Microwave-Assisted Extraction of Phenolics from Hibiscus sabdariffa Flowers: Method Development and Validation †
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Samples
2.3. Extraction of Phenolic Compounds
2.4. Identification and Quantification of Phenolic Compounds
2.5. Experimental Design and Statistical Analysis
2.6. Kinetic Study
2.7. Performance of the Method
3. Result and Discussion
3.1. Identification of Phenolic in Roselle
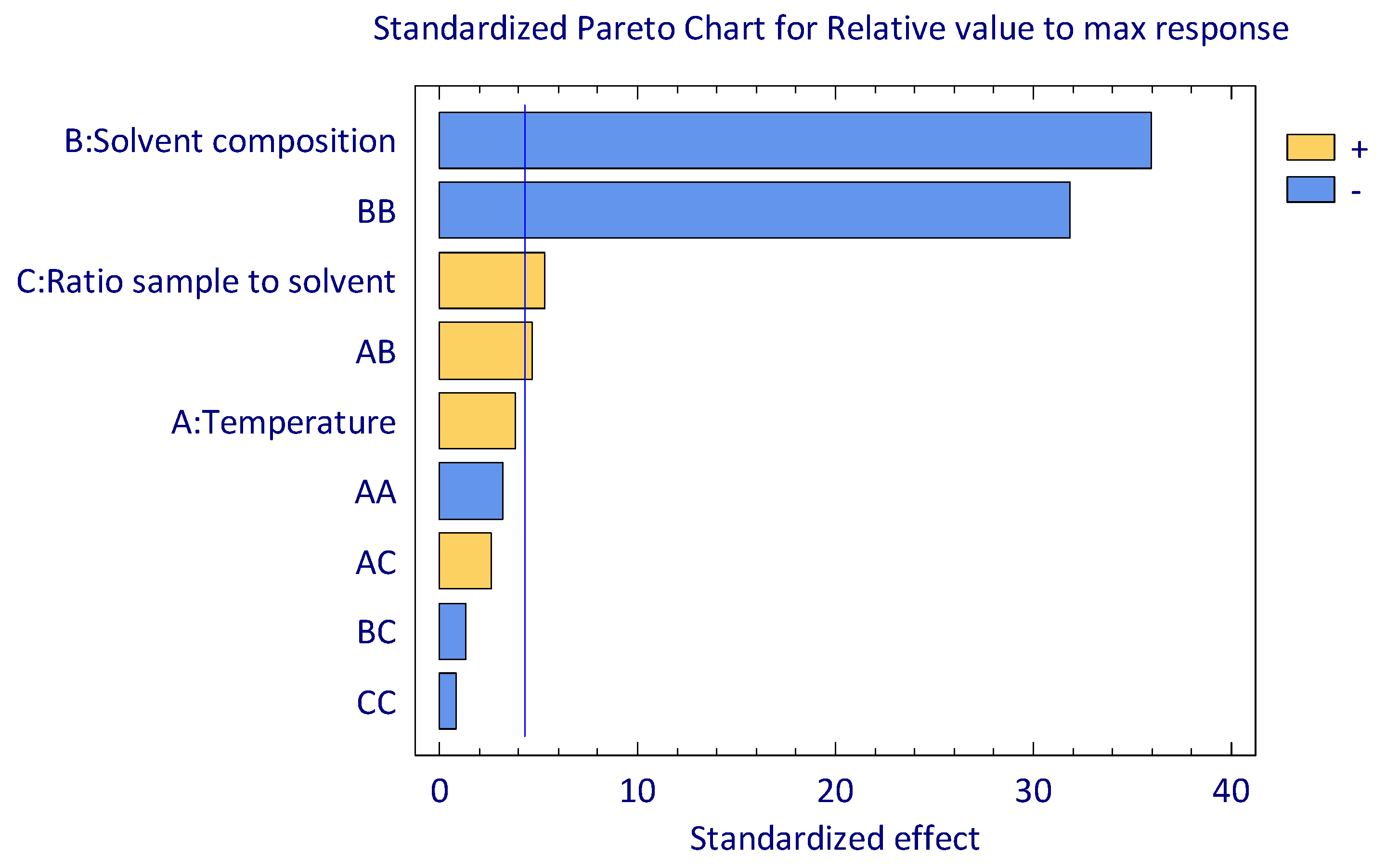
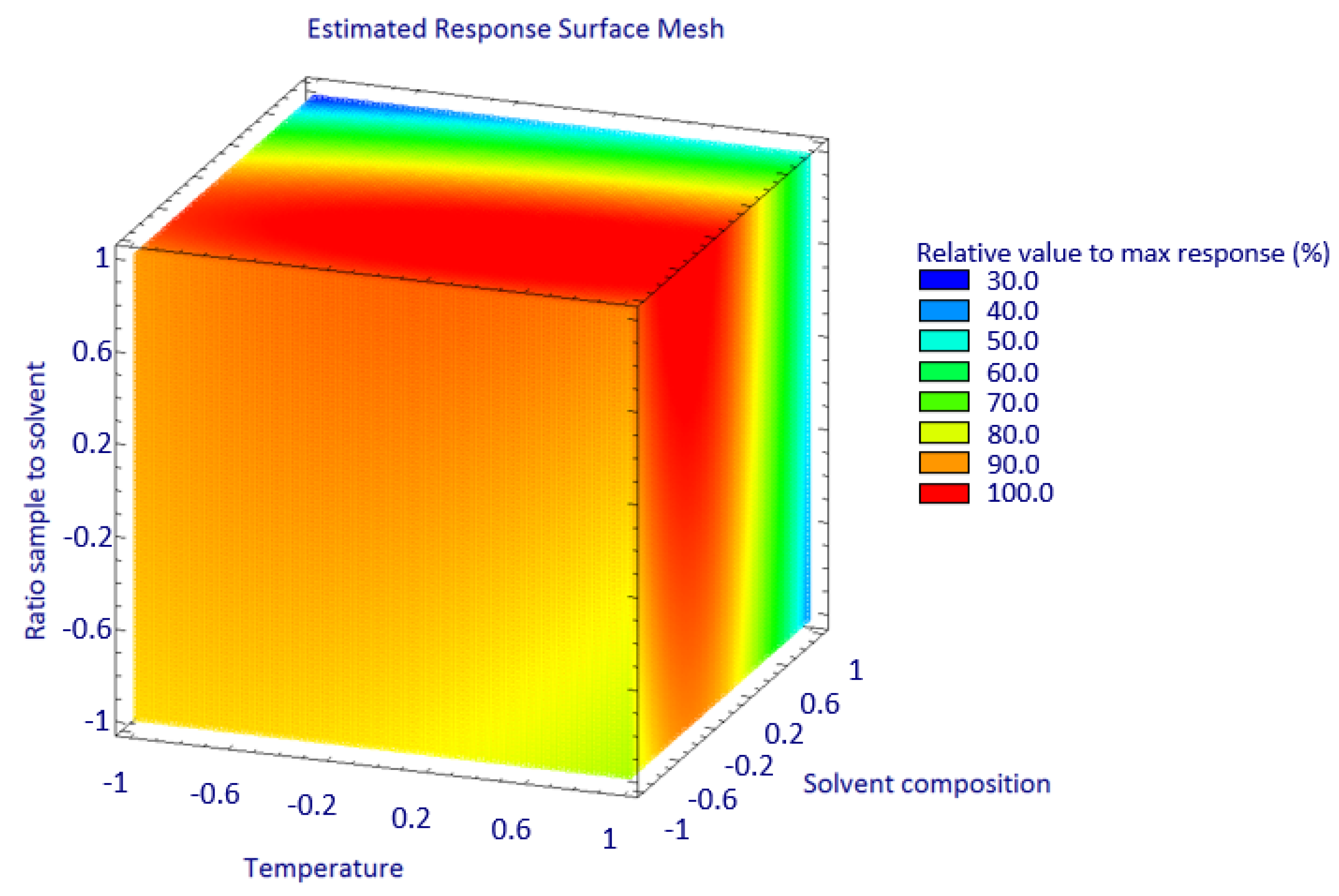
3.2. Effect of MAE Factors
3.3. Optimization of MAE Condition
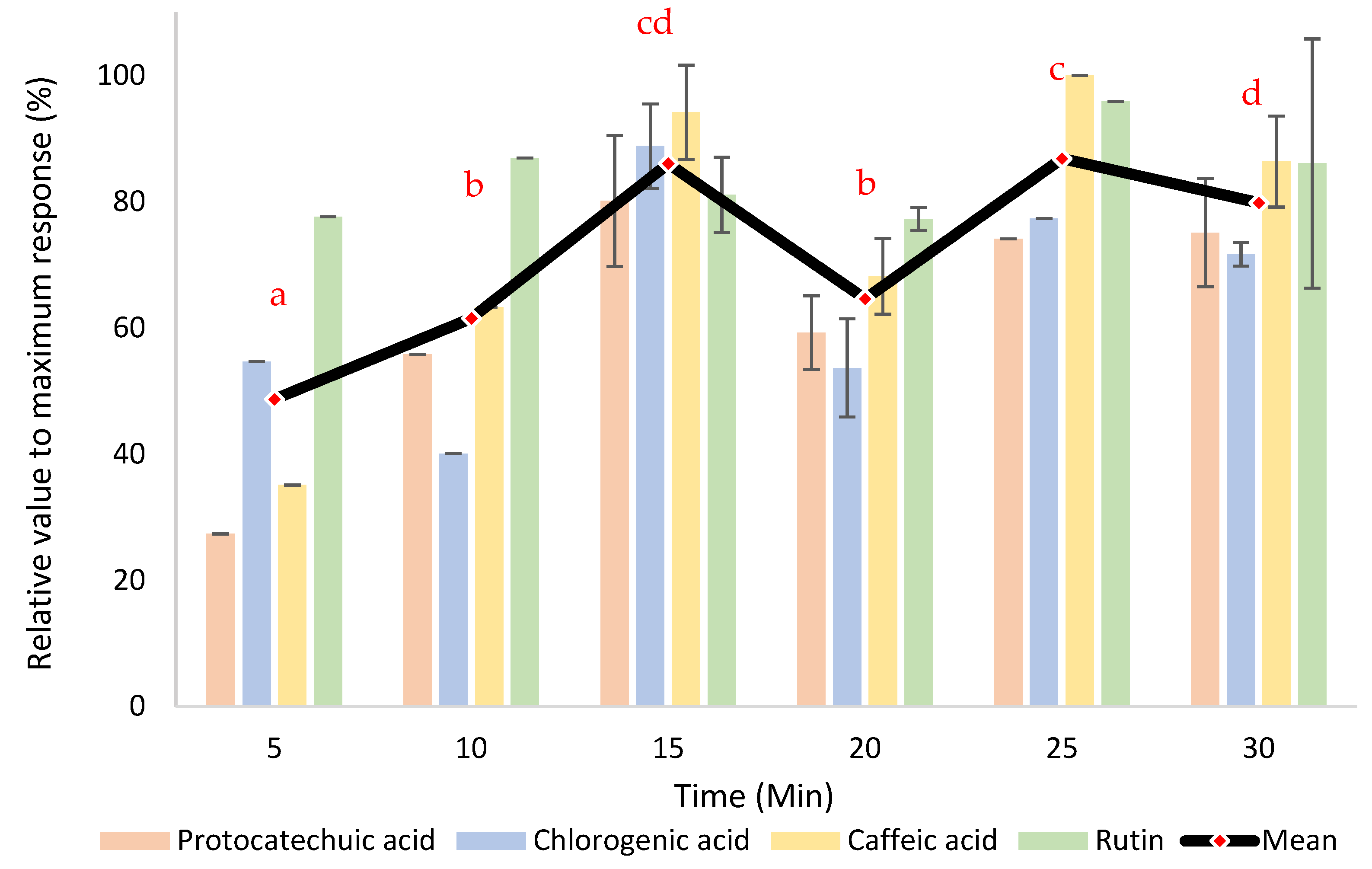
3.4. Kinetics Study
3.5. Method Validation
3.6. Real Sample Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kou, L.; Turner, E.R.; Luo, Y. Extending the Shelf Life of Edible Flowers with Controlled Release of 1-Methylcyclopropene and Modified Atmosphere Packaging. J. Food Sci. 2012, 77, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Hibiscus sabdariffa L. Roselle. Unconv. Oilseeds Oil Sources 2017, 59–65. [Google Scholar] [CrossRef]
- Villani, T.; Juliani, H.R.; Simon, J.E.; Wu, Q.L. Hibiscus sabdariffa: Phytochemistry, quality control, and health properties. ACS Symp. Ser. 2013, 1127, 209–230. [Google Scholar] [CrossRef]
- Thiagarajah, K.; Ong, M.K.; Teh, L.K.; Lye, H.S. Plants Infused Water as Preferred Healthy Drinks; Elsevier Inc.: Amsterdam, Netherlands, 2019; ISBN 9780128152720. [Google Scholar]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Salib, J.Y. Polyphenolic Compounds from Flowers of Hibiscus: Characterization and Bioactivity; Elsevier: Amsterdam, Netherlands, 2014; ISBN 9780123979346. [Google Scholar]
