Optical Properties of Vanadium Oxide/Cellulose Triacetate Photochromic Films †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of Nanocomposites
2.3. Characterisation
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. UV-Vis Spectroscopy
3. Results and Discussion
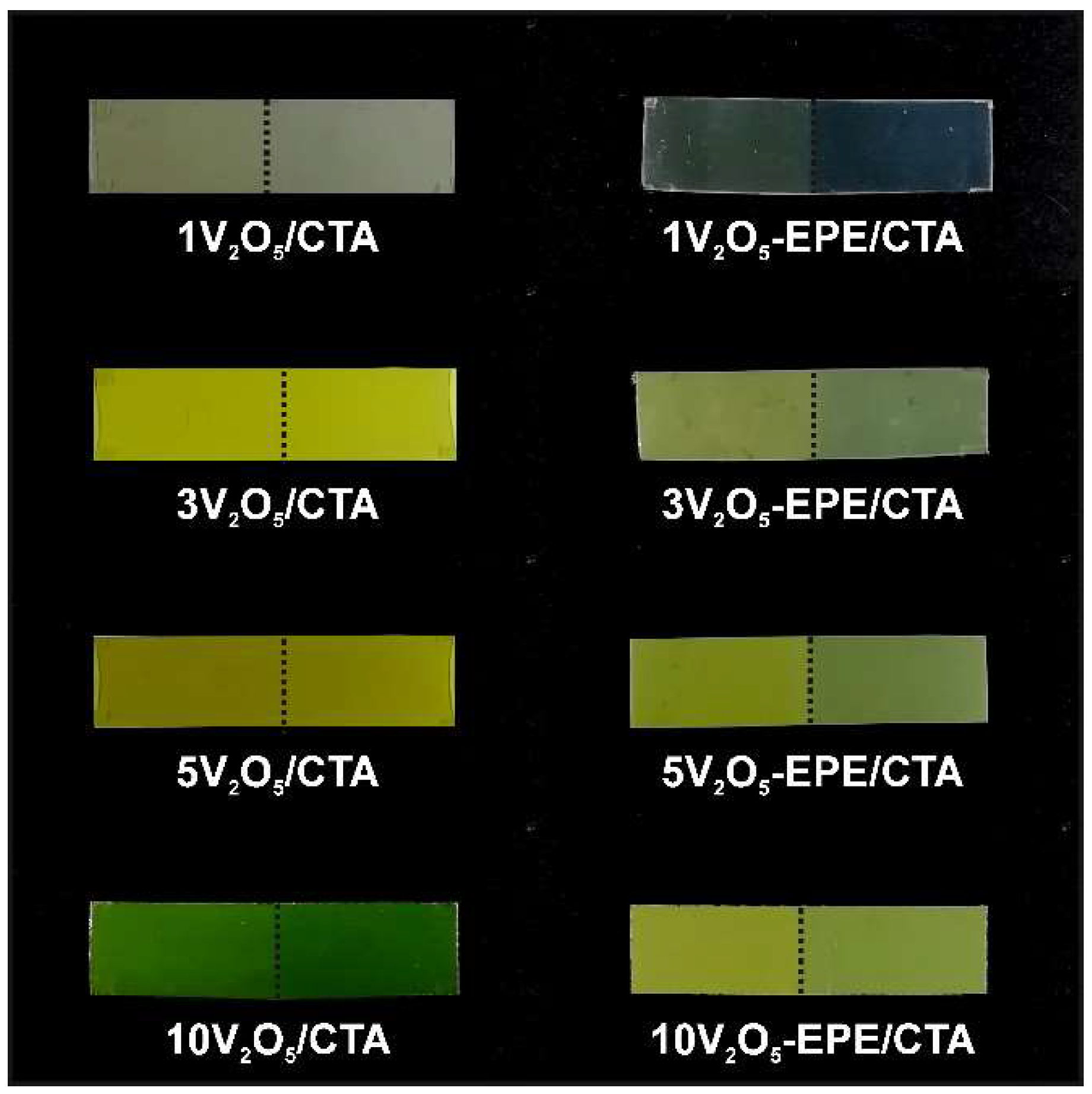
3.1. Appearance of Investigated Films
3.2. FTIR Characterisation
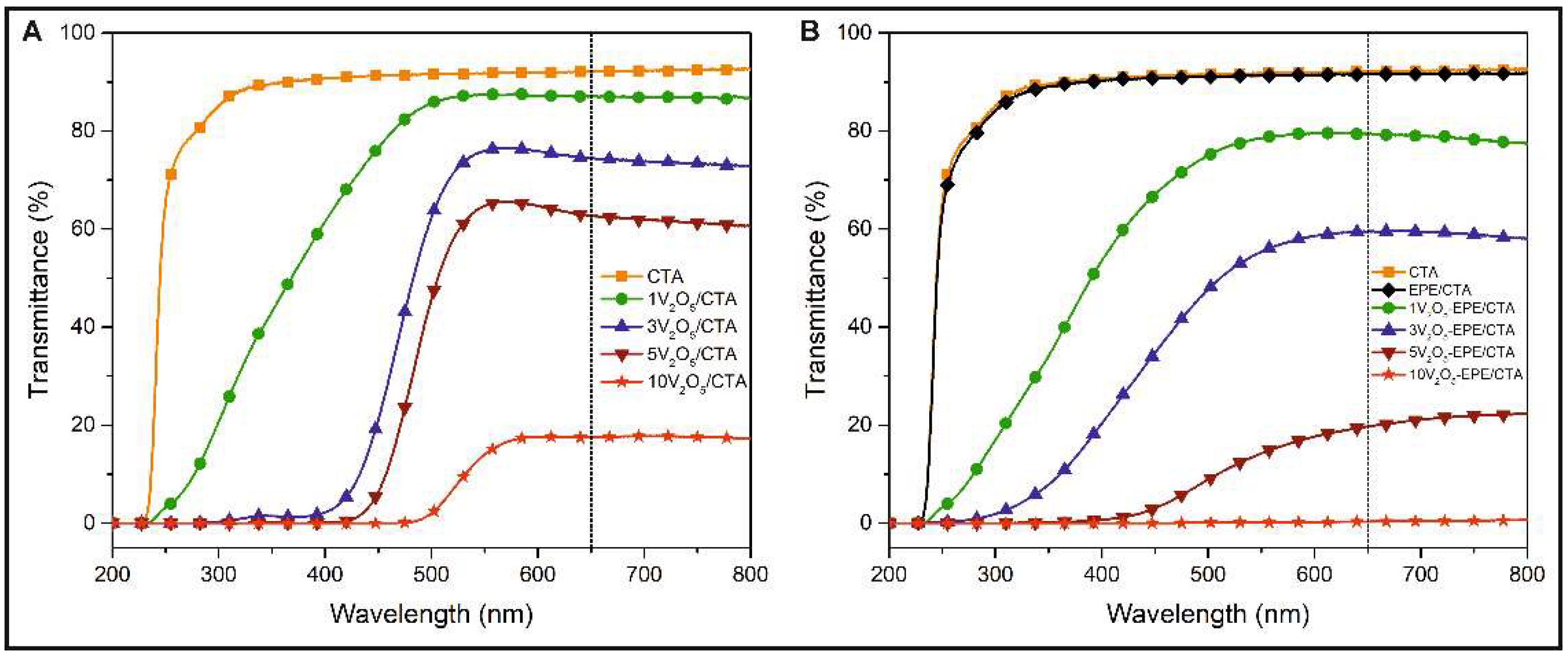
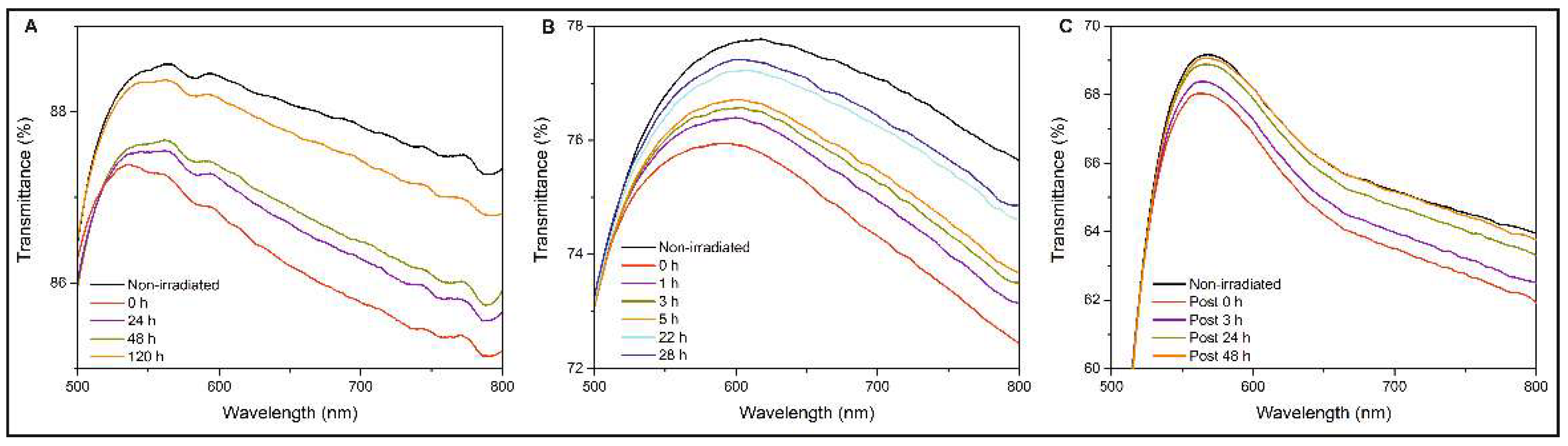
3.3. UV-Vis Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTA | cellulose triacetate |
| EPE | poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) |
| SC | solvent casting |
| SVA | solvent vapour annealing |
| VOIT | vanadium (V) oxytriisopropoxide |
References
- Thiangtham, S.; Runt, J.; Manuspiya, H. Sulfonation of dialdehyde cellulose extracted from sugarcane bagasse for synergistically enhanced water solubility. Carbohydr. Polym. 2019, 208, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dean, D. 3-D printed porous cellulose acetate tissue scaffolds for additive manufacturing. Addit. Manuf. 2020, 31, 100927. [Google Scholar] [CrossRef]
- Cheng, H.N.; Dowd, M.K.; Selling, G.W.; Biswas, A. Synthesis of cellulose acetate from cotton byproducts. Carbohydr. Polym. 2010, 80, 449–452. [Google Scholar] [CrossRef]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 2019, 20, 100302. