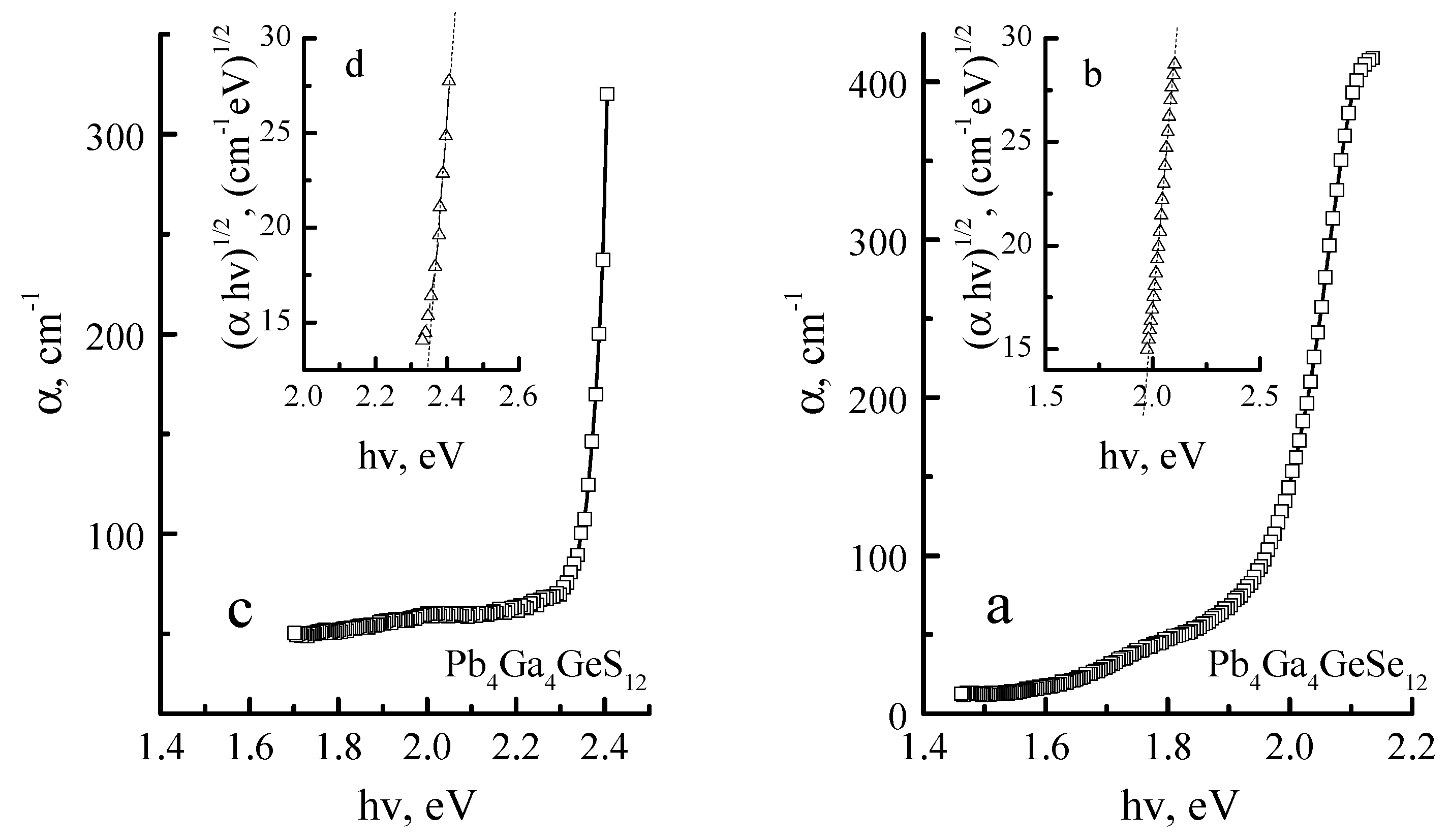1. Introduction
Recently, a series of new compounds were discovered (4-4-1-12 type: Pb
4Ga
4Ge(S,Se)
12 [
1], and 1-2-1-6 type: PbGa
2Ge(Si)Se
6, SnGa
2Ge(S,Se)
6, PbGa
2GeS
6 [
2,
3,
4,
5]) that possess qualitatively new physical properties and may serve as a basis for the design of non-linear optical materials. The 4-4-1-12 quaternary compounds feature non-linear coefficients exceeding those of AgGa(S,Se)
2 (record second harmonic generation (SHG) efficiencies) and an order of magnitude higher laser damage threshold. Additionally, they have a much longer-wave limit of IR transparency (up to 23 μm) and more suitable double refraction for phase synchronism of the converted radiation in the 1–10 μm range. The authors [
1] reported that the Pb
4Ga
4GeS(Se)
12 compounds are isostructural and crystallize in the tetragonal symmetry, space group
P–421c, with the unit cell parameters
a = 1.2673(2),
c = 0.6128(2) nm for Pb
4Ga
4GeS
12, and
a = 1.3064(7),
c = 0.6310(5) nm for Pb
4Ga
4GeSe
12. They have bandgap energy of 2.35 and 1.91 eV and are transparent in a wide range of the IR spectrum at 0.80–22.5 and 0.75–22.5 μm, respectively.