- Tsai, P.J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Sánchez, A.M.; Lorenzo, C.; López-Córcoles, H.; Alonso, G.L. Quality determination of Crocus sativus L. flower by high-performance liquid chromatography. J. Food Compos. Anal. 2020, 93, 103613. [Google Scholar] [CrossRef]
- Abidi, J.; Ammar, S.; Ben Brahim, S.; Skalicka-Woźniak, K.; Ghrabi-Gammar, Z.; Bouaziz, M. Use of ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry system as valuable tool for an untargeted metabolomic profiling of Rumex tunetanus flowers and stems and contribution to the antioxidant activity. J. Pharm. Biomed. Anal. 2019, 162, 66–81. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Segura-Carretero, A.; Puertas-Mejía, M.A.; Cortacero-Ramírez, S.; Beltrán, R.; Alonso-Villaverde, C.; Joven, J.; Dinelli, G.; Fernández-Gutiérrez, A. Selective extraction, separation, and identification of anthocyanins from Hibiscus sabdariffa L. using solid phase extraction-capillary electrophoresis-mass spectrometry (time-of-flight/ion trap). Electrophoresis 2008, 29, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, I.; Javad, S.; Ansari, M.; Ghaffar, N.; Tariq, A. Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology. J. King Saud Univ. Sci. 2020, 32, 1451–1458. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crops Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Dragović-Uzelac, V.; Režek Jambrak, A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Fu, X.Q.; Ma, N.; Sun, W.P.; Dang, Y.Y. Microwave and enzyme co-assisted aqueous two-phase extraction of polyphenol and lutein from marigold (Tagetes erecta L.) flower. Ind. Crops Prod. 2018, 123, 296–302. [Google Scholar] [CrossRef]
