holma, R.; Müllertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Valacchi, G.; Cortesi, R. Lipid nanostructures for antioxidant delivery: A comparative preformulation study. Beilstein J. Nanotechnol. 2019, 10, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ravani, L.; Drechsler, M.; Mariani, P.; Contado, C.; Roukolainen, P.; Ratano, P.; Campolongo, P.; Trezza, V.; Nastruzzi, C.; et al. Cannabinoid antagonist in nanostructured lipid carriers (NLC): Design, characterization and in vivo study. Mater. Sci. Eng. C 2015, 48, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Drechsler, M.; Cortesi, C.; Nastruzzi, C. Encapsulation of cannabinoid drugs in nanostructured lipid carriers. Eur. J. Pharm. Biopharm. 2016, 102, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Manduca, A.; Servadio, M.; Campolongo, P.; Palmery, M.; Trabace, L.; Vanderschuren, L.J.; Cuomo, V.; Trezza, V. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur. Neuropsychopharmacol. 2014, 24, 1337–1348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trezza, V.; Vanderschuren, L.J.M.J. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology 2008, 197, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, J.; Wang, Z.; You, W.; Wu, L.; Ji, C.; Chen, G. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. J. Neurosurg. 2015, 123, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 2016, 14, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Bitarafan, S.; Mohammadpour, Z.; Jafarirad, S.; Harirchian, M.-H.; Yekaninejad, M.S.; Saboor-Yaraghi, A.A. The effect of retinyl-palmitate on the level of pro and anti-inflammatory cytokines in multiple sclerosis patients: A randomized double blind clinical trial. Clin. Neurol. Neurosurg. 2019, 177, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Servadio, M.; Melancia, F.; Manduca, A.; di Masi, A.; Schiavi, S.; Cartocci, V.; Pallottini, V.; Campolongo, P.; Ascenzi, P.; Trezza, V. Targeting anandamide metabolism rescues core and associated autistic-like symptoms in rats prenatally exposed to valproic acid. Transl. Psychiatry 2016, 6, e902. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, K.; Shukla, R.R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr. Drug Deliv. 2019, 16, 887–901. [Google Scholar] [CrossRef] [PubMed]
