Effect of Heat Treatment on Smoothie Quality by Response Surface Methodology †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Smoothie Preparation
2.2. Experimental Analysis and Validation of Optimized Condition of Heat Treatment
2.3. Physical-Chemical Analysis
2.3.1. Color, pH and Solid Soluble Contents
2.3.2. Polyphenoloxidase (PPO) Enzymatic Activity
2.3.3. Antioxidant Capacity and Total Phenolic Content
2.4. Statistical Analysis
2.4.1. Model Fitting and Statistical Analysis
2.4.2. Quality Evaluation of Untreated and Heat-Treated Smoothies
3. Results and Discussion
3.1. Model Fitting
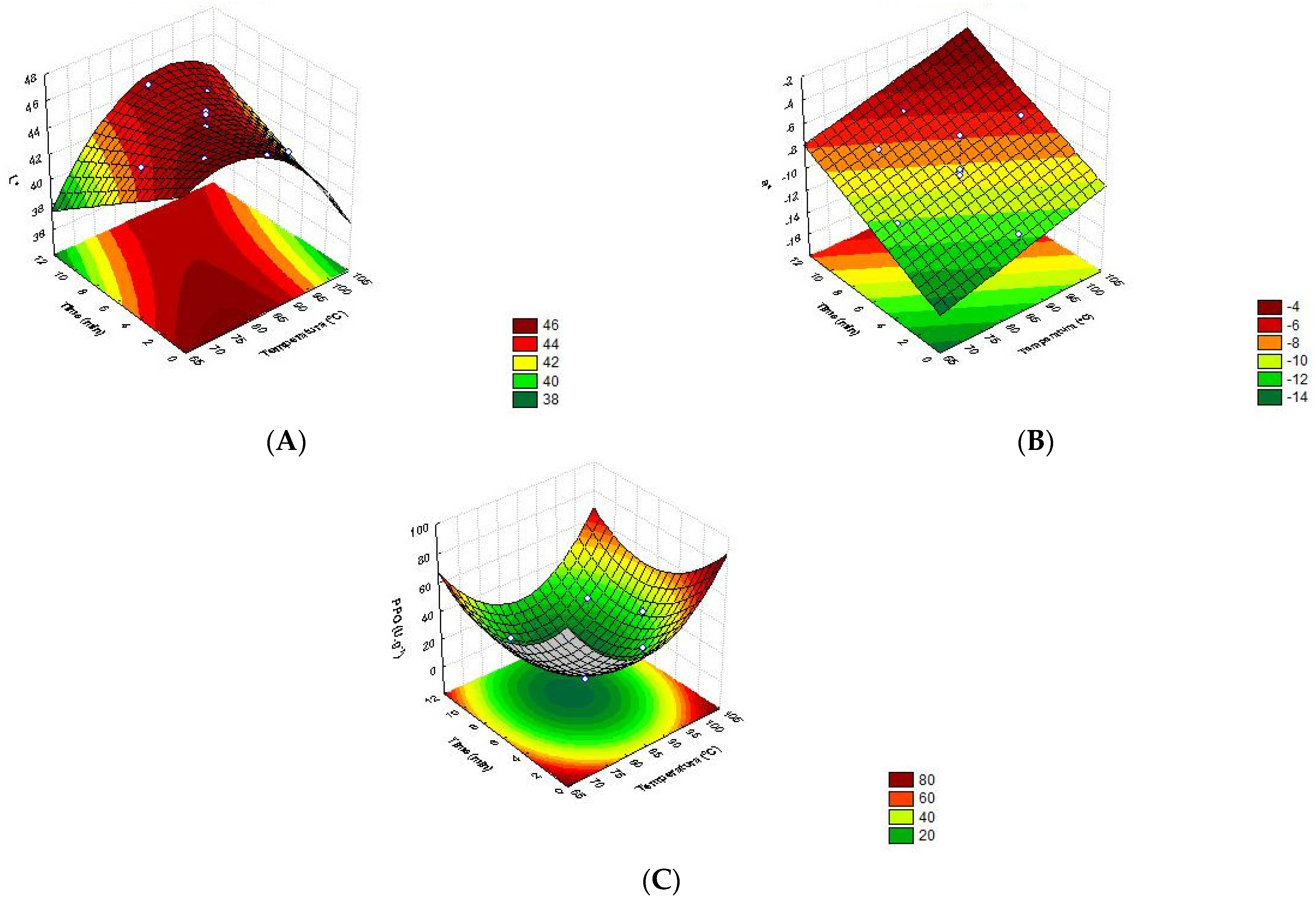
3.2. Response Surface Analysis
3.3. Validation Study of Optimzed Heat Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bates, R.P.; Morris, J.R.; Crandall, P.G. Principles and Practices of Small- and Medium- Scale Fruit JUICE Processing; FAO: Rome, Italy, 2001; p. 146. [Google Scholar]
- Cano-Lamadrid, M.; Hernández, F.; Nowicka, P.; Carbonell-Barrachina, A.A.; Wojdylo, A. Formulation and storage effects on pomegranate smoothie phenolic composition, antioxidante capacity and color. LWT 2018, 96, 322–328. [Google Scholar] [CrossRef]
- Maina, J.W. Analysis of the factos that determine food acceptability. Pharm. Innov. 2018, 7, 253–257. [Google Scholar]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Verónica, F.M.; Bengardino, M.; Jagus, R.J.; Agüero, M.V. Enrichment and preservation of a vegetable smoothie with an antioxidant and antimicrobial extract obtained from beet by-products. LWT 2020, 117, 108622. [Google Scholar]
- García-Torres, R.; Ponagandla, N.R.; Rouseff, R.L.; Goodrich-Schneider, R.M.; Reyes-De-Coucuera, J.I. Effects of Dissolved Oxygen in Fruit Juices and Methods of Removal, Comprehensive Rev. Food Sci. Food Saf. 2009, 8, 409–423. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Ma, Y.; Zhao, X.; Zhang, C. Effect of Thermal Treatments on Quality and Aroma of Watermelon Juice. J. Food Qual. 2018, 2018, 9242675. [Google Scholar] [CrossRef]
- Pinheiro, J.; Ganhão, R.; Gonçalves, M.; Silva, C.L.M. Assessment of thermosonication as postharvest treatment applied on whole tomato fruits: Optimization and validation. Foods 2019, 8, 649. [Google Scholar] [CrossRef]
- Ganhão, R.; Pinheiro, J.; Tino, C.; Faria, H.; Gil, M.M. Characterization of nutritional, physicochemical, phytochemicals composition and antioxidant capacity of three strawberry “Fragaria x ananassa Duch” cultivars (‘Primoris’, ‘Endurance’, ‘Portola’), from western region of Portugal. Foods 2019, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.M.; Raposo, I.; Pinheiro, J.; Alegria, C.; Moldão, M.; Abreu, M. Quality changes during thermal processing of two mixed formulas of fruits and vegetables pulps. Emir. J. Food Agric. 2020, 32, 271–280. