Abstract
Co-products from the agro-food industry can be used as novel and natural ingredients in the reformulation of traditional foods to reduce the use of synthetic additives or improve their final quality. The aim of this study was to enrich pork liver pâté with persimmon flour co-products at two concentrations (3% and 6%) and to compare their total cholesterol (high-performance liquid chromatography (HPLC)), fatty acid (Gas Chromatography (GC)) phenolic compound (HPLC) profiles, and lipid oxidation (thiobarbituric acid-reactive substance (TBARS) assay) after in vitro digestion (INFOGEST consensus method) with the control pâté. The cholesterol content in pâté samples was significantly reduced in a dose-dependent way (control > pâté 3% > pâté 6%; 98 ± 8; 89 ± 3; 68 ± 11 mg/100 g pâté, respectively), probably due to the fiber and cholesterol interactions. Gallic, caffeic acids, glycosylated gallic acid, glycosylated coumaric acid, and glycosylated quercetin were detected in the enriched pâtés. The sum of all these compounds was 74 and 239 µg/g pâté in the pâtés with 3% and 6% of persimmon flour, respectively. Oleic, palmitic, and linoleic acids were the majority of fatty acids found in all pâtés. The increase of lipid oxidation after in vitro digestion was higher in the control pâté than in the enriched pâtés. In conclusion, the enrichment of pâté with persimmon flours caused a reduction in their total cholesterol content and lipid oxidation after in vitro digestion, without modifications in their fatty acid profile to what the phenolic compounds could be contributing.
1. Introduction
Currently, there is an increasing demand for natural ingredients with minimal processing. In this context, agro-food co-products could be a useful resource to natural ingredients with different technological properties, such as antioxidants, emulsifier agents, colorants, or water bindings. Furthermore, some properties have high amounts of nutrients such as fiber, vitamins, minerals, and phytochemicals [1,2,3,4,5]. Pork liver pâté is widely consumed in Europe, as it provides protein, vitamins, and fat. Due to its high-fat content and low natural antioxidants, they suffer from lipid oxidations [6], which have negative connotations to health. Therefore, adding antioxidants to the pork liver matrix could prevent or reduce that process.
The persimmon fruit originated in Asia. It has a high amount of sugar, vitamins, fiber, carotenoids, and polyphenolic compounds [7]. Around the world, different foods (e.g., juice, dehydrated pieces, wine, jam, cakes, etc.) are made with persimmons. The co-products derived to obtain the mentioned food-based persimmon, such as its peel and pulp, continue to have a high amount of nutrients and technological properties, such as colorants, antioxidants, nitrite reduce agents, or cholesterol blinding [2,3,5]. Therefore, the aim of this study was to enrich pork liver pâté with persimmon flour co-products at two concentrations (3% and 6%) and to compare their total cholesterol, fatty acid polyphenolic compound profiles, and lipid oxidation after in vitro digestion with the control pâté.
2. Materials and Methods
2.1. Manufacture of Liver Pâtés
For each studied sample, three different batches were made: control pork liver pâté (CP) (without persimmon flour), pork liver pâté enrichment with 3% of persimmon flour cv. “Rojo Brillante” (P-3RB), and pork liver pâté enrichment with 6% of persimmon flour cv. “Rojo Brillante” (P-6RB), following the procedure described by Lucas-González et al. [5].
2.2. Fatty Acid Profile
Fat extraction was carried out following the Folch method [8]. Derivatization, detection, identification, and quantification of fatty acids was carried out following the procedure describe by Pellegrini et al. [9].
2.3. Total Cholesterol
In the crude pork liver pâté, the total cholesterol formulations following the procedure described by Essaka was determined [10].
2.4. Polyphenolic Compound Determination
Polyphenol compounds extraction was carried out following the methodology by Mpofu, Sapirstein, and Beta [11]. Polyphenol detection, identification, and quantification were carried out as described by Lucas-González et al. [12].
2.5. Simulated In Vitro Gastrointestinal Digestion
The in vitro digestion assay was carried out following the INFOGEST consensus method [13].
2.6. Lipid Oxidation: 2-Thiobarbituric Acid-Reactive Substances (TBARS) Test
Crude and digested samples (after gastric and intestinal steps) were carried using the thiobarbituric acid-reactive substance (TBARS) assay [14]. The results were expressed as a lipid oxidation increase, compared to the corresponding raw sample.
2.7. Statistical
Results were expressed as mean ± standard deviations of three repetitions. To know the differences between the studied samples, we used a simple ANOVA test. Significant statistical differences were considered when p-values were <0.05 after Tukey’s post hoc test.
3. Results and Discussion
3.1. Fatty Acid Profile
The fatty acid profile of pâtés samples (CS, P-3RB and P-6RB) were monounsaturated, saturated, and polyunsaturated. The majority of fatty acids found in liver pork pâté formulations were oleic, palmitic, and linoleic acids. Adding persimmon flour did not modify the fatty acid profile of pork liver pâté.
3.2. Total Cholesterol
The total cholesterol decreased in pâté samples when persimmon flour increased (Table 1). This could be due to the ability of persimmon flour to join fat and bile acid [2]. If the cholesterol was bound to the matrix during gastrointestinal digestion, less cholesterol was available in the bloodstream.

Table 1.
Total cholesterol in pork liver pâté samples.