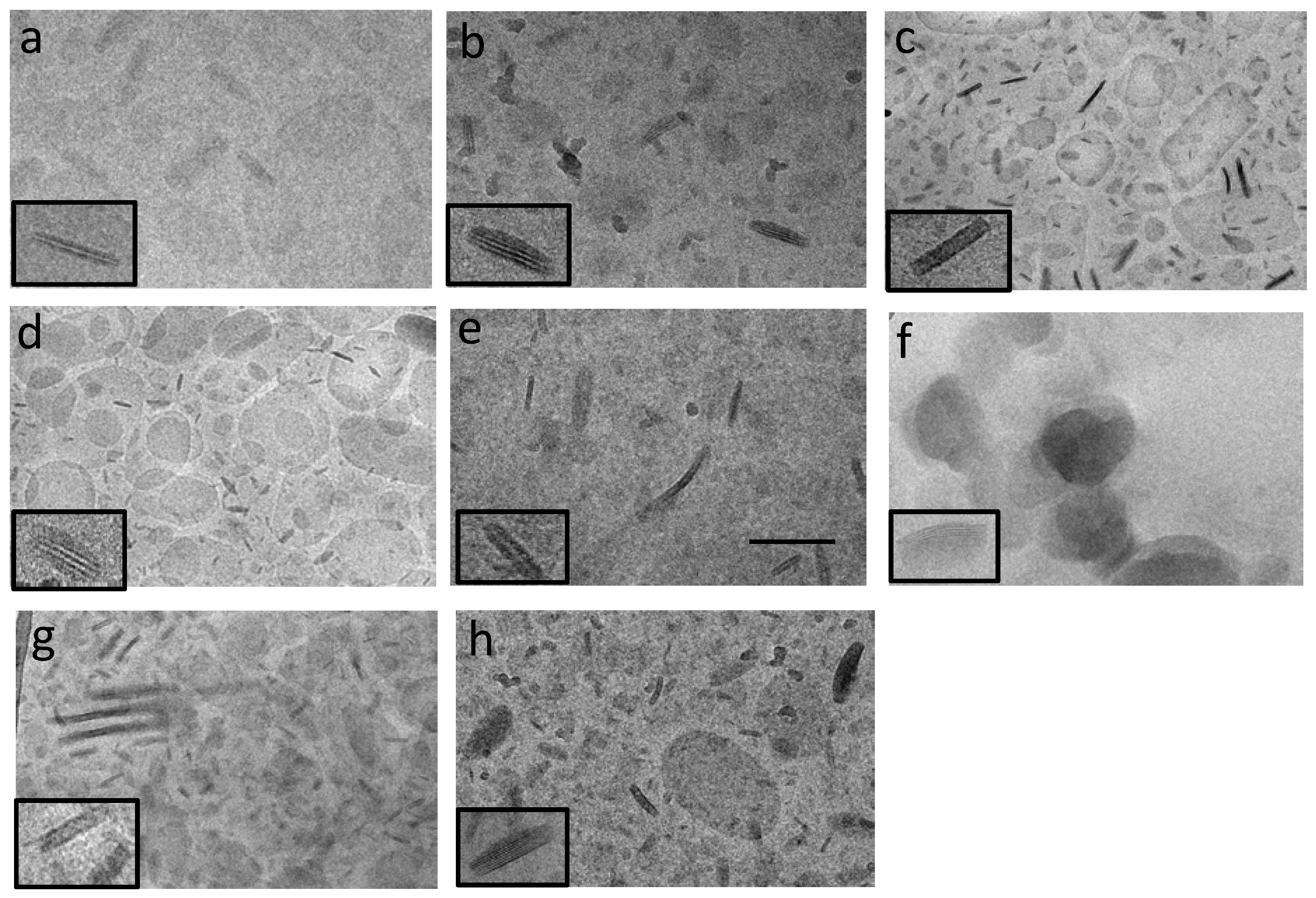
| Nanoparticle | Z Average (nm) | Dispersity | X-ray Diffraction Interlamellar Distance (nm) | EE 1 (%) |
|---|---|---|---|---|
| SLN | 147 ± 52 | 0.26 ± 0.01 | 3.74 | - |
| SLN/P80 | 176 ± 25 | 0.25 ± 0.08 | 4.55 | - |
| NLC | 179 ± 55 | 0.27 ± 0.01 | 4.55 | - |
| SLN-DMF | 254 ± 28 | 0.25 ± 0.01 | 3.81 | 85.2 ± 0.1 |
| NLC-DMF | 195 ± 20 | 0.25 ± 0.01 | 4.49 | 71.2 ± 0.3 |
| SLN-RP | 192 ± 46 | 0.27 ± 0.04 | 4.60 | 45.1 ± 0.1 |
| NLC-RP | 129 ± 22 | 0.24 ± 0.03 | 4.68 | 48.3 ± 0.2 |
| SLN-PRG | 160 ± 70 | 0.30 ± 0.02 | 3.80 | 72.5 ± 0.3 |
| NLC-PRG | 187 ± 64 | 0.29 ± 0.03 | 4.52 | 73.8 ± 0.1 |
| SLN-URB597 | 246 ± 33 | 0.28 ± 0.03 | 4.50 | 93.0 ± 0.2 |
| SLN/P80-URB597 | 273 ± 20 | 0.30 ± 0.03 | 4.48 | 93.0 ± 0.1 |
| NLC--URB597 | 242 ± 45 | 0.29 ± 0.01 | 4.69 | 92.8 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Trezza, V.; Cortesi, R.; Nastruzzi, C. Nanotechnological Strategies for Administration of Poorly Soluble Neuroactive Drugs. Proceedings 2021, 78, 21. https://doi.org/10.3390/IECP2020-08678
Esposito E, Sguizzato M, Drechsler M, Mariani P, Trezza V, Cortesi R, Nastruzzi C. Nanotechnological Strategies for Administration of Poorly Soluble Neuroactive Drugs. Proceedings. 2021; 78(1):21. https://doi.org/10.3390/IECP2020-08678
Chicago/Turabian StyleEsposito, Elisabetta, Maddalena Sguizzato, Markus Drechsler, Paolo Mariani, Viviana Trezza, Rita Cortesi, and Claudio Nastruzzi. 2021. "Nanotechnological Strategies for Administration of Poorly Soluble Neuroactive Drugs" Proceedings 78, no. 1: 21. https://doi.org/10.3390/IECP2020-08678
APA StyleEsposito, E., Sguizzato, M., Drechsler, M., Mariani, P., Trezza, V., Cortesi, R., & Nastruzzi, C. (2021). Nanotechnological Strategies for Administration of Poorly Soluble Neuroactive Drugs. Proceedings, 78(1), 21. https://doi.org/10.3390/IECP2020-08678