Our research was focused on the technology of the single crystal growth of these compounds, and their possible use in active elements for linear and non-linear optics and optoelectronics.
2. Materials and Methods
Polycrystalline alloys of the Pb4Ga4GeS(Se)12 compositions were preliminarily synthesized by co-melting the elements in the stoichiometric ratio. The batches were composed of high-purity elements Pb, 99.99 wt.%, Ga, 99.9997% (SmiLab Ltd., Svitlovodsk, Ukraine), Ge, 99.9999 wt.%, S, Se 99.999 wt.% (Alfa Aesar GmbH & Co KG, Karlsruhe, Germany). Lead was additionally purified by dripping through crushed quartz under static conditions. The total batch mass was 20 g. The sulfur-containing quartz ampoule was evacuated to 10−2 Pa and was locally heated in the oxygen-gas burner flame to complete bonding of sulfur under visual observation of the reaction process. Considering high vapor pressure, Pb4Ga4GeS12 was synthesized in 5 g batches followed by the transfer of the powdered obtained alloy to the growth container. Pb4Ga4GeSe12 was synthesized already in the evacuated growth container. Quartz ampoules with conical bottom were used for the crystal growth.
The results of preliminary experiments suggest the critical importance of the homogeneity of the starting batch and rather high temperature gradient at the solid–melt interface. This is because lead reacts rather poorly with chalcogens. Initial interaction of the elements yields binary lead chalcogenide, which surrounds molten lead and prevents further reaction with other elements or binary chalcogenides of other metals. Additionally, the melts of these systems have high viscosity, as one of the system components (GeS(Se)2) is a known glass-forming agent. High viscosity hinders the diffusion mixing of the melt above the growing crystal which may lead to the formation of blocs, admixtures of other phases, uncontrolled occlusion, all of which affect the properties of single crystals. Therefore, operations of forced homogenization are necessary, such as furnace rotation or vibration, or crushing the alloy into powder before loading it into the growth container.
Homogenization of the alloys of both phases was achieved by placing the ampoules with the alloys in a rotation furnace and heating to 1170 K at the rate of 50 K/h. The furnace was rotated at this temperature for 24 h. After stopping the furnace in the vertical position, the alloys were cooled at 20 K/h to 670 K and annealed for 240 h. The synthesis process was finished by cooling to room temperature in the inertial mode. The obtained alloys were compact ingots.
The crystal growth was performed by the Bridgman method [
6] in a two-zone furnace with steady temperature profile. The growth containers with the synthesized batches were placed in the pre-heated furnace. The temperature of the upper isothermal zone was 1170 K, and that of the lower zone varied from 620 to 670 K. The temperature in the growth zone was controlled with ±0.5 K accuracy. Independent temperature control and selection of metal discs between the two zones permitted the variation of temperature gradient at the solid–melt interface of 1.2–3 K/cm. The growth rate varied in the range of 5–6 mm/day. After the compete crystallization of the melts, the furnace was cooled to room temperature at the rate of 5 K/h.