- López-Hortas, L.; Conde, E.; Falqué, E.; Domínguez, H. Flowers of Ulex europaeus L.-Comparing two extraction techniques (MHG and distillation). C. R. Chim. 2016, 19, 718–725. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Rombaut, N.; Tixier, A.-S.; Billy, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery Natacha. Biofuels Bioprod. Biorefin. 2014. [Google Scholar] [CrossRef]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Microwave-Assisted Extraction, 3rd ed.; Elsevier Inc.: Amsterdam, Netherlands, 2018; ISBN 9780124095472. [Google Scholar]
- Setyaningsih, W.; Duros, E.; Palma, M.; Barroso, C.G. Optimization of the ultrasound-assisted extraction of melatonin from red rice (Oryza sativa) grains through a response surface methodology. Appl. Acoust. 2016, 103, 129–135. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M.; Barroso, C.G. Multiresponse optimization of a UPLC method for the simultaneous determination of tryptophan and 15 tryptophan-derived compounds using a Box-Behnken design with a desirability function. Food Chem. 2017, 225, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M. Optimisation of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019, 288, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Palma, M.; Barroso, C.G. A new microwave-assisted extraction method for melatonin determination in rice grains. J. Cereal Sci. 2012, 56, 340–346. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M.; García-Barroso, C. Fast Determination of Phenolic Compounds in Rice Grains by Ultraperformance Liquid Chromatography Coupled to Photodiode Array Detection: Method Development and Validation. J. Agric. Food Chem. 2019, 67, 3018–3027. [Google Scholar] [CrossRef]
- ICH. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crops Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Stability of 40 phenolic compounds during ultrasound-assisted extractions (UAE). AIP Conf. Proc. 2016, 1755, 080009. [Google Scholar]