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Santos, D.I.; Correia, M.J.N.; Mateus, M.M.; Saraiva, J.A.; Vicente, A.A.; Moldão, M. Fourier Transform Infrared (FT-IR) spectroscopy as a possible rapid tool to evaluate abiotic stress effects on pineapple by-products. Appl. Sci. 2019, 9, 4141. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Embrapa Agroindústria Tropical, 2006; Comunicado técnico 125. 1–4, ISSN 1679-6535. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
| Coded Independent Variables | Decoded Independent Variables | ||
|---|---|---|---|
| X1 | X2 | Treatment (°C) | Time (min) |
| −1.41421 | −1.41421 | 70 | 0.5 |
| −1 | −1 | 75 | 2 |
| 0 | 0 | 85 | 5.5 |
| 1 | 1 | 95 | 9 |
| 1.41421 | 1.41421 | 100 | 10.5 |
| Eq. | Parameter | Model Equations | R2 | R2adj |
|---|---|---|---|---|
| (1) | PPO | PPO = 414.70 − 8.42 x T + 0.05 x T2 − 1.22 x t + 0.55 x t2 − 0.09 x T x t | 0.97 | 0.96 |
| (2) | L* | L* = −12.52 + 1.57 x T − 0.010 x T2 − 2.58 x t + 0.029 x T x t | 0.84 | 0.77 |
| (3) | a* | a* = −28.56 + 0.18 x T + 1.89 x t − 0.021 x t2 − 0.013 x T x t | 0.86 | 0.79 |
| (4) | hue | °h = 166.43 − 0.99 x T + 0.004 x T2 − 2.71 x t + 0.091 x t2 + 0.01 x T x t | 0.92 | 0.87 |
| Quality Parameter | Untreated | Heat-Treated |
|---|---|---|
| CIE Lab | ||
| L* | 42.14 ± 0.35 a | 43.94 ± 0.60 b |
| a* | −16.14 ± 0.49 a | −7.73 ± 0.42 b |
| b* | 29.95 ± 1.14 a | 29.51 ± 0.59 a |
| hue | 118.33 ± 0.30 a | 104.67 ± 0.60 b |
| Antioxidant capacity (μmol Trolox.100g −1) | ||
| FRAP | 5230.49 ± 177.10 a | 5911.44 ± 216.81 b |
| DPPH | 6321.29 ± 441.15 a | 7443.79 ± 448.85 b |
| ABTS | 1564.32 ± 183.00 a | 2350.56 ± 82.07 b |
| Total phenolic content (mg GAE.100g −1) | 77.68 ± 2.05 a | 85.34 ± 4.51 b |
| PPO activity (U.g −1) | 28.12 ± 2.66 a | 2.46 ± 0.96 b |
| pH | 3.57 ± 0.01 a | 3.57 ± 0.01 a |
| Solids soluble content (°Brix) | 10.51 ± 0.06 a | 10.61 ± 0.06 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, J.; Santos, D.I.; Gonçalves, E.M.; Abreu, M.; Moldão-Martins, M. Effect of Heat Treatment on Smoothie Quality by Response Surface Methodology. Proceedings 2021, 70, 6. https://doi.org/10.3390/foods_2020-07626
Pinheiro J, Santos DI, Gonçalves EM, Abreu M, Moldão-Martins M. Effect of Heat Treatment on Smoothie Quality by Response Surface Methodology. Proceedings. 2021; 70(1):6. https://doi.org/10.3390/foods_2020-07626
Chicago/Turabian StylePinheiro, Joaquina, Diana I. Santos, Elsa M. Gonçalves, Marta Abreu, and Margarida Moldão-Martins. 2021. "Effect of Heat Treatment on Smoothie Quality by Response Surface Methodology" Proceedings 70, no. 1: 6. https://doi.org/10.3390/foods_2020-07626
APA StylePinheiro, J., Santos, D. I., Gonçalves, E. M., Abreu, M., & Moldão-Martins, M. (2021). Effect of Heat Treatment on Smoothie Quality by Response Surface Methodology. Proceedings, 70(1), 6. https://doi.org/10.3390/foods_2020-07626