3. Results
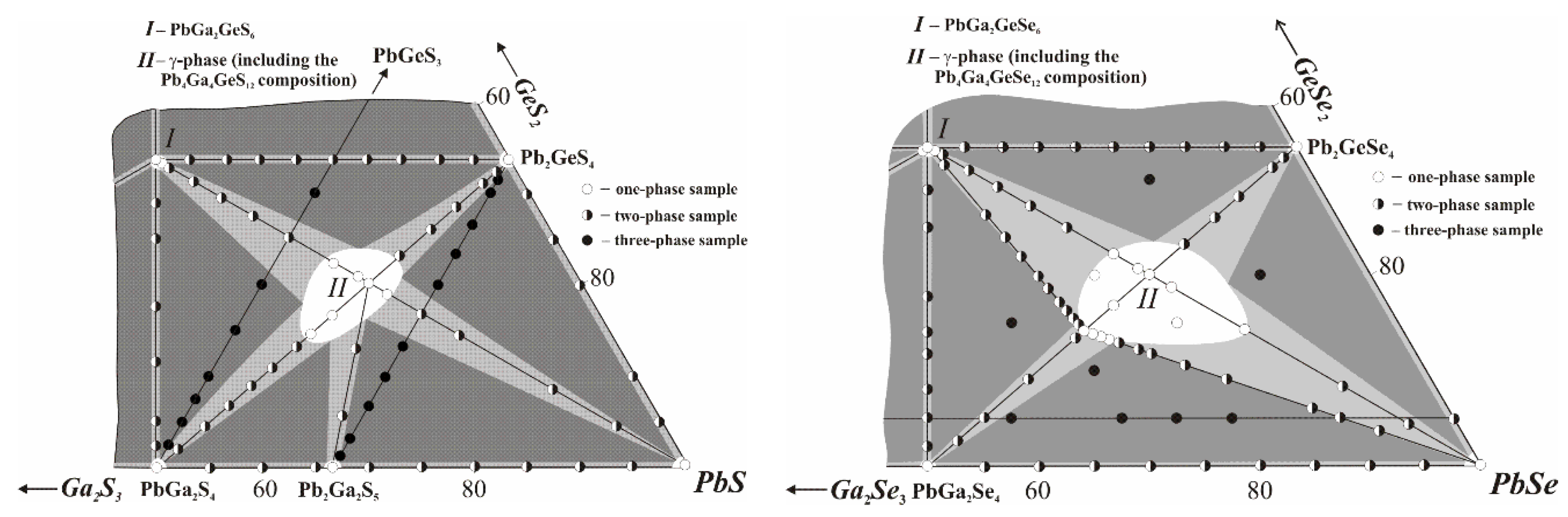
3.1. Phase Equilibria Related to the Formation of Pb3Ga2GeS(Se)8
Our investigations of the quasi-ternary systems PbS(Se)−Ga
2S(Se)
3−GeS(Se)
2 using the XRD and differential thermal analysis (DTA) methods (to be published in detail elsewhere) showed that the Pb
4Ga
4GeS(Se)
12 compositions belong to the solid solution ranges of the Pb
3Ga
2GeS(Se)
8 compounds that form at the crossing of the sections PbGa
2S(Se)
4−Pb
2GeS(Se)
4 and PbS(Se)−PbGa
2GeS(Se)
6 (
Figure 1).