- Krishnan, R.Y.; Rajan, K.S. Influence of microwave irradiation on kinetics and thermodynamics of extraction of flavonoids from Phyllanthus emblica. Braz. J. Chem. Eng. 2017, 34, 885–899. [Google Scholar] [CrossRef]
- Amirah; Reddy Prasad, D.M.; Khan, M.R. Comparison of extraction techniques on extraction of gallic acid from stem bark of Jatropha curcas. J. Appl. Sci. 2012, 12, 1106–1111. [Google Scholar] [CrossRef]
- Christian, K.R.; Jackson, J.C. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J. Food Compos. Anal. 2009, 22, 663–667. [Google Scholar] [CrossRef]
| Factors | −1 | 0 | +1 | Unit |
|---|---|---|---|---|
| x1, temperature | 30 | 55 | 80 | °C |
| x2, solvent composition | 40 | 70 | 100 | % methanol in water |
| x3, solvent to sample ratio | 10:1 | 15:1 | 20:1 | (v/w) |
| Run | x1, Temperature | x2, Solvent Composition | x3, Solvent to Sample Ratio | Relative Values to the Maximum Response (%) |
|---|---|---|---|---|
| 1 | 0 | 1 | −1 | 45.08 |
| 2 | 0 | −1 | 1 | 85.96 |
| 3 | 1 | −1 | 0 | 88.98 |
| 4 | −1 | 0 | 1 | 92.94 |
| 5 | 0 | −1 | −1 | 80.08 |
| 6 | 0 | 0 | 0 | 97.21 |
| 7 | 0 | 0 | 0 | 93.65 |
| 8 | −1 | −1 | 0 | 90.08 |
| 9 | −1 | 0 | −1 | 87.48 |
| 10 | 0 | 0 | 0 | 94.86 |
| 11 | 1 | 0 | 1 | 100.00 |
| 12 | 1 | 0 | −1 | 85.15 |
| 13 | −1 | 1 | 0 | 26.75 |
| 14 | 1 | 1 | 0 | 42.65 |
| 15 | 0 | 1 | 1 | 46.19 |
| Analytes | Range (mg L−1) | Linear Equation | R2 | Limits (mg L−1) | |
|---|---|---|---|---|---|
| LOD | LOQ | ||||
| Protocatechuic acid | 0.5–10 | 0.9995 | 0.256 | 0.852 | |
| 10–100 | 0.9980 | ||||
| Chlorogenic acid | 0.5–10 | 0.9990 | 0.374 | 1.245 | |
| 10–100 | 0.9968 | ||||
| Caffeic acid | 0.5–10 | 0.9997 | 0.219 | 0.730 | |