3.3. Profile of Polyphenolic Compounds
In crude enrichment pâté samples, five polyphenolic compounds were detected (Figure 1), which were contributed by persimmon flour. Gallic acid was the largest, whereas a trace amount of quercetin glycoside was also detected. The increase in the number of compounds was dose-dependent.
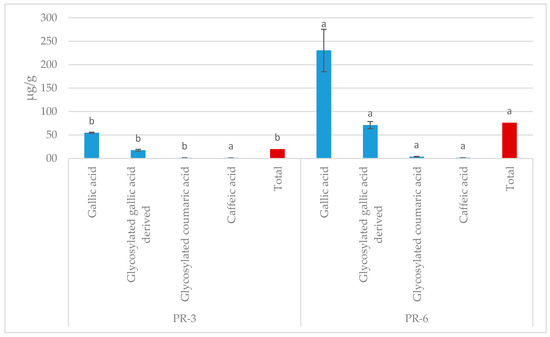
Figure 1.
The polyphenol profile in pâté enriched with persimmon flour. CP: control pâté; P-3RB: pork liver pâté enrichment with 3% of persimmon flour cv. “Rojo Brillante”; P-6RB: pork liver pâté enrichment with 6% of persimmon flour cv. “Rojo Brillante”. Total: Sum of four polyphenols quantified. Different case lower letters (a,b) for each individual phenolic compound and the total indicate significant differences between the two studied samples.
3.4. Lipid Oxidation after In Vitro Digestion
The incorporation of persimmon flour to pork liver pâté reduced lipid oxidation after in vitro gastrointestinal digestion (Figure 2). The phytochemicals presented in persimmon flour, such as carotenoids and polyphenols, could be responsible for a reduction in lipid oxidation after in vitro digestion.
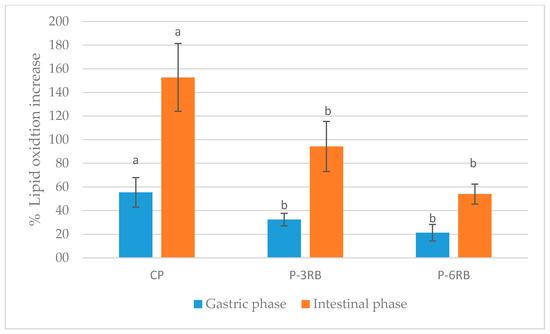
Figure 2.
Lipidic oxidation increase (%) of pork liver pâté samples after in vitro gastrointestinal digestion. CP: control pâté; P-3RB: pork liver pâté enrichment with 3% of persimmon flour cv. “Rojo Brillante”; P-6RB: pork liver pâté enrichment with 6% of persimmon flour cv. “Rojo Brillante”. Different case lower letters (a,b) for each simulated digestion phase indicate significant differences between the three studied samples.
4. Conclusions
The enrichment of pâté with persimmon flour caused a reduction in their total cholesterol content and lipid oxidation after in vitro digestion, without modifying their fatty acid profile to what the phenolic compounds contributed.
Author Contributions
Conceptualization, R.L.-G., J.F.-L., and M.V.-M.; methodology, R.L.-G. and M.V.-M.; investigation, R.L.-G. resources, J.Á.P.-Á.; writing—original draft preparation, R.L.-G.; writing—review and editing, J.F.-L. and M.V.-M.; supervision, P.J.A.; funding acquisition, P.J.A. and J.F.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grant of R. Lucas-González from UMH-Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lorente-Mento, J.M.; Lucas-González, R.; Sayas-Barbera, E.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Turrón Coproducts as Source of Bioactive Compounds: Assessment of Chemical, Physico-Chemical, Techno-Functional and Antioxidant Properties. Foods 2020, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Gonzalez, R.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Fernandez-Lopez, J. Evaluation of particle size influence on proximate composition, physicochemical, techno-functional and physio-functional properties of flours obtained from Persimmon (Diospyros kaki Trumb.) coproducts. Plant Foods Hum. Nutr. 2017, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Gonzalez, R.; Fernandez-Lopez, J.; Perez-Alvarez, J.A.; Viuda-Martos, M. Effect of particle size on phytochemical composition and antioxidant properties of two persimmon flours from Diospyros kaki Thunb. vars. ‘Rojo Brillante’ and ‘Triumph’ co-products. J. Sci. Food Agric. 2018, 98, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT Food Sci. Technol. 2019, 114, 7. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pellegrini, M.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Fernández-López, J. Persimmon (Diospyros kaki Thunb.) coproducts as a new ingredient in pork liver pâté: Influence on quality properties. Int. J. Food Sci. Technol. 2019, 54, 1232–1239. [Google Scholar] [CrossRef]
- Russell, E.A.; Lynch, A.; Lynch, P.B.; Kerry, J.P. Quality and shelf life of duck liver pâtes as influenced by dietary supplementation with α-tocopheryl acetate and various fat sources. J. Food Sci. 2003, 68, 799–802. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542–561. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crop Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Essaka, D.C. Reversed-Phase HPLC Determination of Colesterol in Foods Ítems. Master’s Thesis, East Tennessee State University, Johnson City, TN, USA, 2007. Available online: https://dc.etsu.edu/etd/2034 (accessed on 12 October 2020).
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Casal, S.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M. Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and in vitro gastrointestinal digestion. Food Chem. Toxicol. 2020, 141, 111401. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).