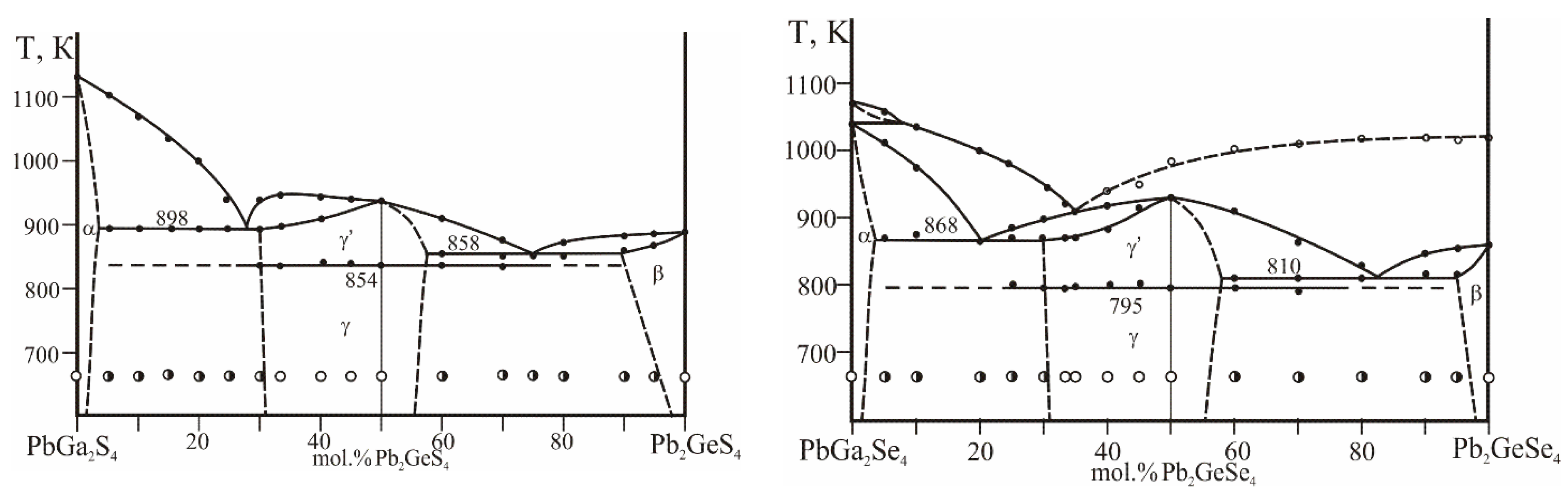
Vertical sections of PbGa
2S(Se)
4−Pb
2GeS(Se)
4 were investigated to consider the conditions for the crystal growth of Pb
4Ga
4GeS(Se)
12 as the sections demonstrate the nature of the formation of these phases (
Figure 2). The sulfur-containing section PbGa
2S
4−Pb
2GeS
4 is quasi-binary in the entire concentration and temperature range, while the selenide section PbGa
2Se
4−Pb
2GeSe
4 is quasi-binary only in the sub-solidus region due to incongruent melting of the starting ternary compounds PbGa
2Se
4 and Pb
2GeSe
4. Each of the 4-4-1-12 compounds is dimorphous, with the phase transitions taking place at 854 K for the sulfide and 795 K for the selenide. The Pb
4Ga
4GeS(Se)
12 phases each occupy at the studied sections of PbGa
2S(Se)
4−Pb
2GeS(Se)
4 the range of ~32–57 mol.% Pb
2GeS(Se)
4.
The quaternary sulfide melts congruently at 943 K. The melting point maximum falls onto the Pb4Ga4GeS12 composition, while the stoichiometric composition of the quaternary solid solution corresponds to Pb3Ga2GeS8. The eutectic between PbGa2S4 and HT-Pb4Ga4GeS12 lies at 27 mol.% Pb2GeS4, 898 K; the eutectic between HT-Pb4Ga4GeS12 and Pb2GeS4 is at 75 mol.% Pb2GeS4, 858 K.
The crystallization of the Pb4Ga4GeSe12 phase is related to the ternary peritectic process Lp1 + PbSe ↔ PbGa2Se4 (α-solid solutions) + Pb3Ga2GeSe8 (γ׳-solid solutions) at 868 K in the PbSe−Ga2Se3−GeSe2 system. The other ternary peritectic process Lp2+ PbSe ↔ Pb3Ga2GeSe8 (γ׳-solid solutions) + Pb2GeSe4 (β-solid solutions) takes place at 810 K.
3.2. Optical Properties of the Pb3Ga2GeS(Se)8 Single Crystals
Two experiments examining the growth of each of the crystals were performed. The increase in the temperature gradient proved quite advantageous. Using optimized growth condition, crystals of up to 11 mm diameter and 58 mm (Pb4Ga4GeS12) and 55 mm length (Pb4Ga4GeSe12) were obtained. It should be noted that the lower conical bottom of the selenide sample (up to 2 mm diameter) contains blocs of other phase composition, due to the field of the secondary crystallization L + δ + γ. The XRD results confirm that the low-temperature modifications of these compounds have non-centrosymmetric crystal structure (tetragonal symmetry, space group P–421c).
Obtained boules were cut using Ni-Cr wire into separate plates that were polished to parallel-plane, optical quality samples of 0.2 mm thickness. These samples were used for the measurements of physical properties such as optical absorption and photoconductivity.