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Tang, R.; Yan, Q.; Tian, W.; Zhang, L. Enhanced permeability, mechanical and antibacterial properties of cellulose acetate ultrafiltration membranes incorporated with lignocellulose nanofibrils. Int. J. Biol. Macromol. 2020, 151, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Charvet, A.; Vergelati, C.; Long, D.R. Mechanical and ultimate properties of injection molded cellulose acetate/plasticizer materials. Carbohydr. Polym. 2019, 204, 182–189. [Google Scholar] [CrossRef]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and characterization of cellulose acetate from rice husk: Eco-friendly condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef]
- Kaya, G.G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.-M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Prog. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environ. Impact Assess. Rev. 2020, 85, 106438. [Google Scholar] [CrossRef]
- Leibler, L. Nanostructured plastics: Joys of self-assembling. Prog. Polym. Sci. 2005, 30, 898–914. [Google Scholar] [CrossRef]
- An, M.; Zhang, Q.; Ye, K.; Lin, Y.; Wang, D.; Chen, W.; Yin, P.; Meng, L.; Li, L. Structural evolution of cellulose triacetate film during stretching deformation: An in-situ synchrotron radiation wide-angle X-Ray scattering study. Polymer 2019, 182, 121815. [Google Scholar] [CrossRef]
- Songsurang, K.; Miyagawa, A.; Manaf, M.E.A.; Phulkerd, P.; Nobukawa, S.; Yamaguchi, M. Optical anisotropy in solution-cast film of cellulose triacetate. Cellulose 2013, 20, 83–96. [Google Scholar] [CrossRef]