| 10–100 | 0.9971 | ||||
| Rutin | 0.5–10 | 0.9996 | 0.242 | 0.808 | |
| 10–100 | 0.9982 | ||||
| Compound | Precision (CV, %) | Recovery (%) | |
|---|---|---|---|
| Intra-Day (n = 9) | Inter-Day (n = 3 × 3) | ||
| Protocatechuic acid | 5.16 | 10.36 | 91.54 |
| Chlorogenic acid | 5.30 | 6.67 | 104.46 |
| Caffeic acid | 4.82 | 7.48 | 118.79 |
| Rutin | 6.46 | 5.94 | 105.54 |
| Samples | Concentration (mg 100 g−1 dried sample) | ||||
|---|---|---|---|---|---|
| Protocatechuic Acid | Chlorogenic Acid | Caffeic Acid | Rutin | Total | |
| Pink Roselle | 1.33±0.01 | 41.08±0.04 | 3.59±0.01 | 49.26±0.04 | 13.534 |
| Red Roselle | 0.46±0.01* | 118.86±0.03 | 5.72±0.01 | 138.98±0.03 | 3.361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathimah, R.N.; Setyaningsih, W.; Carrera, C.; Palma, M. Microwave-Assisted Extraction of Phenolics from Hibiscus sabdariffa Flowers: Method Development and Validation. Proceedings 2021, 70, 51. https://doi.org/10.3390/foods_2020-07681
Fathimah RN, Setyaningsih W, Carrera C, Palma M. Microwave-Assisted Extraction of Phenolics from Hibiscus sabdariffa Flowers: Method Development and Validation. Proceedings. 2021; 70(1):51. https://doi.org/10.3390/foods_2020-07681
Chicago/Turabian StyleFathimah, Rohmah Nur, Widiastuti Setyaningsih, Ceferino Carrera, and Miguel Palma. 2021. "Microwave-Assisted Extraction of Phenolics from Hibiscus sabdariffa Flowers: Method Development and Validation" Proceedings 70, no. 1: 51. https://doi.org/10.3390/foods_2020-07681
APA StyleFathimah, R. N., Setyaningsih, W., Carrera, C., & Palma, M. (2021). Microwave-Assisted Extraction of Phenolics from Hibiscus sabdariffa Flowers: Method Development and Validation. Proceedings, 70(1), 51. https://doi.org/10.3390/foods_2020-07681