The data from experimental absorption spectra (
Figure 3) are comparable to the theoretical calculations of the band gap energy [
7]. The band gap of the studied materials was determined by the extrapolation of the linear part of the absorption curve to the energy axis (
Figure 3b,d). The obtained bandgap energy value is equal to 2.28 and 1.86 eV for Pb
4Ga
4GeS
12 and Pb
4Ga
4GeSe
12 crystals, respectively, which is close to the results of [
1].
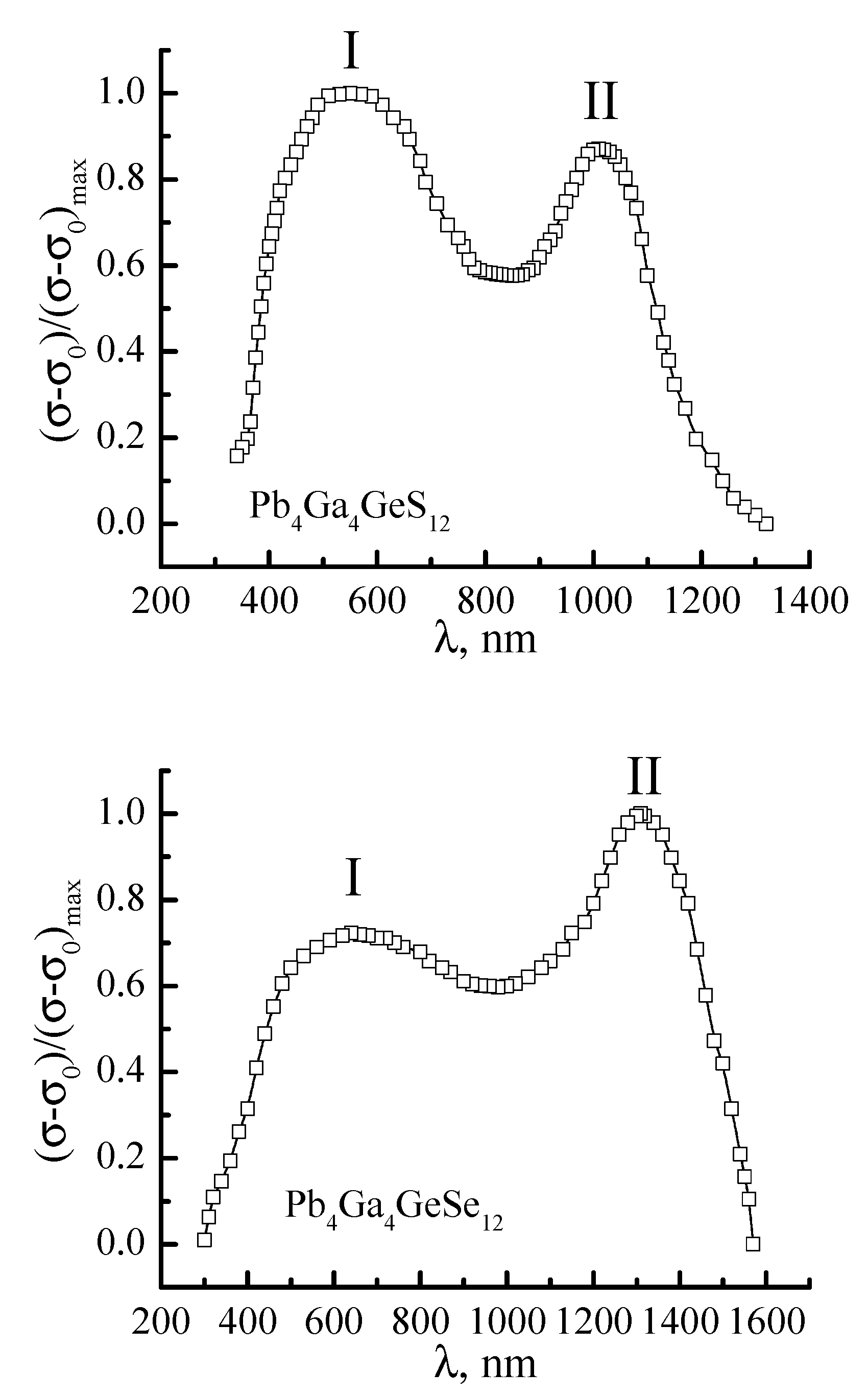
A study of the spectral distribution of photoconductivity for Pb
4Ga
4GeS
12 and Pb
4Ga
4GeSe
12 crystals was undertaken for additional confirmation of the obtained results (
Figure 4). Experimental measurements were performed using Keithley 6514 Sub-Femtoamp SourceMeter electrometer. Sensitivity of experimental setup was not worse than 1 pA. An MDR-206 monochromator with the diffraction grating with 600 lines/mm and spectral resolution 0.2 nm was used as a spectral device. The electrical contacts were applied by fusing of Ga-In eutectic and were ohmic for all considered compositions.