- Kloster, G.A.; Mosiewicki, M.A.; Marcovich, N.E. Chitosan/iron oxide nanocomposite films: Effect of the composition and preparation methods on the adsorption of congo red. Carbohydr. Polym. 2019, 221, 186–194. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Veer, A.; Shirsath, S.R.; Sonawane, S.H. Ultrasound assisted preparation, characterization and adsorption study of ternary chitosan-ZnO-TiO2 nanocomposite: Advantage over conventional method. Ultrason. Sonochem. 2018, 52, 120–130. [Google Scholar] [CrossRef]
- Muller, P.; Kapin, É.; Fekete, E. Effects of preparation methods on the structure and mechanical properties of wet conditioned starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2014, 113, 569–576. [Google Scholar] [CrossRef]
- Kang, M.; Oh, E.; Kim, I.; Kim, S.W.; Ryu, J.-W.; Kim, Y.-G. Optical characteristics of amorphous V2O5 thin films colored by an excimer laser. Curr. Appl. Phys. 2012, 12, 489–493. [Google Scholar] [CrossRef]
- Kwon, J.H.; Fatimah, S.; Baek, S.H.; Kim, Y.G.; Yang, H.W.; Ko, Y.G. Electrochemical response of VxOy-Al2O3 composite layer with dark-green color achieved by plasma electrolytic oxidation. J. Alloy. Compd. 2020, 827, 154367. [Google Scholar] [CrossRef]
- Sajitha, S.; Aparna, U.; Deb, B. Ultra-thin manganese dioxide-encrusted vanadium pentoxide nanowire mats for electrochromic energy storage applications. Adv. Mater. Interfaces 2019, 6, 1901038. [Google Scholar] [CrossRef]
- Such, G.K.; Evans, R.A.; Davis, T.P. Rapid photochromic switching in a rigid polymer matrix using living radical polymerization. Macromolecules 2006, 39, 1391–1396. [Google Scholar] [CrossRef]
- Malic, N.; Campbell, J.A.; Ali, A.S.; York, M.; D’Souza, A.; Evans, R.A. Controlling molecular mobility in polymer matrices: Synchronizing switching speeds of multiple photochromic dyes. Macromolecules 2010, 43, 8488–8501. [Google Scholar] [CrossRef]
- Ratner, J.; Kahana, N.; Warshawsky, A.; Krongauz, V. Photochromic polysulfones. 2. Photochromic properties of polymeric polysulfone carrying pendant spiropyran and spirooxazine groups. Ind. Eng. Chem. Res. 1996, 35, 1307–1315. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Hermoso-de-Mendoza, J.; Gutierrez, J.; Tercjak, A. Optical Properties of Vanadium Oxide/Cellulose Triacetate Photochromic Films. Proceedings 2021, 69, 37. https://doi.org/10.3390/CGPM2020-07182
Gomez-Hermoso-de-Mendoza J, Gutierrez J, Tercjak A. Optical Properties of Vanadium Oxide/Cellulose Triacetate Photochromic Films. Proceedings. 2021; 69(1):37. https://doi.org/10.3390/CGPM2020-07182
Chicago/Turabian StyleGomez-Hermoso-de-Mendoza, Joseba, Junkal Gutierrez, and Agnieszka Tercjak. 2021. "Optical Properties of Vanadium Oxide/Cellulose Triacetate Photochromic Films" Proceedings 69, no. 1: 37. https://doi.org/10.3390/CGPM2020-07182
APA StyleGomez-Hermoso-de-Mendoza, J., Gutierrez, J., & Tercjak, A. (2021). Optical Properties of Vanadium Oxide/Cellulose Triacetate Photochromic Films. Proceedings, 69(1), 37. https://doi.org/10.3390/CGPM2020-07182