A characteristic feature of the spectral distribution of photoconductivity is the presence of two spectral maxima. The first lies in the region of λ
1 = 570 (2.17 eV) and 680 nm (1.82 eV) for the Pb
4Ga
4GeS
12 and Pb
4Ga
4GeSe
12 crystals, respectively, and show a good match with the band gap estimated from the spectral dependence of the absorption coefficient. Therefore, we can assert that peak I of the spectral dependence Δσ/Δσ
max is due to the intrinsic photoconductivity of the studied crystals. The impurity level is located in the region of λ
2 = 1030 (1.20 eV) and 1340 nm (0.92 eV) for Pb
4Ga
4GeS
12 and Pb
4Ga
4GeSe
12 crystals, respectively. One can see from the obtained results that the location of impurity maximum satisfactorily agrees well with the calculated energy positions of intrinsic defects vs. and V
Se [
7], which is close to the center of the band gap. The lower formation energy of V
Se in comparison with the energy of vs. formation favors the prevalence of peak II of the impurity photoconductivity in the Pb
4Ga
4GeSe
12 crystals.
Author Contributions
Conceptualization, L.P. and H.M.; methodology, L.P., Y.K. and H.M.; sample synthesis, H.B. and O.N.; single crystal growth, Y.K. and H.B.; DTA and XRD data collection and analysis, Y.K. and H.B.; optical absorption and photoconductivity data collection and analysis, O.N. and H.M.; writing—original draft preparation, H.B., Y.K. and O.N.; writing—review and editing, Y.K., L.P. and H.M.; supervision, H.M.; project administration, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Y.K.; Chen, M.C.; Zhou, L.J.; Chen, L.; Wu, L.M. Syntheses, structures, and nonlinear optical properties of quaternary chalcogenides: Pb4Ga4GeQ12 (Q = S, Se). Inorg. Chem. 2013, 52, 8334–8341. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-Z.; Lin, C.-S.; Cui, H.-H.; Long, W. PbGa2MSe6 (M = Si, Ge): Two Exceptional Infrared Nonlinear Optical Crystals. Chem. Mater. 2015, 27, 914–922. [Google Scholar] [CrossRef]
- Fedorchuk, A.O.; Parasyuk, O.V.; Cherniushok, O.; Andriyevsky, B.; Myronchuk, G.L.; Khyzhun, O.Y.; Lakshminarayana, G.; Jedryka, J.; Kityk, I.V.; El-Naggar, A.M.; et al. PbGa2GeS6 crystal as a novel nonlinear optical material: Band structure aspects. J. Alloys Compd. 2018, 740, 294–304. [Google Scholar] [CrossRef]
- Yousaf, N.; Khan, W.; Khan, S.H.; Yaseen, M.; Laref, A.; Murtaza, G. Electronic, optical and thermoelectric properties of SnGa2GeX6 (X = S, Se) compounds. J. Alloys Compd. 2018, 737, 637–645. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Zhang, H.; Lin, C.-S.; Cheng, W.-D.; Guo, Z.; Chai, G.-L. PbGa2GeS6: An Infrared Nonlinear Optical Material Synthesized by an Intermediate-Temperature Self-Fluxing Method. Cryst. Growth Des. 2018, 18, 1162–1167. [Google Scholar] [CrossRef]
- Vilke, K.-T. Crystal Growth; Nedra: Leningrad, Russia, 1977. [Google Scholar]
- Piasecki, M.; (Jan Dlugosz University, Czestochowa, Poland); Rudysh, M.; (Ivan Franko National University of Lviv, Lviv, Ukraine). Personal communication, 2019.
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).